Introduction
Coronary heart disease (CHD), a leading cause of
mortality worldwide (1), often
develops as a result of myocardial ischemia/hypoxia, secondary to
coronary atherosclerosis (CAS)-induced stenosis (2). In 2009, the mortality of CHD patients
in China was ~94.9/100,000 in cities, which was higher than that in
the rural areas (71.3/100,000) (3). Furthermore, the rate of mortality
attributable to CHD in the USA in 2009 was 236.1/100,000 (4). Although, the overall mortality is
lower in the Chinese population, the World Health Organization
predicts an increase in the global burden of CHD in China, and the
number of patients succumbing to cardiovascular diseases is
projected to be ~4 million by 2020 (5).
In patients with acute myocardial infarction (MI),
ventricular arrhythmias may lead to heart failure and finally,
mortality (6). The incidence of
premature ventricular contraction, ventricular tachycardia and
ventricular fibrillation is ~10–93, 3–39 and 4–36%, respectively
(7,8). Currently, CHD is predominantly
treated with therapeutic agents, interventional procedures and
surgery with the aim of improving the myocardial ischemia/hypoxia,
or controlling the risk factors of CAS, and symptomatically
managing the mechanical dysfunction and arrhythmia to maintain a
favorable cardiac perfusion (9,10).
Despite numerous treatment options, prognosis for the prevention of
recurrence of CHD continues to remain poor. Recent advancement in
the field of angiogenesis in myocardial ischemia indicates that
therapeutic angiogenesis (TA) may promote the growth of blood
vessels in the ischemic myocardium, which in turn improves cardiac
perfusion (11,12). Improved cardiac perfusion mitigates
ischemic tissue necrosis, leading to reduced infarction size,
improved left ventricular function and ultimately, survival.
Angiogenic therapy, which involves the use of an
exogenous stimulus to promote blood vessel growth, is an attractive
approach for the treatment of ischemic diseases (13). It has been demonstrated in
experimental models that the stimulation of blood vessel growth
leads to growth of the whole vascular tree, improvement of ischemic
tissue perfusion and improved muscle aerobic energy metabolism
(13). In the progression of CHD,
MI-induced angiogenesis is a slow process and may only partially
compensate for the blood supply to the heart following coronary
occlusion (11). However, TA
further promotes the growth of capillaries and stimulates
collateral circulation in the ischemic heart. In recent years,
there has been an increased focus on the influence of traditional
Chinese medicine (TCM) on angiogenesis (14). It has been identified that TCM
stimulates the ischemic heart to produce angiogenic factors, which
promote angiogenesis in a paracrine manner (15).
Astragalus is the dry root of Astragalus
membranaceus (Fisch) Bge var. mongholicus (Beg) Hsiao or Shanxi
Astragalus membranaceus (Fisch) Bge. Astragalus is a sweet
herbal supplement, which according to the TCM theory, is known to
invigorate the spleen and lungs, and exerts anti-inflammatory and
immunomodulatory effects (16,17).
Astragalus contains saponins, flavonoids and polysaccharides, of
which saponins are the predominant active component.
Astragaloside (AST), is the total saponin fraction
isolated from Shanxi Astragalus membranaceus (Fisch) Bge
(17), and is usually applied in
the prevention and therapy of cardiovascular and cerebrovascular
diseases, immune disorders, pulmonary fibrosis, liver cancer,
diabetes, kidney disease and for reducing the signs of aging
(18). AST inhibits platelet
aggregation and causes an increase in prostacyclin and nitric oxide
levels, thereby exerting its anti-thrombotic effect. It has been
proposed that AST may increase microvessel density (MVD) in the
ischemic heart of rats; however, the specific mechanisms remain
poorly understood (19). There is
evidence demonstrating that AST IV may stimulate angiogenesis of
human umbilical vein endothelial cells, which is accompanied by
deposition of the hypoxia-inducible factor-1α protein and
transcription of the VEGF gene (20).
VEGF and bFGF are key factors in promoting the
growth and differentiation of endothelial cells, and are important
in angiogenesis (21). The
expression of VEGF and bFGFs and their receptors in capillary
endothelial cells is altered in response to pathological
conditions, such as ischemia (11). Reduction in oxygen tension leads to
activation of these endothelial mitogenic factors and promotes
endothelial cell growth. While VEGF (also known as vascular
permeability factor) induces differentiation and maturation of
endothelial cells, bFGF upregulates the expression of integrins,
thereby enhancing cell adhesion and migration, which ultimately
results in the formation of new blood vessels (22,23).
These two growth factors have been shown to increase regional
perfusion, tissue metabolism, improve myocardial function and
protect against ischemic damage (13). In the current study, it was
hypothesized that AST-induced angiogenesis may be mediated through
pro-angiogenic agents, such as VEGF and bFGF. Therefore, the aim of
the current study was to investigate the effect of AST on the
expression of VEGF and bFGF in the blood and heart of rats
following MI, and to investigate the potential underlying
mechanisms of AST-induced angiogenesis.
Materials and methods
Animals
Healthy adult male Wistar rats (n=45) weighing
250±30 g were purchased from the Yisi Laboratory Animal Technology
Co., Ltd. (Changchun, China) and maintained in a standard care
facility with free access to water and food until completion of the
study. The rats were treated in accordance with the Guide for the
Care (24) and Use of Laboratory
Animals published by the US National Institutes of Health. The
study was approved by the ethics committee of The Second Affiliated
Clinical Medical College of Harbin Medical University (Harbin,
China).
Instruments and materials
AST (lot no. MUST-13091001) was obtained from
Chengdu Mansite Biology Co., Ltd. (Sichuan, China) and polyclonal
rabbit anti-rat VEGF (cat. no. PB0084) and bFGF (cat. no. BA0259)
antibodies were purchased from Wuhan Boster Biotech Co., Ltd.
