Introduction
MicroRNAs (miRNAs/miRs) are non-coding,
single-stranded RNAs of 21 to 23 nucleotides in length that
post-transcriptionally regulate gene expression (1). miRNAs regulate the expression of an
estimated 30% of all mRNAs in humans (1). To date, thousands of miRNAs have been
identified in the human genome, and were found to have critical
roles in a wide array of physiological and pathological processes
(2). Of note, it has been revealed
that the dysregulation of miRNAs is implicated in cancer (3–7). The
miR-33 family, including miR-33a and miR-33b, are encoded within
the intronic sequences of the Srebp genes in organisms (8–11).
miR-33a is located in intron 16 of the Srebp-2 gene on chromosome
22 and miR-33b is present in intron 17 of the Srebp-1 gene on
chromosome 17 (12). The miR-33
family were reported to be involved in the regulation and balancing
of cholesterol metabolism, fatty acid oxidation and insulin
signaling (12–15). Several recent studies reported that
miR-33a was involved in several malignancies, including lung cancer
(16), hepatocellular carcinoma
(17), colon cancer (18), osteosarcoma (19) and supraglottic carcinoma (20). However, studies regarding miR-33a
expression in various tumor types are controversial. Certain
studies reported miR-33a to be upregulated in supraglottic
(20) and intestinal-type gastric
cancer tissues (21), while other
studies showed that miR-33a was down-regulated in metastatic lung
cancer (22). Contradictory
results were also reported regarding the functions of miR-33a in
cancer. While certain studies reported that miR-33a promoted
tumorigenesis (23), cancer
progression (18) and drug
resistance (19), of human colon
cancer, osteosarcoma and hematopoietic stem cells, others reported
that miR-33a inhibited the proliferation (24,25),
migration and invasion (16,22)
of lung cancer and lymphoma. Gastric cancer is the fourth most
common malignancy in the world and the second leading cause of
cancer mortality. More than 70% of new cases and mortalities occur
in developing countries, particularly in China (26). However, to the best of our
knowledge, the roles of miR-33a in gastric cancer have not been
studied. The expression and functions of miR-33a in gastric cancer,
particularly in clinical specimens, have remained to be
elucidated.
Although surgery is the most effective treatment for
localized gastric cancer, ~50% of all patients have recurrences
following curative resection. Even though traditional chemotherapy
is effective in these cases, the prognosis of gastric cancer
patients remains poor (27–29).
Novel and effective molecular targets, such as miRNAs, for treating
gastric cancer are therefore urgently required. The present study
examined miR-33a expression in gastric cancer specimens and cell
lines and investigated its correlation with clinicopathological
factors as well as gastric cancer-associated antigens and gene
expression. The effects of miR-33a overexpression on the
proliferation and cell cycle progression of the SGC-7901 gastric
cancer cell line as well as gene expression were assessed. A
luciferase reporter assay was employed to identify direct target
proteins of miR-33a. The present study indicated that miR-33a may
be a biomarker and a molecular target for gastric cancer
therapy.
Materials and methods
Reagents and apparatus
In the present study, the following reagents and
apparatus were used: miRNeasy Mini kit (Qiagen, Valencia, CA, USA),
a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA),
GoTaq® qPCR Master mix (Promega Corp., Madison, WI,
USA), RevertAid First Strand RevertAid First Strand cDNA Synthesis
kit (Thermo Fisher Scientific, Waltham, MA, USA), primers (shown in
Table I; Invitrogen Life
Technologies, Inc., Carlsbad, CA, USA), ABI 7500 thermocycler
(Applied Biosystems, Thermo Fisher Scientific), miScript miR-33a
mimic (Qiagen), miScript miR-33a inhibitor (Qiagen), AllStars
Negative Control small interfering (si)RNA (Qiagen), miScript
Inhibitor Negative control (Qiagen), HiperFect transfection reagent
(Qiagen), Transwell inserts (pore size, 8 µm;
Corning-Costar, Corning, NY, USA), Matrigel basement membrane
matrix (BD Biosciences, Bedford, MA, USA), Giemsa (Sigma-Aldrich,
St. Louis, MO, USA), pGL3 control vector (Promega Corp.), pRL-TK
(Promega Corp.), Dual-Luciferase Reporter Assay system (Promega
Corp), Protein bicinchoninic acid (BCA) Assay kit (Bio-Rad
Laboratories Inc., Hercules, CA, USA), polyvinylidene difluoride
membranes (Millipore, Billerica, MA, USA), anti-PIM1 (rabbit
monoclonal, Abcam, Cambridge, MA, USA; cat. no. ab75776; 1:15,000),
anti-p53 (rabbit monoclonal; Abcam; cat. no. ab179477; 1:3,000),
anti-CDK6 (rabbit monoclonal; Abcam; cat. no. ab124821; 1:100,000),
anti-CCND (rabbit monoclonal; Abcam; cat. no. ab16663; 1:200),
anti-β-actin (rabbit polyclonal antibody, Santa Cruz Biotechnology,
Inc., Dallas, TX, USA; cat. no. sc-130657; 1:500) and anti-rabbit
IgG VHH Single Domain antibody (HRP) (Abcam; cat. no. ab191866;
1:5,000).
 | Table ISequences of primers used for
PCR. |
Table I
Sequences of primers used for
PCR.
| Primer name | Primer sequence
(5′–3′) |
|---|
| hsa-miR-33a-RT |
GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTGCAAT |
|
hsa-miR-33a-PCR |
GGCCGTGCATTGTAGTTGC |
| hsa-miR-33b-RT |
GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGCAATG |
|
hsa-miR-33b-PCR |
GGCGTGCATTGCTGTTGC′ |
| U6-RT |
CGCTTCACGAATTTGCGTGTCAT |
| U6-PCR |
GCTTCGGCAGCACATATACTAAAAT |
| UNP |
CAGTGCGTGTCGTGGAGT |
| CDK6-F |
GAGTCTGATTACCTGCTCCGC |
| CDK6-R |
CCGGAGATCGGTCTAGCTTT |
| CCND1-F |
GATGCCAACCTCCTCAACGA |
| CCND1-R |
GGAAGCGGTCCAGGTAGTTC |
| PIM1-F |
CTGGGGAGAGCTGCCTAATG |
| PIM1-R |
AAGAGATCTTGCACCGGCTC |
| P53-F |
CTGCCCTCAACAAGATGTTTTG |
| P53-R |
CTATCTGAGCAGCAGCGCTCATGG |
| 18S-F |
CAGCCACCCGAGATTGAGCA |
| 18S-R |
TAGTAGCGACGGGCGGTGTG |
| CDK6 3′UTR-F |
GACTAGTGTCCCCCTCAGCAAGC |
| CDK6 3′UTR-R |
CCCAAGCTTCAAATCAGGCCCGGCAGCTGC |
| CCND1 3′UTR-F |
GACTAGTAGAGAACAGG |
| CCND1 3′UTR-R |
CCCAAGCTTATTGTTTTTTTCCACCT |
| PIM1 3′UTR-F |
GACTAGTTGTCAGATGCCCGAGGG |
| PIM1 3′UTR-R |
CCCAAGCTTAATAAGATCTCTTTTATTCCCCTGT |
| P53 3′UTR-F |
GACTAGTCCTCCGGAGTAGCTGG |
| P53 3′UTR-R |
CCCAAGCTTGATCGATATAAAAATGGG |
Cell culture and clinical specimen
collection
The human gastric carcinoma cell lines MKN-28,
SGC-7901, BGC-823 and HGC-27 and the gastric epithelial cell line
GES-1 were provided by the Research Center of the Fourth Hospital
of Hebei Medical University (Shijiazhuang, China), obtained from
the Tumor Cell Bank of Chinese Academy of Medical Sciences
(Beijing, China). Cells were cultured in 10-cm2 dishes
(BD Biosciences, Franklin Lakes, NJ, USA) in Dulbecco's modified
Eagle's medium or RPMI-1640 (Gibco Life Technologies, Carlsbad, CA,
USA) and incubated at 37°C with humidified 5% CO2. The
protocol for the collection of clinical specimens and the study
protocol were approved by the Ethics Committee of Hebei Medical
University (Shijiazhuang, China). All participants provided written
informed consent. A total of 57 gastric carcinoma tissues and
paired adjacent tissues were retrieved from surgical pathology
files at the Forth Hospital of Hebei Medical University
(Shijiazhuang, China). None of the patients had received
pre-operative irradiation or chemotherapy. Information regarding
age, gender, histological grade, differentiation status of
adenocarcinoma, stage, tumor size/invasion depth (T), tumor
embolus, degree of lymph node invasion (N) and distant metastasis
(M) was retrieved from patient charts. The pre-treatment of the
tissues was performed as previously described (30). Only sections containing a minimum
of 90% carcinoma cells by examination with hematoxylin-eosin
staining (Promega Corp.) were used for total RNA preparation
(Fig. 1).
Total RNA isolation and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA, including miRNA, was isolated from frozen
gastric carcinoma tissues, the paired adjacent tissues and cell
lines using the miRNeasy Mini kit according to the manufacturer's
instructions. RNA purity and concentration were confirmed by
spectrophotometry using the NanoDrop ND-1000. RT-qPCR analysis was
performed using the RevertAid First Strand cDNA Synthesis kit and
the GoTaq® qPCR Master Mix according to the
manufacturer's instructions in an ABI 7500 thermocycler. PCR
primers are shown in Table I. The
thermocycling conditions were as follows: 95°C for 2 min, 95°C for
15 sec for 40 cycles, 60°C for 1 min for 40 cycles, 95°C for 15
sec, 60°C for 15 sec and 95°C for 15 sec. The fold-change for each
miRNA and mRNA, relative to U6 and 18S, were calculated using the
2−ΔΔCt method. Three independent RT-PCR reactions were
performed.
Transfection of miScript miRNA mimic and
inhibitor
SGC-7901 cells (1×105) were transfected
with 5 nM miScript miR-33a mimic, 50 nM miScript miR-33a inhibitor,
5 nM AllStars Negative Control siRNA or 50 nM miScript Inhibitor
Negative Control using a HiperFect transfection Reagent according
to the manufacturer's instructions.
Cell proliferation assay
SGC-7901 cells transfected with miR-33a mimics (5
nM), inhibitor (50 nM) or Allstars negative control siRNA (50 nM)
were seeded in 96-well plates at a density of 5×103
cells per well for the indicated time intervals. Cell proliferation
was assessed using the Cell Counting Kit-8 (CCK-8; Dojindo,
Kumamoto, Japan) as described previously (31).
Cell cycle analysis
SGC-7901 cells transfected with miR-33a mimics (5
nM), inhibitor (50 nM) or Allstars negative control siRNA (50 nM)
were seeded in cell culture plates and incubated for 24, 48 and 72
h. At the end of the incubation, the cells were washed twice with
ice-cold phosphate-buffered saline (PBS), fixed in 70% cold
ethanol, treated with 100 µg/ml ribonuclease A (Roche
Diagnostics, Basel, Switzerland) and labeled with 50 µg/ml
propidium iodide (PI; Sigma-Aldrich) for 30 min at 37°C. The cells
were analyzed using the FACScalibur™system (BD Biosciences) in
conjunction with CellQuest software (BD Biosciences) (32).
Luciferase reporter assays
The 3′ untranslated regions (UTR) in the gene
promoters of PIM1, P53, CDK6 and CCND1 were
PCR-amplified from genomic DNA and inserted into the pMIR control
vector using the SpeI and HindIII restriction sites.
PCR primers used to amplify the PIM1, P53, CDK6 and CCND1 3′UTRs
are listed in Table I. SGC-7901
cells were co-transfected with 2 µg firefly luciferase
reporter vector and 0.5 µg control vector containing
Renilla luciferase pRL-TK vector. For each group, 20 nM of
the miR-33a mimic or AllStars Negative Control siRNA was used.
Firefly and Renilla luciferase activities were measured
consecutively using the dual luciferase system at 48 h after
transfection.
Western blot analysis
Cell extracts were prepared and protein
concentration in the lysates was measured using the Protein BCA
Assay kit. Western blot analysis was performed as described
previously (33). The antibodies
used for western blotting were PIM1, P53, CDK6 and CCND1. An
anti-β-actin antibody was used as a protein loading control.
Primary antibodies were applied at 4°C overnight. The secondary
antibody was incubated at room temperature for 1 h.