(Hubei, China), while the polyclonal rabbit anti-rat VIII antibody
(cat. no. bs-0434R) was obtained from Beijing Bo'ao Hengxin Biotech
Co., Ltd. (Beijing, China). The ELISA kits for VEGF and bFGF were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA). A
small animal ventilator (model, 55-705B) was provided by Harvard
University (Cambridge, MA, USA) and the Leica RM 2135 rotary
microtome was from Leica Microsystems GmbH (Wetzlar, Germany). The
image acquisition system (Moticam 3000) was purchased from Motic,
Inc., Ltd. (Hong Kong, China) and the Tecan Infinite®
Pro 200 microplate reader was obtained from Tecan Schweiz AG,
(Männedorf, Switzerland). TRIzol (cat. no. 15596-026) was purchased
from Gibco Life Technologies (Carlsbad, CA, USA), and a DyNAmo
Flash SYBR Green PCR (cat. no. F-415XL) and reverse transcription
kits (cat. no. K1622) were obtained from Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). In addition, an Applied
Biosystems®-7500 Real-Time PCR System (Applied
Biosystems Life Technologies, Foster City, CA, USA), X-ray film
(cat. no. 6535876; Kodak, Rochester, NY, USA), a Mini-Protean 3
Electrophoresis system (Bio-Rad, Hercules, CA, USA) and a dark box
(Guangdong Yuehua Medical Instrument Factory Co., Ltd., Guangdong,
China) were used in the present study.
Induction of MI
Rats were weighed and anesthetized via
intra-peritoneal (i.p.) injection of 1% sodium pentobarbital (40
mg/kg; Merck Millipore, Darmstadt, Germany). Following tracheal
intubation, aerobic positive pressure ventilation was performed. An
incision was made at the fourth left intercostal space and a
thoracotomy was performed. The heart was exposed out of the
thoracic cavity by pressing the right thoracic cavity at the back
of the pericardium. The left anterior descending coronary artery
was ligated, the heart was replaced in the thoracic cavity, and the
wound was closed. Upon regaining spontaneous respiration, the
tracheal intubation was removed and the rats were returned to their
home cages. In the sham surgery group, the coronary artery was
exposed using the same protocol, however, it was not ligated.
Grouping
At 24 h after induction of MI, six rats had
succumbed. Subsequently, seven rats succumbed during the
experiments; two from each of the control, sham surgery, and
low-dose groups, and one rat from the high-dose group. At four
weeks, 32 rats remained. Analysis of the causes of mortality in the
rats revealed that it may have been caused by insufficient
postoperative respiratory care, arrhythmia or heart failure,
amongst other factors. The surviving rats were divided into four
groups: Low-dose (n=8), high-dose (n=8), control (n=8) and sham
surgery (n=8). In the low- and high-dose groups, the rats were
administered with 2-ml i.p. injections of AST at 2.5 and 10
mg/kg/d, respectively. In the control and sham surgery groups,
normal saline of equal volume was administered via i.p. injection.
This treatment was continued for four weeks.
Histological analysis
Four weeks after MI induction and/or AST treatment,
the rats were sacrificed and the heart was collected. Briefly, 10%
potassium chloride (Tianjin Kemiou Chemical Reagent Co., Tianjin,
China) was injected into the heart to arrest the heart in the
diastolic phase. The heart was separated and the atrium, major
blood vessels, extracardiac connective tissue and aortic arch were
removed. These were rinsed in phosphate-buffered saline (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China), and
dried on filter paper. The left ventricle was weighed on electronic
scales and the left ventricular mass index (LVMI) was calculated as
follows: Left ventricular weight (mg)/body weight (g). The
myocardium was incised at the borderline of the ischemic heart at
the cross-sectional level of the papillary muscle, fixed in 10%
neutral formalin solution (Tianjin Kemiou Chemical Reagent Co.) and
routine processing for histological examination was then performed.
Following a series of dehydration steps in ethanol (Tianjin Kemiou
Chemical Reagent Co.), a final tissue clearing in xylene was
conducted, and the heart was embedded in paraffin (Tianjin Kemiou
Chemical Reagent Co.) and sliced into 4-µm sections. The
paraffin sections were further processed and stained with
hematoxylin and eosin (H&E; Beyotime Institute of
Biotechnology, Shanghai, China), prior to mounting and microscopic
evaluation using a light microscope (CH20BIMF200; Olympus, Tokyo,
Japan) at ×400 magnification.
Detection of serum VEGF and bFGF
levels
At 24 h and four weeks following MI induction, 5 ml
blood was collected from the posterior orbital venous plexus,
without anticoagulants, and was centrifuged at 1,800×g for 10 min.
The supernatant was collected and stored at −80°C. The serum
contents of VEGF and bFGF were measured by ELISA according to the
manufacturer's instructions.
Detection of mRNA expression of VEGF and
bFGF in the ischemic heart by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted according to the
manufacturer's instruction and as previously described (25). Briefly, 100 mg heart tissue samples
were placed in a 5-ml test tube, followed by the addition of 1 ml
cold TRIzol. The heart tissue samples were homogenized and
centrifuged according to the manufacturer's instructions. cDNA was
then reverse transcribed. RNA (4 µl) was thawed and mixed
with 5X reverse transcription buffer (4 µl), 0.5 µl
dNTPs, 1 µl Moloney murine leukemia virus (M-MLV; Thermo
Fisher Scientific, Inc.), 0.5 µl oligo (dT), and 10
µl diethylpyrocarbonate-treated water (total volume, 20
µl; JRDUN Biotechnology Co., Ltd., Shanghai, China). Reverse
transcription was performed at 37°C for 1 h and 95°C for 5 min to
inactivate M-MLV. Finally, qPCR was conducted. The obtained cDNA
underwent extension by PCR. Data were analyzed using ABI Prism 7300
SDS software (Applied Biosystems Life Technologies,). Data obtained
from RT-qPCR were calculated according to the 2−∆∆Ct
method and experiments were repeated three times. The primers and
their corresponding sizes were as follows: Sense, 5′-GAG TCT GTG
CTC TGG GAT TTG-3′ and antisense, 5′-TCC TGC TAC CTC TTT CCT
CTG-3′, for VEGF (length, 188 bps); sense, 5′-TCT GTC TCC CGC ACC
CTATC-3′ and antisense, 5′-ACC AGC CTT CCA CCC AAAGC-3′ for bFGF
(length, 118 bps); and sense, 5′-GTC GGT GTG AAC GGA TTTG-3′ and
antisense, 5′-TCC CAT TCT CAG CCT TGAC-3′ for GAPDH (length, 181
bps).