Statistical analysis
The expression levels of miRNAs from tissues were
compared by using the Wilcoxon Signed Ranks test, the Mann-Whitney
U test or the Kruskal-Wallis test. A Multiple linear regression
analysis was performed to analyze the combined effects of
clinicopathological features. The cell experimental data were
analyzed by using the t-test. A P-value of less than 0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
miR-33a is downregulated in gastric
cancer
To evaluate the expression of miR-33 family members
in gastric cancer tissues, RT-qPCR was used to detect the
expression levels in 57 pairs of tumor tissues and their matched
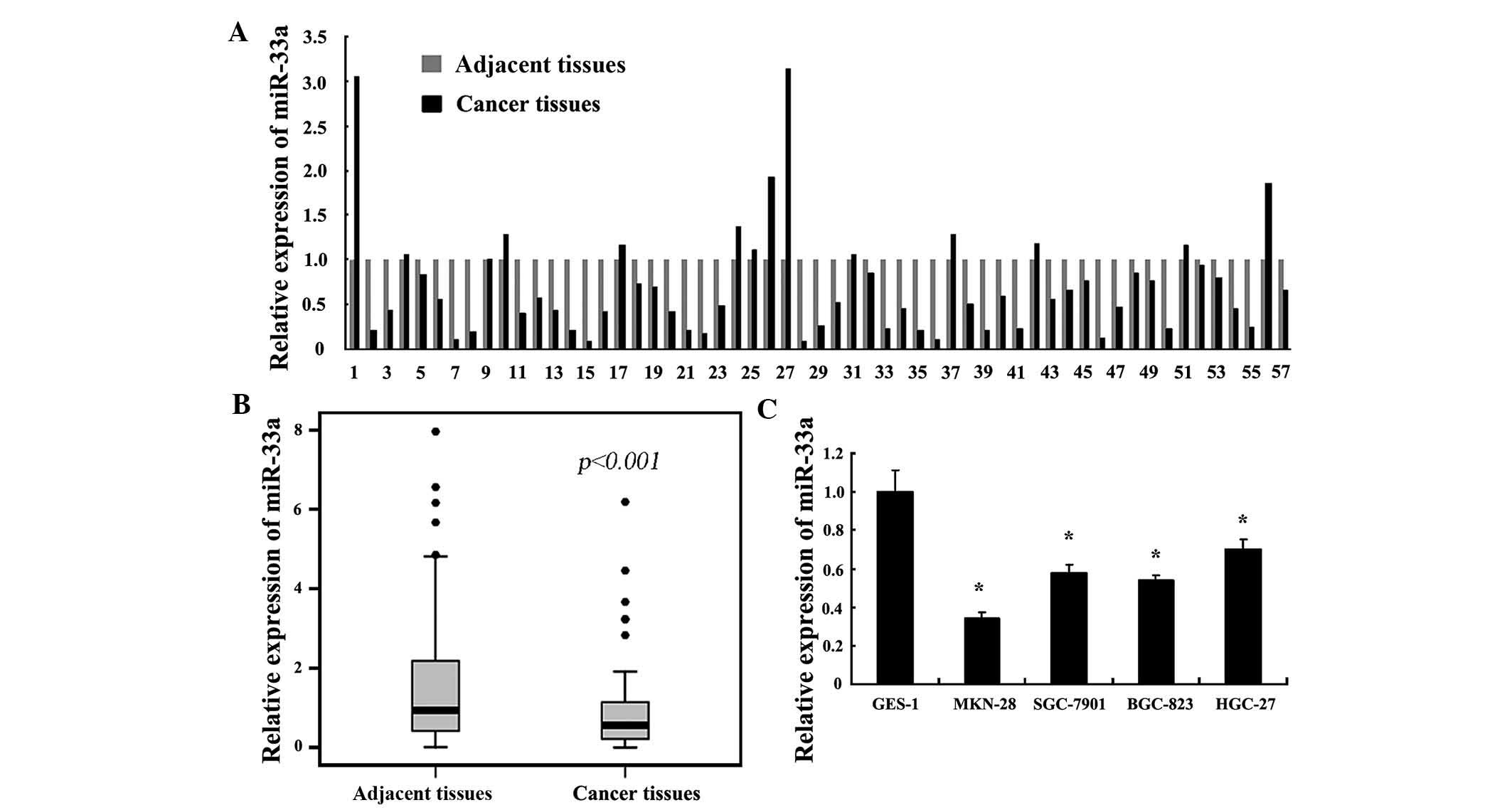
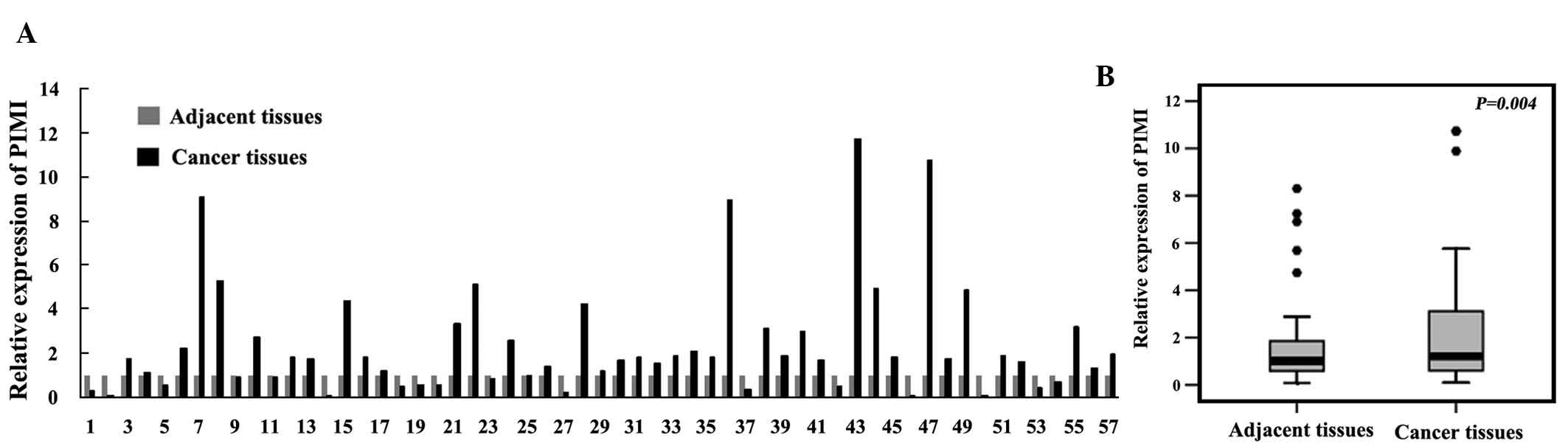
adjacent tissues. In comparison with the adjacent tissues, miR-33a
was downregulated in 75.4% (43/57) of the tumor samples. The
expression of miR-33a in gastric cancer tissues was significantly
lower than that in their matched adjacent tissues (Fig. 2A and B). While miR-33b was
downregulated in 56.1% (32/57) of the tumor samples, its expression
was not significantly different between gastric cancer tissues and
normal adjacent tissues (data not shown). Similarly to the
expression in cancer tissue specimens, miR-33a was reduced in the
gastric cancer cell lines compared to that in the normal gastric
cell line GES-1 (Fig. 2C).
miR-33a levels are inversely correlated
with pathological differentiation and distant metastasis (M)
Next, the present study determined the potential
clinicopathological implications of altered miR-33a expression in
gastric cancer. All data of the patients who donated the tissue
samples, including age, gender, pathological differentiation,
tumor-nodes-metastasis (TNM) stage, tumor size/invasion depth (T),
lymph node metastasis (N), M and venous invasion were obtained from
clinical and pathological records. In the 57 patients with gastric
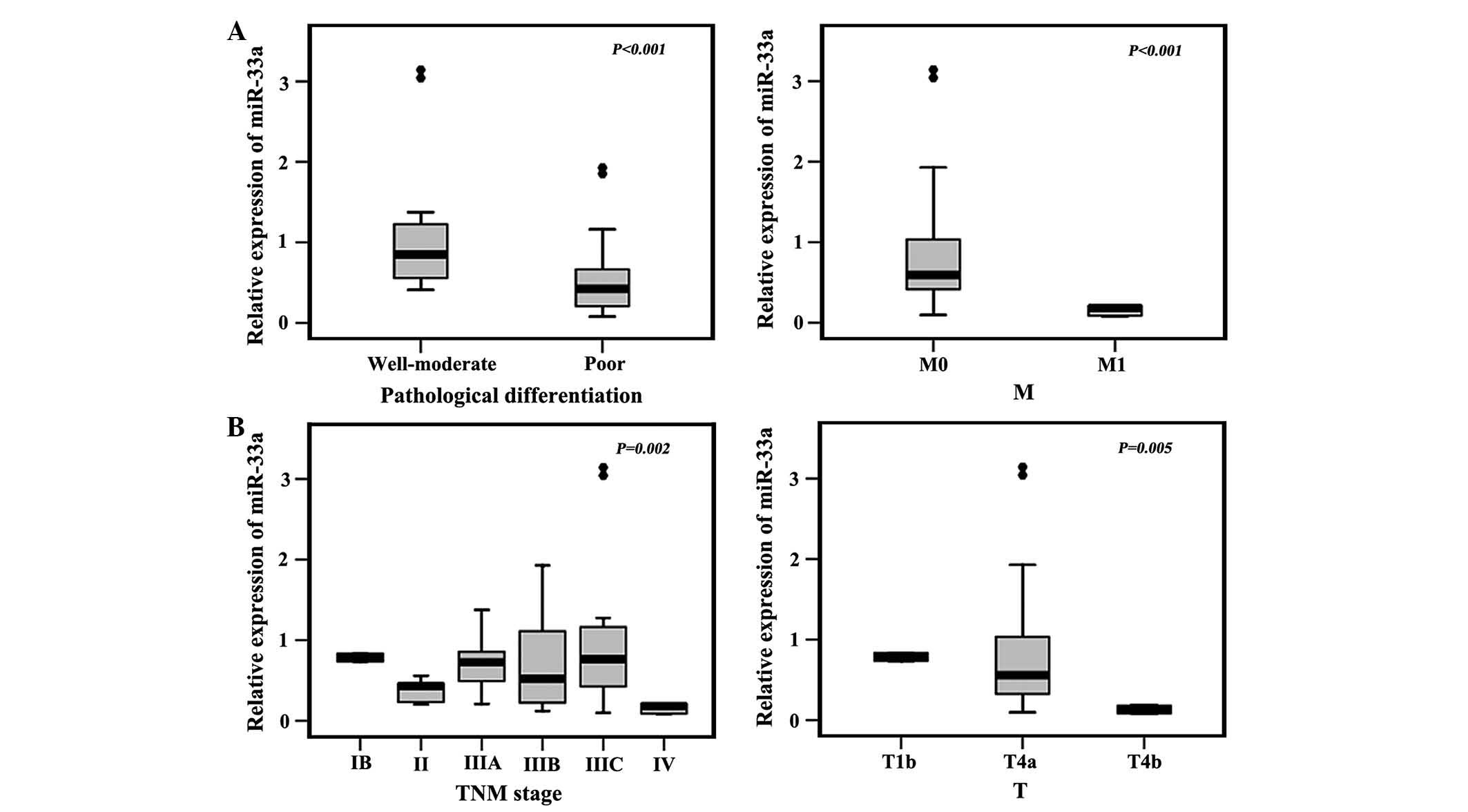
cancer, miR-33a expression was associated with pathological
differentiation, TNM stage, T and M, but not with age, gender, N
and venous invasion (Table II and
Fig. 3). To analyze the combined
effects of pathological differentiation, T, N, M and venous
invasion on miR-33a expression, a multiple linear regression
analysis was performed using these clinicopathological features.
The results showed that miR-33a expression was inversely correlated
with pathological differentiation and M (Table III). In addition, the expression
of miR-33a was negatively correlated with gastric cancer biomarker
CA199 (Table IV). These results
suggested that miR-33a had critical roles in the proliferation,
invasion and metastasis of gastric cancer.
 | Table IICorrelation between the expression of
microRNA-33a in gastric cancer specimens with clinicopathological
features. |
Table II
Correlation between the expression of
microRNA-33a in gastric cancer specimens with clinicopathological
features.
| Clinicopathological
feature | Number | Median | Interquartile
range | P-value |
|---|
| Gender | | | | 0.211a |
| Male | 53 | 0.560 | 0.768 | |
| Female | 4 | 0.433 | 0.656 | |
| Age (years) | | | | 0.127a |
| ≤65 | 41 | 0.554 | 0.707 | |
| >65 | 16 | 0.728 | 0.633 | |
| Pathological
differentiation | | | | 0.001a |
| Well/moderate | 20 | 0.899 | 0.619 | |
| Poor | 37 | 0.431 | 0.538 | |
| TNM stage | | | | 0.002b |
| IB | 2 | 0.833 | 0.092 | |
| IIB | 6 | 0.431 | 0.328 | |
| IIIA | 14 | 0.789 | 0.450 | |
| IIIB | 12 | 0.575 | 0.837 | |
| IIIC | 17 | 0.761 | 0.712 | |
| IV | 6 | 0.190 | 0.119 | |
| T | | | | 0.005b |
| T1b | 2 | 0.833 | 0.092 | |
| T4a | 57 | 0.595 | 0.748 | |
| T4b | 4 | 0.139 | 0.102 | |
| N | | | | 0.113b |
| N0 | 8 | 0.495 | 0.483 | |
| N1 | 14 | 0.789 | 0.450 | |
| N2 | 12 | 0.574 | 0.837 | |
| N3a | 17 | 0.525 | 0.938 | |
| N3b | 6 | 0.190 | 0.673 | |
| M | | | | 0.001a |
| M0 | 51 | 0.666 | 0.576 | |
| M1 | 6 | 0.190 | 0.119 | |
| Venous
invasion | | | | 0.643a |
| Negative | 36 | 0.557 | 0.703 | |
| Positive | 21 | 0.595 | 0.932 | |
 | Table IIICombined effects of pathological
differentiation, T/N/M and venous invasion on microRNA-33a
expression. |
Table III
Combined effects of pathological
differentiation, T/N/M and venous invasion on microRNA-33a
expression.
| Model | Standardized
coefficient
| P-value |
|---|
| Β | t |
|---|
| Constant | | 3.422 | <0.001 |
| Histological
grade | −0.105 | −3.164 | 0.003 |
| T | −0.431 | −0.169 | 0.867 |
| N | −0.247 | 1.745 | 0.087 |
| M | −0.186 | −2.016 | 0.049 |
| Venous
invasion | −0.265 | 0.297 | 0.768 |
 | Table IVCorrelation of the expression of
microRNA-33a with the levels of antigens CA50, CEA, CA199 and CA724
in gastric cancer specimens. |
Table IV
Correlation of the expression of
microRNA-33a with the levels of antigens CA50, CEA, CA199 and CA724
in gastric cancer specimens.