Detection of VEGF and bFGF protein
expression in the heart by western blot assay
The heart tissues were cut into blocks and mixed
with lysis buffer (20 mg tissues/150–250 µl lysis buffer;
JRDUN Biotechnology Co., Ltd.) containing protease and phosphatase
inhibitor, which was followed by homogenization. Following lysis,
samples were centrifuged at 4°C for 15 min at 1,800×g and the
supernatant was collected for protein quantification. Proteins were
separated and transferred onto polyvinylidene fluoride membranes
(Hangzhou MAILV Filtration Equipment Co., Ltd., Hangzhou, China).
Following methanol treatment (Tianjin Kemiou Chemical Reagent Co.),
the membranes were washed with Tris-buffered saline and Tween-20
(TBST), and then blocked with skimmed milk powder (JRDUN
Biotechnology Co., Ltd.). Subsequently, the membrane was incubated
with primary antibody (dilutions: VEGF, 1:1,000; bFGF, 1:300;
GAPDH, 1:1,500) according to the manufacturer's instructions. The
antibodies were diluted with blocking buffer and incubation of the
membranes was conducted at room temperature for 2 h or at 4°C
overnight. The membranes were then washed three times with TBST
(5-min washes), and treated with horseradish peroxidase-conjugated
antibody at 1:2,000 at 37°C for 1 h. Following three 5-min washes
in TBST, visualization was performed using an enhanced
chemiluminescence method (EMD Millipore, Billerica, MA, USA).
Protein expression was normalized to that of the internal reference
(GAPDH) to give the relative expression. The experiments were
repeated three times.
Detection of MVD
An immunohistochemical streptavidin-peroxidase
method (Beyotime Institute of Biotechnology) was used to detect
coagulation factor VIII expression levels in the ischemic heart of
the MI rats. Coagulation factor VIII is expressed in the cytoplasm
of vascular endothelial cells. According to the method described by
Weidner (26), sections were
observed under a microscope (magnification, ×100) and five fields
with a high number of microvessels were selected around the
ischemic heart. The microvessels were counted at a magnification of
x400. Three sections were used for analysis in each rat, and five
randomly selected fields were selected from each section. The
number of microvessels was determined and averaged, and the mean
number of microvessels per field was designated as the MVD.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Continuous variables are presented
as means and standard deviations. One-way analysis of variance
(ANOVA) with least significant difference post hoc tests were
performed to compare the differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Gross cardiac morphology, pathological
myocardial changes and changes in left ventricular mass in
rats
LVMI is an important indicator of myocardial
hypertrophy (27). Following MI,
cardiomyocyte hypertrophy is an important feature of myocardial
remodeling and is involved in the occurrence and development of
heart failure. Thus, therapeutic modalities, which target
pathological cardiomyocyte hypertrophy are crucial for the
prevention of myocardial remodeling, and the occurrence and
development of heart failure (28). On completion of the study, the rats
were sacrificed and their hearts were collected. Macroscopic
observations revealed grey infarct regions of different sizes. The
mean left ventricular mass and LVMI were found to be significantly
higher in the control, and low- and high-dose groups when compared
with the sham surgery group (left ventricular mass: 850.44, 811.95
and 786.81 mg vs. 653.04 mg, respectively; P<0.001. LVMI: 2.75,
2.56 and 2.49 mg/g vs. 2.07 mg/g; P<0.001). Furthermore, the
mean left ventricular mass and LVMI were significantly higher in
the control group when compared with the low- and high-dose groups
(left ventricular mass: 850.44 mg vs. 811.95 and 786.81 mg;
P≤0.033. LVMI: 2.75 mg/g vs. 2.56 and 2.49 mg/g; P≤0.003). There
were no significant differences identified between the groups with
regard to rat weight (Fig. 1 and
Table I).
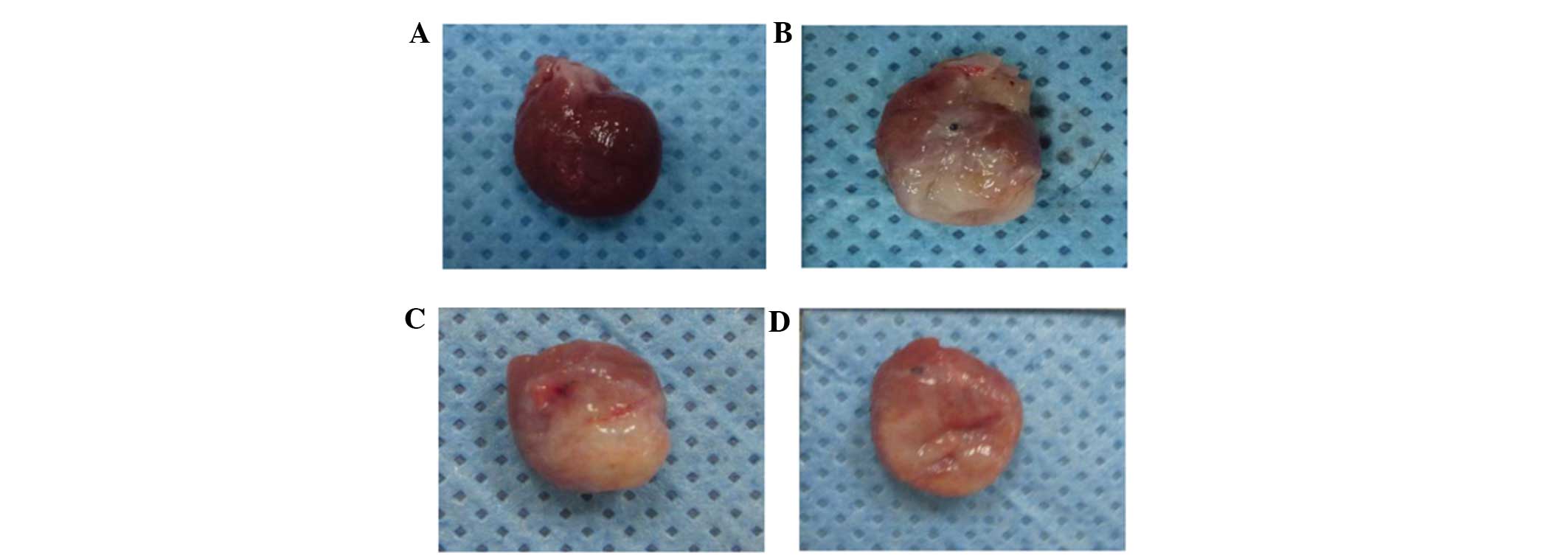 | Figure 1Infarct size of rats four weeks
subsequent to induction of myocardial infarction. (A) Rat heart
specimen of sham surgery group, (B) Rat heart specimen of control
group, (C) Rat heart specimen of low-dose astragaloside group,
revealing light yellow color in the lesion tissue and blur
boundaries between normal and lesion tissue and (D) Rat heart
specimen of high-dose astragaloside group, demonstrating that the
color of the lesion site was slightly different from the normal
tissue, and the light yellow region was markedly smaller than
hearts in other groups. In the ischemic heart, the myocardium
exhibited disordered arrangement. In the non-ischemic heart, the
myocardium exhibited ordered arrangement and a small quantity of
inflammatory cell infiltration. In the ischemic heart, the
myocardial cells were lysed and fractured, the myocardial structure
was disordered, the nucleus was absent and there was infiltration
of fibroblasts. There was a clear boundary between the ischemic
heart and the intact heart. |
 | Table IWeight comparisons between the
groups. |
Table I
Weight comparisons between the
groups.