| Antigen | Number | Median | Interquartile
range | Spearman test
|
|---|
| r | P-value |
|---|
| CA50 (IU/ml) | 51 | 7.40 | 7.45 | −0.214 | 0.132 |
| CEA (ng/ml) | 53 | 2.42 | 2.36 | 0.067 | 0.632 |
| CA199 (U/ml) | 53 | 15.64 | 21.02 | −0.407 | 0.002 |
| CA724 (U/ml) | 53 | 2.11 | 8.17 | −0.100 | 0.477 |
miR-33a inhibits cell proliferation and
causes G1-phase arrest
To investigate the biological effects of miR-33a,
the present study assessed the changes in cell proliferation, cell
cycle of the gastric cancer cell line SGC-7901 transfected with
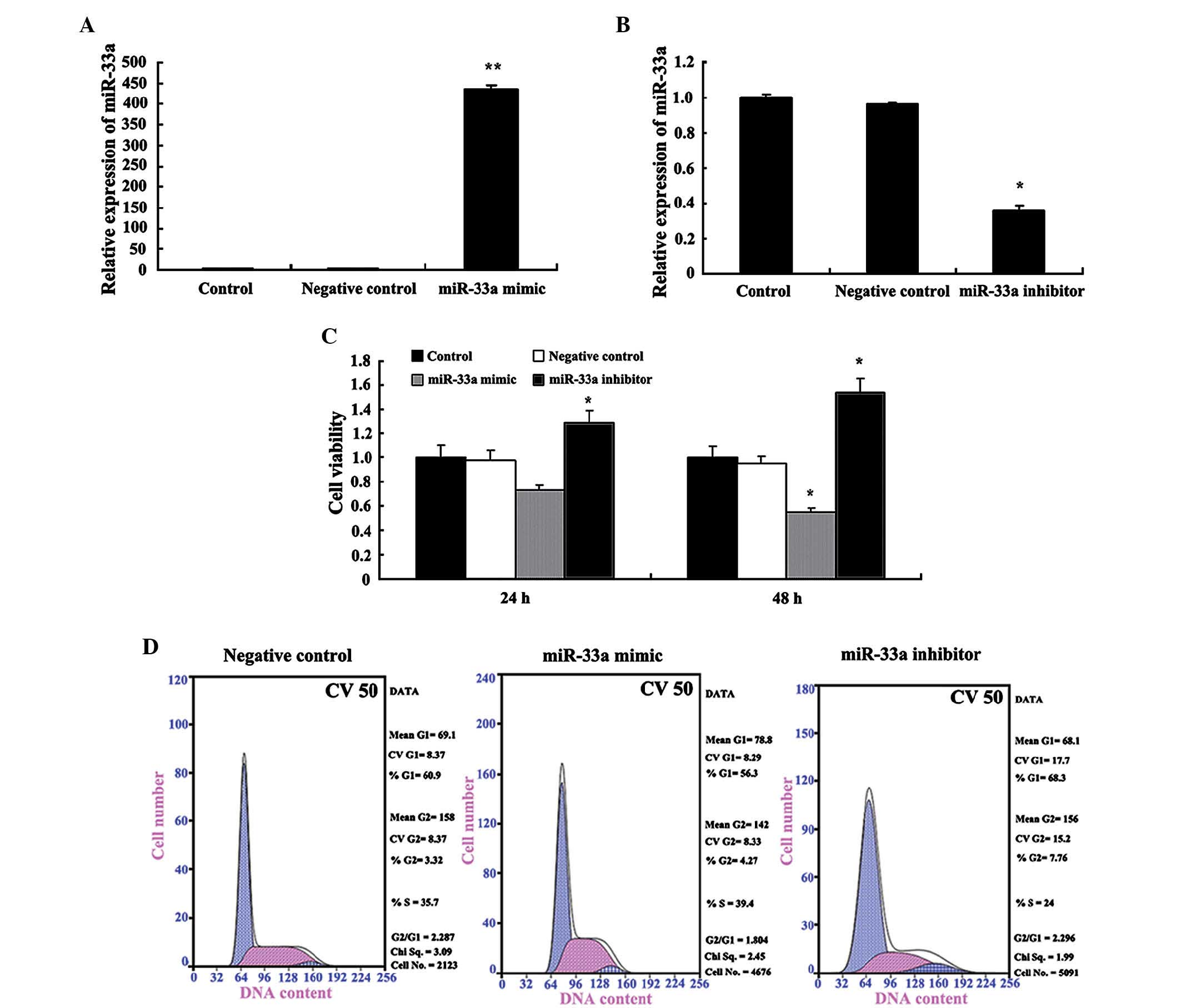
vectors expressing miR-33a mimic or inhibitor. RT-qPCR analysis
showed that the forced overexpression (Fig. 4A) and knockdown (Fig. 4B) of miR-33a were successful.
Transfection of miR-33a mimic significantly inhibited the capacity
of SGC-7901 cells to proliferate and arrested cells in
G1 phase, while the transfection with miR-33a inhibitor
significantly increased cell proliferation (Fig. 4B and C). In addition, the effects
of miR-33a overexpression or knockdown on the migratory and
invasive properties of SGC-7901 cells were assessed using Transwell
and wound healing assays; however, no differences between
transfected and control cells were observed (data no shown).
miR-33a mimic significantly arrested cells in the G1 phase.
miR-33a directly targets CDK6, CCND1 and
PIM1 in SGC-7901 gastric cancer cells
To reveal how miR-33a suppresses gastric cancer cell
proliferation, Targetscan (http://www.targetscan.org/) and Miranda (http://www.microrna.org/) were used to identify
miR-33a targets in human gastric cancers. Among these candidate
target genes, cyclin-dependent kinase (CDK6), cyclin (CCN)D1, PIM1
and p53 were predicted to be miR-33a targets (Fig. 5A). CDK6 and CCND1 regulate cell
cycle progression from G1 to S phase (34). Downregulation of CDK6 and CCND1
leads to cell cycle arrest in G1 phase (35). PIM1 is a positive regulator at the
G2/M transition of the cell cycle (36) and mediates resistance to AKT
inhibition (37). p53 as a tumor
suppressor inhibits cell proliferation by inducing apoptosis and G1
arrest (38,39). To test the direct targeting of
CDK6, CCND1, PIM1 and p53 by miR-33a, their complementary site
sequences were inserted into plasmids downstream of a firefly
luciferase reporter gene, which were then co-transfected into
SGC-7901 cells together with miR-33a mimic or scrambled
oligonucleotide. Compared to the scrambled oligonucleotide
transfection, the co-transfection with miR-33a caused a marked
reduction in the luciferase activity of the CDK6, CCND1 and PIM1
reporter constructs (Fig. 4B).
Following transfection with miR-33a mimics, protein and mRNA levels
of CDK6, CCND1 and PIM1 were significantly decreased, while they
were significantly increased following miR-33a inhibition
(P<0.01) (Fig. 5C and D). In
the miR-33a mimics group, CCND1 and CDK6 were downregulated to a
greater extent than PIM1. The above results may indicate that
miR-33a inhibited gastric cancer cell proliferation via the
downregulation of CDK6, CCND1 and PIM1.