| Parameter | Sham surgery
(n=8) | Control (n=8) | Low-dose (n=8) | High-dose (n=8) | P-value |
|---|
| Weight (g) | 315.44±4.56 | 310.05±8.72 | 317.10±10.03 | 316.84±6.58 | 0.253 |
| Left ventricular mass
(mg) | 653.04±45.46 |
850.44±32.00a |
811.95±25.41a,b |
786.81±31.14a,b | <0.001 |
| Left ventricular
mass index (mg/g) | 2.07±0.14 | 2.75±0.13a | 2.56±0.07a,b | 2.49±0.11a,b | <0.001 |
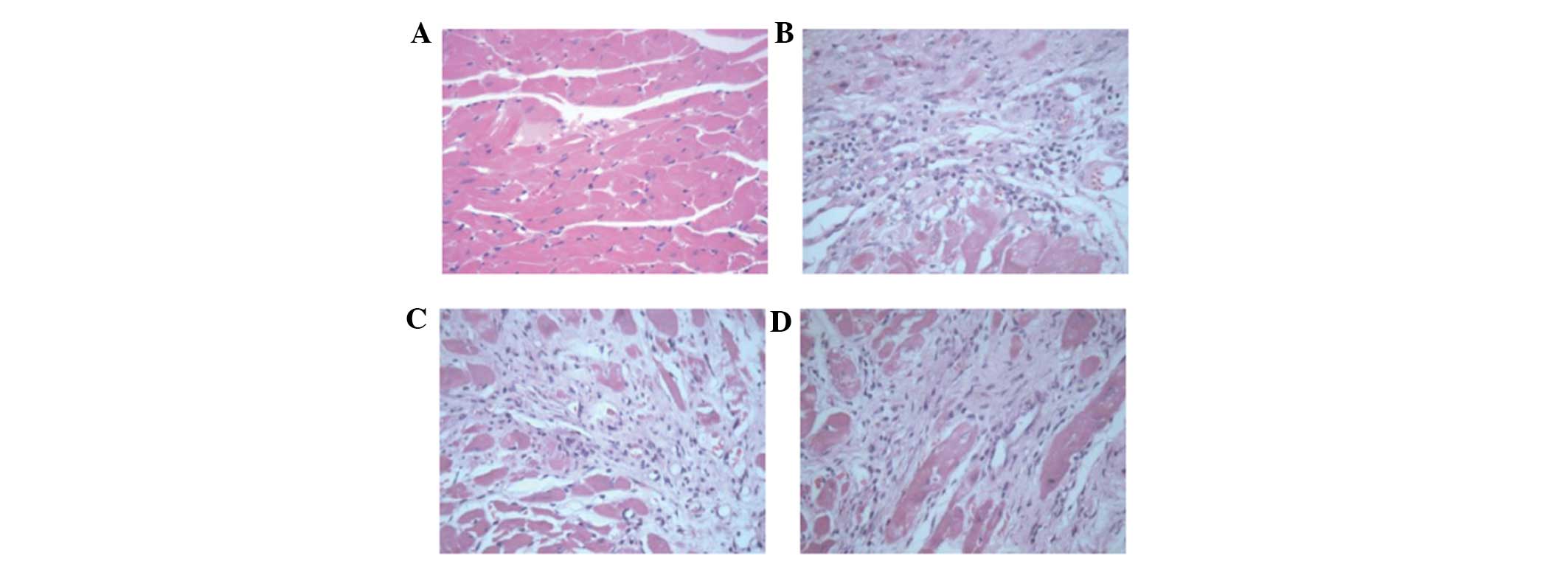
The pathological changes in the myocardium were
examined by H&E staining of the heart. At four weeks after
induction of MI, samples of heart tissue were collected for H&E
staining. Under a light microscope, the myocardium revealed ordered
arrangement and a small number of inflammatory cells, which had
infiltrated into the heart in the sham surgery group. In the
control group and low- and high- dose group, the cardiomyocytes
were lysed or fractured, the myocardial structure was disordered,
their nuclei were absent, and a small quantity of fibroblasts had
infiltrated into the heart. There was a clear boundary between the
infarct region and the intact region (Fig. 2).
Serum levels of VEGF and bFGF among the
groups at different time points
A comparison between the different groups of VEGF
and bFGF serum levels is presented in Table II. No significant differences were
noted between the sham surgery, the control, and the low- and
high-dose groups in serum VEGF and bFGF levels 24 h after surgery
(P>0.05).
 | Table IIComparisons between groups at various
time points in serum VEGF and bFGF levels. |
Table II
Comparisons between groups at various
time points in serum VEGF and bFGF levels.