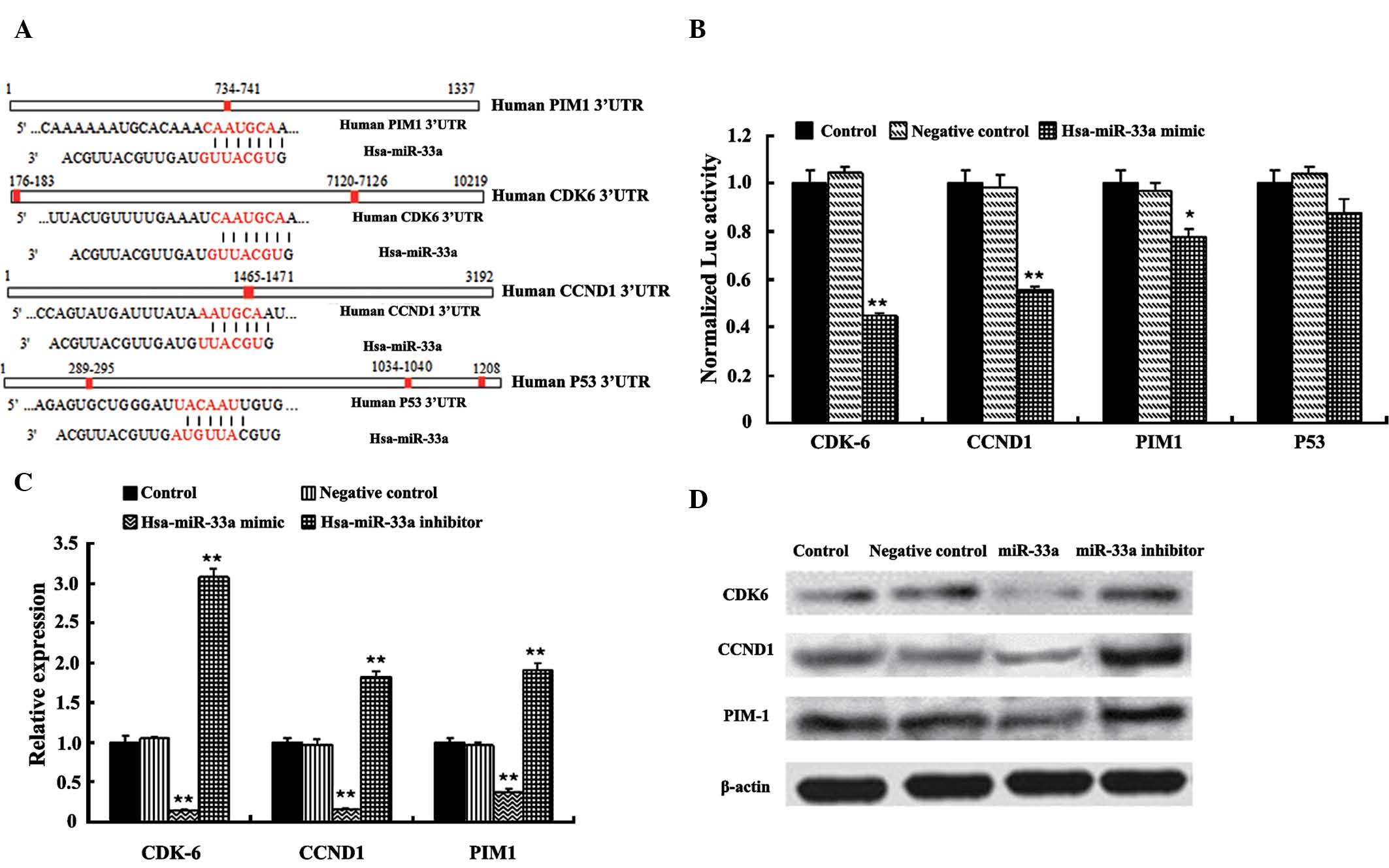 | Figure 5miR-33a directly targets CDK6, CCND1
and PIM1. (A) The 3′UTRs of CDK6, CCND1, PIM1 and P53 have putative
binding sites for miR-33a. (B) Normalized activity of luciferase
reporter with the CDK6, CCND1, PIM1 and P53 3′UTR in SGC-7901 cells
in the presence of co-transfected Allstars negative control small
interfering RNA or miR-33a mimic. Effects of miR-33a on the (C)
mRNA and (D) protein expression of CDK6, CCND1 and PIM1 in SGC-7901
were detected by reverse transcription quantitative polymerase
chain reaction and western blot analysis, respectively. Values are
expressed as the mean ± standard deviation of three independent
experiments. *P<0.05, **P<0.01 vs.
control. miR, microRNA; UTR, untranslated region; CCND1, cyclin D1;
Luc, luciferase; Hsa, Homo sapiens. |
PIM1 is upregulated in gastric cancer
tissues
Since the present study showed that CDK6, CCND1 and
PIM1 were targets of miR-33a, the expression of CDK6, CCND1 and
PIM1 in gastric cancer tissues was evaluated. To verify the direct
association of miR-33a with CDK6, CCND1 and PIM1 in gastric cancer,
a correlation analysis was performed, revealing that the levels of
miR-33a were negatively correlated to the expression of CDK6, CCND1
and PIM1 in gastric cancer specimens (Table V). In comparison with the adjacent
tissues PIM1 was upregulated in 68.4% (39/57) of the tumor samples
(Fig. 6A). Furthermore, CDK6 and
CCND1 were upregulated in 45.6% (26/57) and 54.4% (31/57) of tumor
tissues, respectively, compared with those in adjacent tissues
(results not shown). Quantification of the results showed that
expression of PIM1 but not CDK6 and CCND1 was significantly higher
in gastric cancer tissues than that in their matched adjacent
normal tissues (Fig. 6B). These
results indicated that miR-33a most likely has a role in gastric
cancer by targeting PIM1.
 | Table VSpearman correlation of miR-33a with
the expression of CDK6, CCND1, PIM1and P53 in gastric cancer
specimens (n=57). |
Table V
Spearman correlation of miR-33a with
the expression of CDK6, CCND1, PIM1and P53 in gastric cancer
specimens (n=57).
| Gene | r | P-value |
|---|
| CDK6 | −0.353 | 0.010 |
| CCND1 | −0.323 | 0.014 |
| PIM1 | −0.282 | 0.033 |
| P53 | −0.095 | 0.482 |
Discussion
It is widely known that the miR-33 family has a
critical role in cholesterol metabolism, fatty acid oxidation and
regulation of insulin signaling (12,17,40,41).
An increasing number of studies reported that miR-33a participates
in the initiation and progression of malignancies (16–20).
However, previous results regarding miR-33a expression were
contradictory (21,22). Numerous studies have demonstrated
that miR-33a expression is dysregulated in various human cancer
types (16–20). However, to the best of our
knowledge, miR-33a has not been previously studied in gastric
cancer. The present study reported that miR-33a expression in
gastric cancer tissues was significantly lower than that in their
adjacent tissues. Similarly, the expression of miR-33a was reduced
in gastric cancer cell lines compared to that in a normal gastric
cell line. In addition, the expression of miR-33a was inversely
correlated with pathological differentiation and M. Furthermore,
miR-33a expression was negatively correlated with cancer biomarker
CA199, which is one of the most sensitive and relevant tumor
biomarkers for gastric cancer. These results collectively indicated
that miR-33a may be a critical factor of proliferation, migration
and invasion, as well as a potential therapeutic target for the
inhibition of these processes in gastric cancer. Furthermore,
similarly to CA199, miR-33a may be a useful predictive biomarker of
gastric cancer.