A, Serum VEGF
|
|---|
| Time point | Serum level (pg/ml)
| P-value |
|---|
| Sham surgery
(n=8) | Control (n=8) | Low-dose (n=8) | High-dose
(n=8) |
|---|
| 24 h after
surgery | 92.36±13.06 | 93.78±13.28 | 95.86±6.10 | 96.52±11.60 | 0.878 |
| 4 weeks after
surgery | 90.24±7.08 | 93.04±13.08 |
109.79±10.74a,b |
115.10±12.21a,b | <0.001 |
B, Serum bFGF
|
|---|
| Time point | Serum level (pg/ml)
| P-value |
|---|
| Sham surgery
(n=8) | Control (n=8) | Low-dose (n=8) | High-dose
(n=8) |
|---|
| 24 h after
surgery | 5.17±1.99 | 4.77±1.70 | 4.78±2.13 | 5.31±2.20 | 0.932 |
| 4 weeks after
surgery | 4.69±2.32 | 4.32±1.23 | 9.00±2.37a,b | 9.56±2.93a,b | <0.001 |
The mean serum VEGF and bFGF levels (Table II) four weeks after surgery were
significantly higher in the low- and high-dose groups, as compared
with those in the sham surgery and control groups (serum VEGF:
109.79 and 115.1 pg/ml vs. 90.24 and 93.04 pg/ml; P≤0.005. Serum
bFGF: 9 and 9.56 pg/ml vs. 4.69 and 4.32 pg/ml; P≤0.001).
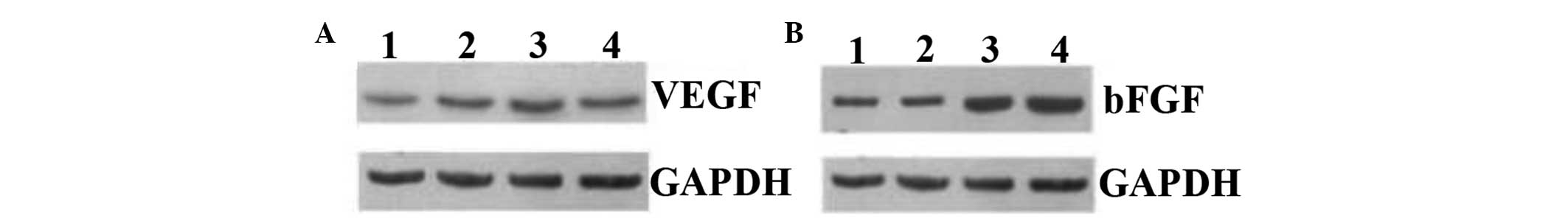
mRNA and protein expression levels of
VEGF and bFGF in the four groups
The mean VEGF mRNA levels were significantly higher
in the low- and high-dose groups, compared with those in the sham
surgery and control groups (0.015 and 0.02 vs. 0.003 and 0.002;
P≤0.012). Similarly, the mean bFGF mRNA level was significantly
higher in the high-dose group, as compared with that in the sham
surgery, control and low-dose groups (0.019 vs. 0.003, 0.003 and
0.010; P≤0.028) (Table III).
However, in contrast to the VEGF mRNA levels, a dose-dependent
effect of AST was observed in the bFGF mRNA levels, with a
significantly higher mRNA expression level observed in the
high-dose group compared with the low-dose group (0.019±0.014 vs.
0.010±0.008).
 | Table IIIComparisons between groups in MVD,
VEGF and bFGF protein expression, and VEGF and bFGF mRNA
expression. |
Table III
Comparisons between groups in MVD,
VEGF and bFGF protein expression, and VEGF and bFGF mRNA
expression.
A, Myocardial
infarction marginal zone
|
|---|
| Parameter | Sham surgery
(n=8) | Control (n=8) | Low-dose (n=8) | High-dose
(n=8) | P-value |
|---|
| MVD (per visual
field) | 5.25±1.39 | 20.5±5.24a | 26.88±4.32a,b | 29.88±3.36a,b | <0.001 |
| VEGF protein
expression | 0.23±0.15 | 0.25±0.13 | 0.52±0.27a,b | 0.58±0.36a,b | 0.015 |
| bFGF protein
expression | 0.48±0.13 | 0.62±0.14 | 0.88±0.19a,b | 0.88±0.42a,b | 0.006 |
B, Ischemic
myocardium
|
|---|
| Parameter | Sham surgery
(n=8) | Control (n=8) | Low-dose (n=8) | High-dose
(n=8) | P-value |
|---|
| VEGF mRNA
expression | 0.003±0.004 | 0.002±0.003 | 0.015±0.007a,b | 0.02±0.015a,b | <0.001 |
| bFGF mRNA
expression | 0.003±0.003 | 0.003±0.003 | 0.010±0.008 | 0.019±0.014a,b,c | 0.013 |
Similar to the mRNA expression levels, VEGF and bFGF
protein expression (Table III
and Fig. 3) were significantly
higher in the AST-treated rats (low- and high-dose), as compared
with those in the sham surgery and control groups (VEGF: 0.52 and
0.58 vs. 0.23 and 0.25; P≤0.039. bFGF: 0.88 and 0.88 vs. 0.48 and
0.62; P≤0.049). Although a dose-dependent increase was observed in
the bFGF mRNA expression levels, no such change in bFGF protein
expression was identified between the two AST treatment groups.