Previous studies regarding the function of miR-33a
in cancer have reported conflicting results. A recent study
reported that miR-33a was upregulated in human colon cancer stem
cells (SW1116csc) (18). miR-33a
induced tumorigenesis by decreasing p53-mediated apoptosis
(23). In addition, a recent study
indicated that miR-33a promoted osteosarcoma-cell resistance to
cisplatin by downregulating TWIST (19). By contrast, a few studies
demonstrated that miR-33a functioned as tumor-suppressor to inhibit
metastasis (16,22) and proliferation (24,25,42)
in numerous cancer types. These findings indicated that the
function of miR-33a in cancer is not universal and that it may be
cell type-specific. The present study further investigated the
roles of miR-33a in gastric cancer, indicating that miR-33a
inhibited the proliferation of SGC-7901 gastric cancer cells due to
causing cell cycle arrest in G1 phase.
miRNAs have roles of post-transcriptional regulation
by inhibiting the expression of their target genes. miR-33a
regulates cholesterol levels by targeting Abca1, Abcg1 and Npc1
(9–11) as well as fatty acid metabolism by
targeting Ampk, Cpt1a, Crot, Hadhb and Sirt6 (8,12).
However, various studies reported a diversity of miR-33a target
genes involved in cancer. For instance, p53 was reported as one of
the target genes of miR-33a (23).
Another study reported that PIM1 was one of the target genes of
miR-33a, but not p53 or CDK6 (25). A further study showed that CDK6 and
CCND1 were the target genes of miR-33a (24). In the present study, Luciferase
reporter assays showed that in SGC-7901 cells, miR-33a directly
targeted CDK6, CCND1 and PIM1, but not p53. These results suggested
that the effect of miR-33 on target genes and cancer cell viability
may be cell type-specific. In SGC-7901 cells overexpressing
miR-33a, mRNA and protein levels of CDK6 and CCND1 were decreased
to a greater extent than PIM1, which may be the reason for the
observed cell cycle arrest in G1 phase, but not in G2 phase. To
confirm this pathway in gastric cancer patients, the expression of
CDK6, CCND1 and PIM1 was assessed in gastric cancer specimens,
showing that miR-33a expression was negatively correlated to CDK6,
CCND1 and PIM1. The expression levels of PIM1, but not those of
CDK6 and CCND1, were significantly higher in gastric cancer tissues
than those in their matched adjacent tissues. As to the differences
between the results for cells and clinical specimens, additional
mechanisms to the miR-33a/CDK6/CCND1 pathway, possibly associated
with the tumor microenvironment, may have been involved in tumors
from patients with gastric cancer, which require to be further
investigated. A previous study reported that transforming growth
factor (TGF)-β inhibited the expression of CDK6 (43), and our group also identified that
TGF-β was upregulated in gastric cancer tissues (data not shown).
The results of the present study proved that miR-33a exerted its
effects by directly interacting with CDK6, CCND1 and PIM1 in
gastric cancer cells and mostly through PIM1 in gastric cancer
patients.
In conclusion, the present study showed that miR-33a
inhibits the proliferation of gastric cancer cells by directly
targeting CDK6, CCND1 and PIM1. Analysis of tumor samples from
gastric cancer patients showed that the miR-33a/PIM1 interaction
was the most clinically relevant pathway involved. miR-33a may be
utilized as a valuable biomarker for gastric cancer and may
resemble a potent therapeutic target.
References
|
1
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi M, Liu D, Duan H, Shen B and Guo N:
Metastasis-related miRNAs, active players in breast cancer invasion
and metastasis. Cancer Metastasis Rev. 29:785–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng F, Henson R, Wehbe-Janek H, Smith H,
Ueno Y and Patel T: The MicroRNA let-7a modulates
interleukin-6-dependent STAT-3 survival signaling in malignant
human cholangiocytes. J Biol Chem. 282:8256–8264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fassina A, Cappellesso R, Simonato F, Siri
M, Ventura L, Tosato F, Busund LT, Pelizzo MR and Fassan M: A
4-MicroRNA signature can discriminate primary lymphomas from
anaplastic carcinomas in thyroid cytology smears. Cancer
Cytopathol. 122:274–281. 2014. View Article : Google Scholar
|
|
5
|
Wang Q, Huang Z, Guo W, Ni S, Xiao X, Wang
L, Huang D, Tan C, Xu Q, Zha R, et al: MicroRNA-202-3p inhibits
cell proliferation by targeting ADP-ribosylation factor-like 5A in
human colorectal carcinoma. Clin Cancer Res. 20:1146–1157. 2014.
View Article : Google Scholar
|
|
6
|
Nohata N, Sone Y, Hanazawa T, Fuse M,
Kikkawa N, Yoshino H, Chiyomaru T, Kawakami K, Enokida H, Nakagawa
M, et al: miR-1 as a tumor suppressive microRNA targeting TAGLN2 in
head and neck squamous cell carcinoma. Oncotarget. 2:29–42. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S, Chen Y, Wu W, Ouyang N, Chen J,
Li H, Liu X, Su F, Lin L and Yao Y: miR-150 promotes human breast
cancer growth and malignant behavior by targeting the pro-apoptotic
purinergic P2X7 receptor. PLoS One. 8:e807072013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerin I, Clerbaux LA, Haumont O, Lanthier
N, Das AK, Burant CF, Leclercq IA, MacDougald OA and Bommer GT:
Expression of miR-33 from an SREBP2 intron inhibits cholesterol
export and fatty acid oxidation. J Biol Chem. 285:33652–33661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marquart TJ, Allen RM, Ory DS and Baldán
A: miR-33 links SREBP-2 induction to repression of sterol
transporters. Proc Natl Acad Sci USA. 107:12228–12232. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Najafi-Shoushtari SH, Kristo F, Li Y,
Shioda T, Cohen DE, Gerszten RE and Näär AM: MicroRNA-33 and the
SREBP host genes cooperate to control cholesterol homeostasis.
Science. 328:1566–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rayner KJ, Suárez Y, Dávalos A, Parathath
S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ and
Fernández-Hernando C: MiR-33 contributes to the regulation of
cholesterol homeostasis. Science. 328:1570–1573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dávalos A, Goedeke L, Smibert P, Ramírez
CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U,
Pastor-Pareja JC, et al: miR-33a/b contribute to the regulation of
fatty acid metabolism and insulin signaling. Proc Natl Acad Sci
USA. 108:9232–9237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wijesekara N, Zhang LH, Kang MH, Abraham
T, Bhattacharjee A, Warnock GL, Verchere CB and Hayden MR: miR-33a
modulates ABCA1 expression, cholesterol accumulation and insulin
secretion in pancreatic islets. Diabetes. 61:653–658. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramírez CM, Goedeke L, Rotllan N, Yoon JH,
Cirera-Salinas D, Mattison JA, Suárez Y, de Cabo R, Gorospe M and
Fernández-Hernando C: MicroRNA 33 regulates glucose metabolism. Mol
Cell Biol. 33:2891–2902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goedeke L, Vales-Lara FM, Fenstermaker M,
Cirera-Salinas D, Chamorro-Jorganes A, Ramírez CM, Mattison JA, de
Cabo R, Suárez Y and Fernández-Hernando C: A regulatory role for
microRNA 33* in controlling lipid metabolism gene
expression. Mol Cell Biol. 33:2339–2352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rice SJ, Lai SC, Wood LW, Helsley KR,
Runkle EA, Winslow MM and Mu D: MicroRNA-33a mediates the
regulation of high mobility group AT-hook 2 gene (HMGA2) by thyroid
transcription factor 1 (TTF-1/NKX2-1). J Biol Chem.