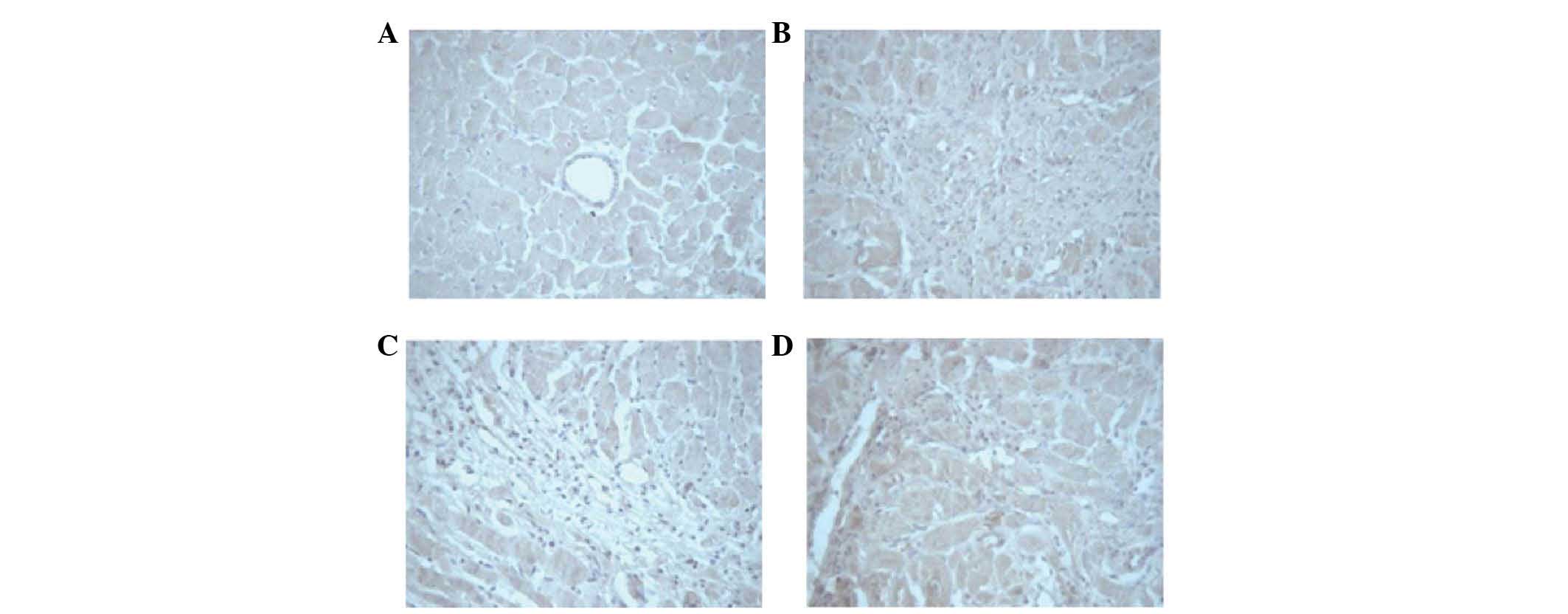
MI induces an increase in MVD
At four weeks following pharmacotherapy,
immunohistochemistry was conducted to identify coagulation factor
VIII. The newly generated capillaries were observed in the
myocardium of rats in all of the groups, particularly at the border
of the infarct region (Fig. 4).
The MVD was calculated in the surrounding margin of the infarct
regions (Table III). The mean
MVD, as indicated by the expression of coagulation factor VIII
(Table III; Fig. 4), was significantly greater in the
control, and the low- and high-dose groups, when compared with that
of the sham surgery group (20.5, 26.88 and 29.88 vs. 5.25/visual
field; P<0.001). When compared with the control group, the MVD
was observed to be significantly higher in the low- and high-dose
groups (26.88 and 29.88 vs. 20.5/visual field; P≤0.003).
Discussion
In the present study, the effect of AST (daily
treatment for four weeks) was evaluated in a rat model of MI. The
results indicate that AST offers substantial cardioprotection in MI
rats. Increases in MVD, observed in AST-treated rats, may indicate
angiogenesis and improved collateral circulation. Furthermore, the
serum levels and mRNA/protein expression levels of VEGF and bFGF
were significantly higher in the AST-treated rats. Although no
dose-dependent effect was observed in the mRNA expression levels of
VEGF, increased bFGF mRNA expression levels were identified in the
high-dose group (10 mg/kg/day AST). Taken together, the current
results indicate that AST improves cardiac function via
upregulation of VEGF and bFGF, which subsequently stimulates
angiogenesis in the MI heart.
Angiogenesis is a physiological process through
which new blood vessels form from pre-existing vessels by budding
in response to angiogenic signals, such as hypoxia, growth factors,
or vasodilators. In addition, angiogenesis is involved in the
formation of new blood vessels during embryonic development, as
well as in tissue repair and tumorigenesis (11,29).
Angiogenesis is crucial in myocardial tissue repair following MI.
The present study demonstrates a considerable change in LVMI and
necrosis in AST-treated rats (Figs.
1 and 2), indicating that AST
inhibits or reduces myocardial hypertrophy, and thus prompts
cardioprotection. Similarly, a significant increase in MVD was also
observed in the AST-treated rats, when compared with the control
and sham surgery group rats (Table
III; P≤0.015 by one-way ANOVA with Bonferroni post hoc tests).
These findings indicate that AST promotes angiogenesis in the heart
of MI rats, which is consistent with previous studies (19,20).
Increases in MVD in the heart may reflect the
formation of coronary collateral circulation to a certain extent.
Following MI, the formation of collateral circulation requires the
stimulation of quiescent endothelial cells by various growth
factors, including VEGF and bFGF. The role of VEGF and bFGF in
angiogenesis is well understood (30,31).
VEGF proteins are homodimeric, with two subunits of ~120–200 amino
acids in length, and bind to Fms-like tyrosine and fetal liver
kinase-1/kinase insert domain receptor on the endothelial cells
(32) to stimulate proliferation
of the endothelial cells to form new microvessels (31). bFGF, however, mediates the
expression of integrins, enhances cell adhesion and migration of
vascular endothelial and smooth muscle cells, promotes the
formation of new blood vessels and increases collateral circulation
(22,23,33).
De novo formation of microvessels has the
potential to salvage ischemic myocardium in the early stages
following MI, and is also essential for long-term left ventricular
remodeling to prevent the transition to heart failure (29). Although the existence of collateral
circulation in CHD patients is associated with improved clinical
outcomes, the net effect is not sufficiently adequate to compensate
for the flow lost as a result of occlusion of the native coronary
arteries (11). The use of a
therapeutic agent that further accelerates collateral vessel growth
may prove to be particularly beneficial in controlling CHD
worldwide. Although experimental studies on the stimulation of
angiogenesis have been promising, to the best of our knowledge, no
single therapeutic agent has been identified as applicable in
clinical practice, either due to a lack of efficacy or negative
side effects (34).
AST is a type of TCM, which has been used for
centuries and implicated in the stimulation of angiogenesis;
furthermore, its safety and efficacy have been well established in
various models (17–20). The findings of the present study
validate the administration of AST in MI to alleviate the
deleterious consequences of ischemia. Daily AST treatment for four
weeks induced a marked increase in the serum VEGF and bFGF levels
(at 24 h), when compared with the baseline levels and was
significantly different from the control and the sham surgery
groups (P<0.05; Table II). In
addition, a clear increase in the mRNA and protein expression
levels of VEGF and bFGF was observed in the AST-treated rats, when
compared with the control group (P<0.05; Table III and Fig. 3), indicating that AST induced the
upregulation of these endothelial mitogenic factors. Though no
dose-dependent effect of AST was noted in the serum level or the
mRNA/protein expression levels of VEGF, a significant increase in
the level of bFGF mRNA expression was observed at the higher dose
of AST (10 mg/kg/day). However, this apparent increase in bFGF mRNA
was not translated to the protein levels (Table III), therefore, it is challenging
to establish whether there is a dose-dependent effect of AST on
these parameters. It is proposed that a dose-dependent effect may
be more evident at a higher dose than that which was used in the
current study.
The increase in MVD, as represented by the increase
in coagulation factor VIII expression (Fig. 4), provides further evidence for
AST-induced angiogenesis and myocardial protection. A significant
increase was observed in the MVD per visual field in AST-treated
rats, when compared with the control and the sham surgery group
rats (P<0.05; Table III).
However, no significant difference between the two different AST
dose groups was identified, which may be attributed to the slight
variability in demarcating the ischemic heart tissue, which was
collected for histological analysis, or due to the procedures that
were used for MVD detection.
In a normal myocardium, only traces of VEGF and bFGF
expression are observed. However, numerous clinical and
experimental procedures, such as artificial heart surgery,
myocardial ischemia, hypoxia, thoracotomy and anesthesia may induce
feedback mechanisms and cause increases in the expression of VEGF
and bFGF, which may have affected the outcomes of the current
study. This presents a study limitation; however, care was taken
during the current study regarding the surgical methods and
attempts were made to maintain the inter-group variability at a
minimum. An additional limitation of the present study is that
growth of microcirculation in experimental animals may vary between
species and the results may not correlate with a different animal
model, such as a porcine or dog model of MI.
Collectively, the present data indicate that AST
promotes angiogenesis in the heart of MI-induced rats, which may be
ascribed to the upregulation of VEGF and bFGF levels in the blood
and heart tissue. Experimental studies on in vitro and in
vivo models have demonstrated that the pro-angiogenic effects
of AST are mediated through VEGF and downstream Akt signaling
pathways (35). Recent
identification of a novel VEGF, Notch/Jagged-l, that directly
transmits the Jagged-1/Notch signals to cells to regulate the
VEGFR3-associated angiogenesis (36,37),
indicates that there are additional mechanisms involved. However,
although the functions of Notch and VEGF are different, the two are
complementary in tumor angiogenesis (38). Establishing whether such a
complementary role exists in myocardial angiogenesis may be of
interest for future studies.
In conclusion, AST stimulates MI-induced
angiogenesis of the heart, via the upregulation of endothelial
mitogenic factors, including VEGF and bFGF. However, the
involvement of additional signaling pathways in AST-induced
angiogenesis cannot be disregarded and further studies are required
to extend the understanding of the mechanism of action of this
well-established TCM.
Acknowledgments
The present study was supported by the Science and
Technology Research Project of Department of Education of
Heilongjiang Province. (grant no. 12521297).
References
|
1
|
Orphanou K, Stassopoulou A and Keravnou E:
Risk assessment for primary coronary heart disease event using
dynamic Bayesian networks. Artificial Intelligence in Medicine.
Holmes J, Bellazzi R, Sacchi L and Peek N: 9105. Springer
International Publishing; pp. 161–165. 2015, View Article : Google Scholar
|
|
2
|
Liu Y and Liu RX: The research progress of
Traditional Chinese medicine in treatment of coronary
atherosclerosis heart disease. Hebei J of Trad Chin Med.
35:1476–1478. 2013.In Chinese.
|
|
3
|
Tao HM, Qin S and Zhang DY: Research
progress of coronary heart disease in women. Adv Cardiovasc Dis.
35:250–253. 2014.In Chinese.
|
|
4
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al:
Executive summary: Heart disease and stroke statistics-2013 update:
A report from the American heart association. Circulation.
127:143–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li XY and Fu ZQ: Pharmacotherapy of
coronary heart disease in the elderly. J Med Res. 40:3–5. 2011.In
Chinese.
|
|
6
|
de bakker JM, van capelle FJ, Janse MJ,
Wilde AA, Coronel R, Becker AE, Dingemans KP, van Hemel NM and
Hauer RN: Reentry as a cause of ventricular tachycardia in patients
with chronic ischemic heart disease: Electrophysiologic and
anatomic correlation. Circulation. 77:589–606. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang LM, Zhang SD and Ann YC: Treatment of
ventricular arrhythmia after myocardial infarction. Chinese Journal
of Cardiovascular Review. 6:144–146. 2008.In Chinese.
|
|
8
|
Meng QC: Treatment and prevention of
reperfusion arrhythmia after thrombolytic therapy for acute
myocardial infarction. Journal of Medical Forum. 27:55–57. 2006.In
Chinese.
|
|
9
|
Bates ER and Topol EJ: Limitations of
thrombolytic therapy for acute myocardial infarction complicated by
congestive heart failure and cardiogenic shock. J Am Coll Cardiol.
18:1077–1084. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kones R: Primary prevention of coronary
heart disease: Integration of new data, evolving views, revised
goals and role of rosuvastatin in management. A comprehensive
survey. Drug Des Devel Ther. 5:325–380. 2011. View Article : Google Scholar
|
|
11
|
Ware JA and Simons M: Angiogenesis in
ischemicheart disease. Nat Med. 3:158–164. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rissanen TT, Korpisalo P, Markkanen JE,
Liimatainen T, Ordén MR, Kholová I, de Goede A, Heikura T, Gröhn OH
and Ylä-Herttuala S: Blood flow remodels growing vasculature during
vascular endothelial growth factor gene therapy and determines
between capillary arterialization and sprouting angiogenesis.