288:16348–16360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Y, Feng Y, Wu T, Srinivas S, Yang W,
Fan J, Yang C and Wang S: Aflatoxin B1 negatively regulates
Wnt/ß-catenin signaling pathway through activating miR-33a. PLoS
One. 8:e730042013. View Article : Google Scholar
|
|
18
|
Yu XF, Zou J, Bao ZJ and Dong J: miR-93
suppresses proliferation and colony formation of human colon cancer
stem cells. World J Gastroenterol. 17:4711–4717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Huang Z, Wu S, Zang X, Liu M and
Shi J: miR-33a is up-regulated in chemoresistant osteosarcoma and
promotes osteosarcoma cell resistance to cisplatin by
down-regulating TWIST. J Exp Clin Cancer Res. 33(12)2014.
View Article : Google Scholar
|
|
20
|
Zhang T, Han G, Wang Y, Chen K and Sun Y:
MicroRNA expression profiles in supraglottic carcinoma. Oncol Rep.
31:2029–2034. 2014.PubMed/NCBI
|
|
21
|
Li X, Luo F, Li Q, Xu M, Feng D, Zhang G
and Wu W: Identification of new aberrantly expressed miRNAs in
intestinal-type gastric cancer and its clinical significance. Oncol
Rep. 26:1431–1439. 2011.PubMed/NCBI
|
|
22
|
Kuo PL, Liao SH, Hung JY, Huang MS and Hsu
YL: MicroRNA-33a functions as a bone metastasis suppressor in lung
cancer by targeting parathyroid hormone related protein. Biochim
Biophys Acta. 1830:3756–3766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herrera-Merchan A, Cerrato C, Luengo G,
Dominguez O, Piris MA, Serrano M and Gonzalez S: miR-33-mediated
down-regulation of p53 controls hematopoietic stem cell
self-renewal. Cell Cycle. 9:3277–3285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cirera-Salinas D, Pauta M, Allen RM,
Salerno AG, Ramírez CM, Chamorro-Jorganes A, Wanschel AC, Lasuncion
MA, Morales-Ruiz M, Suarez Y, et al: MiR-33 regulates cell
proliferation and cell cycle progression. Cell Cycle. 11:922–933.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas M, Lange-Grünweller K, Weirauch U,
Gutsch D, Aigner A, Grünweller A and Hartmann R: The proto-oncogene
Pim-1 is a target of miR-33a. Oncogene. 31:918–928. 2012.
View Article : Google Scholar
|
|
26
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang YK, Kang WK, Shin DB, Chen J, Xiong
J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, et al:
Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as
first-line therapy in patients with advanced gastric cancer: A
randomised phase III noninferiority trial. Ann Oncol. 20:666–673.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ajani JA, Rodriguez W, Bodoky G,
Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A,
Lang I and Falcon S: Multicenter phase III comparison of
cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced
gastric or gastro-esophageal adenocarcinoma study: The FLAGS trial.
J Clin Oncol. 28:1547–1553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohtsu A, Shah MA, Van Cutsem E, Rha SY,
Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: A randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo W, Dong Z, Guo Y, Chen Z, Kuang G and
Yang Z: Methylation-mediated repression of GADD45A and GADD45G
expression in gastric cardia adenocarcinoma. Int J Cancer.
133:2043–2053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong D, Zheng T, Zhang M, Wang D, Du S, Li
X, Fang J and Cao X: Static mechanical stress induces apoptosis in
rat endplate chondrocytes through MAPK and mitochondria-dependent
caspase activation signaling pathways. PLoS One. 8:e694032013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang H, Deng M, Tang Y and Xie X, Guo J,
Kong Y, Ye F, Su Q and Xie X: miR-200b and miR-200c as prognostic
factors and mediators of gastric cancer cell progression. Clin
Cancer Res. 19:5602–5612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou X, Sang M, Liu W, Gao W, Xing E, Lü
W, Xu Y, Fan X, Jing S and Shan B: LMO4 inhibits p53-mediated
proliferative inhibition of breast cancer cells through interacting
p53. Life Sci. 91:358–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nurse P: Checkpoint pathways come of age.
Cell. 91:865–867. 1997. View Article : Google Scholar
|
|
35
|
Rivadeneira DB, Mayhew CN, Thangavel C,
Sotillo E, Reed CA, Graña X and Knudsen ES: Proliferative
suppression by CDK4/6 inhibition: Complex function of the
retinoblastoma pathway in liver tissue and hepatoma cells.
Gastroenterology. 138:1920–1930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bachmann M, Hennemann H, Xing PX, Hoffmann
I and Möröy T: The oncogenic serine/threonine kinase Pim-1
phosphorylates and inhibits the activity of Cdc25C-associated
kinase 1 (C-TAK1): A novel role for Pim-1 at the G2/M cell cycle
checkpoint. J Biol Chem. 279:48319–48328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cen B, Mahajan S, Wang W and Kraft AS:
Elevation of receptor tyrosine kinases by small molecule AKT
inhibitors in prostate cancer is mediated by Pim-1. Cancer Res.
73:3402–3411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Waldman T, Kinzler KW and Vogelstein B:
p21 is necessary for the p53-mediated G1 arrest in human cancer
cells. Cancer Res. 55:5187–5190. 1995.PubMed/NCBI
|
|
40
|
Baselga-Escudero L, Arola-Arnal A,
Pascual-Serrano A, Ribas-Latre A, Casanova E, Salvadó MJ, Arola L
and Blade C: Chronic administration of proanthocyanidins or
docosahexaenoic acid reverses the increase of miR-33a and miR-122
in dyslipidemic obese rats. PLoS One. 8:e698172013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang JM, Zhou JJ, Zheng Q, Gan H and Wang
H: Dialysis method alters the expression of microRNA-33a and its
target genes ABCA1, ABCG1 in THP-1 macrophages. Ther Apher Dial.
18:44–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li T, Francl JM, Boehme S and Chiang JY:
Regulation of cholesterol and bile acid homeostasis by the
cholesterol 7α-hydroxylase/steroid response element-binding protein
2/microRNA-33a axis in mice. Hepatology. 58:1111–1121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang F, Taipale M, Heiskanen A and Laiho
M: Ectopic expression of Cdk6 circumvents transforming growth
factor-beta mediated growth inhibition. Oncogene. 20:5888–5896.
2001. View Article : Google Scholar : PubMed/NCBI
|