Circulation. 112:3937–3946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dragneva G, Korpisalo P and Ylä-Herttuala
S: Promoting blood vessel growth in ischemic diseases: Challenges
in translating preclinical potential into clinical success. Dis
Model Mech. 6:312–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Li J, Wang J, Li J, Janicki JS and
Fan D: Effects and mechanisms of Chinese herbal medicine in
ameliorating myocardial ischemia-reperfusion injury. Evid Based
Complement Alternat Med. 2013:9256252013.PubMed/NCBI
|
|
15
|
Liu YF, Liu SW and Liu ZX: Effects of
Ginsenosiee Rb1 on blood vessel regeneration after ischemia and
reperfusion in rats. Chin J Histochem Cytochem. 17:39–44. 2008.In
Chinese.
|
|
16
|
Han D, Zhang Y, Liu M, et al: The
hemodynamic and antioxidant effects of Astragaloside on ventricular
remodeling rats. Chin J Lab Diag. 17:1956–1959. 2013.In
Chinese.
|
|
17
|
Wang YP, Li XY, Song CQ and Hu ZB: Effect
of astragaloside IV on T, B lymphocyte proliferation and peritoneal
macrophage function in mice. Acta Pharmacol Sin. 23:263–266.
2002.PubMed/NCBI
|
|
18
|
Yin Y, Li WP, Gong HL, Zhu FF, Li WZ and
Wu GC: Protective effect of astragaloside on focal cerebral
ischemia/reperfusion injury in rats. Am J Chin Med. 38:517–527.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JS, Yu JM, Ju L, et al: Effects of
Astragalosides on angiogenesis in myocardium infarction rats. Chin
J Prim Med Pharm. 19:215–217. 2012.
|
|
20
|
Zhang L, Liu Q, Lu L, Zhao X, Gao X and
Wang Y: Astragaloside IV stimulates angiogenesis and increases
hypoxia-inducible factor-1α accumulation via phosphatidylinositol
3-kinase/Akt pathway. J Pharmacol Exp Ther. 338:485–491. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Luca A, Carotenuto A, Rachiglio A,
Gallo M, Maiello MR, Aldinucci D, Pinto A and Normanno N: The role
of the EGFR signaling in tumor microenvironment. J Cell Physiol.
214:559–567. 2008. View Article : Google Scholar
|
|
22
|
Böttcher RT and Niehrs C: Fibroblast
growth factor signaling during early vertebrate development. Endocr
Rev. 26:63–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andersson H and Brittebo E: Proangiogenic
effects of environmentally relevant levels of bisphenol A in human
primary endothelial cells. Arch Toxicol. 86:465–474. 2012.
View Article : Google Scholar
|
|
24
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: Special Report: The 1996 Guide for the Care and Use
of Laboratory Animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reddy BY, Greco SJ, Patel PS, Trzaska KA
and Rameshwar P: RE-1-silencing transcription factor shows
tumor-suppressor functions and negatively regulates the oncogenic
TAC1 in breast cancer cells. Proc Natl Acad Sci U S A.
106:4408–4413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
27
|
Aurgemma GP and Gaasch WH: Quantitative
evaluation of left ventricular structure, wall stress, and systolic
function. The Practice of Clinical Echocardiography. Otto CM: 2nd
edition. Elsevier Saunders; Philadelphia, PA: pp. 65–87. 2002
|
|
28
|
Meiners S, Dreger H, Fechner M, Bieler S,
Rother W, Günther C, Baumann G, Stangl V and Stangl K: Suppression
of cardio-myocyte hypertrophy by inhibition of the
ubiquitin-proteasome system. Hypertension. 51:302–308. 2008.
View Article : Google Scholar
|
|
29
|
Cochain C, Channon KM and Silvestre JS:
Angiogenesis in the infarcted myocardium. Antioxid Redox Signal.
18:1100–1113. 2013. View Article : Google Scholar :
|
|
30
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar
|
|
31
|
Pohl-Schickinger A, Koehne P, Schmitz T,
Schmitt KR, Hübler M, Redlin M, Berger F and Stiller B: Vascular
endothelial growth factor and its soluble receptor in infants with
congenital cardiac disease. Cardiol Young. 20:505–508. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shibuya M: VEGF-VEGFR signals in health
and disease. Biomol Ther. 22:1–9. 2014. View Article : Google Scholar
|
|
33
|
Wafai R, Tudor EM, Angus JA and Wright CE:
Vascular effects of FGF-2and VEGF-B in rabbits with bilateral hind
limb ischemia. J Vasc Res. 46:45–54. 2009. View Article : Google Scholar
|
|
34
|
Schirmer SH, van Nooijen FC, Piek JJ and
van Royen N: Stimulation of collateral artery growth: Travelling
further down the road to clinical application. Heart. 95:191–197.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Hu G, Li S, Li ZH, Lam CO, Hong
SJ, Kwan YW, Chan SW, Leung GP and Lee SM: Pro-angiogenic activity
of astragaloside IV in HUVECs in vitro and zebrafish in vivo. Mol
Med Rep. 5:805–811. 2012.
|
|
36
|
Karanu FN, Murdoch B, Gallacher L, Wu DM,
Koremoto M, Sakano S and Bhatia M: The notch ligand jagged-1
represents a novel growth factor of human hematopoietic stem cells.
J Exp Med. 192:1365–1372. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Benedito R, Roca C, Sörensen I, Adams S,
Gossler A, Fruttiger M and Adams RH: The notch ligands Dll4 and
Jagged1 have opposing effects on angiogenesis. Cell. 137:1124–1135.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hernandez SL, Banerjee D, Garcia A,
Kangsamaksin T, Cheng WY, Anastassiou D, Funahashi Y,
Kadenhe-Chiweshe A, Shawber CJ, Kitajewski JK, et al: Notch and
VEGF pathways play distinct but complementary roles in tumor
angiogenesis. Vasc Cell. 5:172013. View Article : Google Scholar : PubMed/NCBI
|