Introduction
Bladder cancer is one of the most common urogenital
cancers worldwide, with a high incidence in developed countries
(1,2). Despite advances in cystoscopy in the
detection and surveillance of bladder cancer, progress in the
development of effective treatments remains limited. Approximately
50–70% of patients treated with endoscopic resection will undergo
recurrence and 10–30% will progress to muscle-invasive disease,
which has led to the use of adjuvant therapy with intravesical
agents (3,4). However, the conventional
chemotherapeutic regimens are often poorly tolerated as a result of
the associated side effects (5).
These factors highlight the requirement for the production of novel
adjuvant agents to improve the efficacy of bladder cancer
treatment.
Apoptosis serves an important role in the treatment
of cancer as it is a common target of numerous treatment strategies
(6–9). Caspases are crucial mediators of
programmed cell death (apoptosis) (10). Among them, caspase-3 is a
frequently activated death protease, catalyzing the specific
cleavage of numerous key cellular proteins (11).
There has been an increase in the discovery of
relatively non-toxic natural compounds with a wide range of
biological activities (12).
Chalcones are ubiquitous natural compounds with anticancer
potential and relatively few side effects, which have been reported
to inhibit cellular proliferation by inducing cell cycle arrest
(5,13) and/or apoptosis of cancer cells
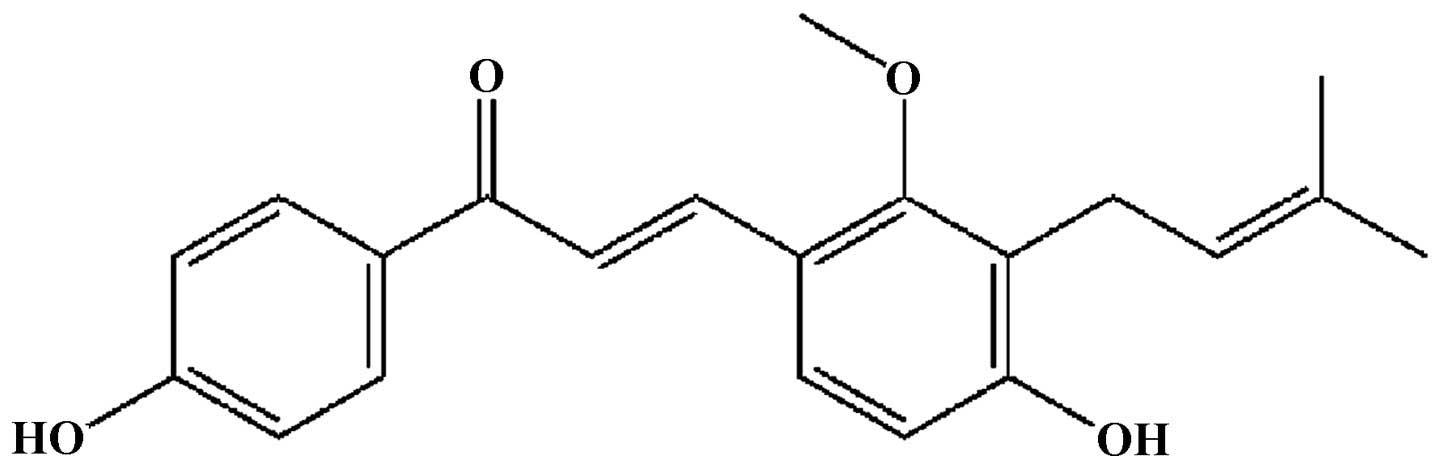
(14,15). Licochalcone C (LC; Fig. 1), a chalcone isolated and
identified from the root of Glycyrrhiza inflata (16,17),
exhibits antibacterial (18) and
anti-inflammatory effects (19),
however the antitumoral activity has not been investigated.
Therefore, the aim of the current study was to elucidate the
inhibitory effects of LC on bladder cancer cells and explore the
underlying mechanisms.
Materials and methods
Cell lines and cell culture
T24, MCF7 and A549 cells were purchased from the
China Center for Type Culture Collection (Wuhan, China). The T24
cells were cultured in RPMI 1640 medium (Gibco Life Technologies,
Carlsbad, CA, USA) and both the MCF7 and A549 cells were cultured
in Dulbecco's modified Eagle's medium (Gibco Life Technologies)
containing 10% fetal bovine serum (FBS; Tianjin Hao Yang Biological
Manufacture Co., Ltd., Tianjin, China) at 37°C with 5%
CO2. The media contained 100 U/ml penicillin
(Sigma-Aldrich, St. Louis, MO, USA) and 100 µg/ml
streptomycin (Sigma-Aldrich). The cells were passaged every 3 days
and were diluted every day prior to each experiment.
Cell viability assay
The LC was purchased from Shanghai Lichen Trading
Co., Ltd. (Shanghai, China). The MTT (Beyotime Institute of
Biotechnology, Haimen, China) assay was used to evaluate viability
of cells, which is based on the conversion of MTT to formazan
crystals by mitochondrial dehydrogenases (20). Cells were seeded onto 96-well
plates (8×104 cells/ml) and incubated overnight in 100
µl of the culture medium. The cells were treated with a
range of concentrations of LC (0, 25, 30, 35, 40 and 45
µg/ml) for 24 h. Following incubation, 20 µl MTT (5
mg/ml) was added to each well, which were then incubated for 4 h
prior to removal of the supernatant. A total of 150 µl
dimethyl sulfoxide (DMSO; Sigma-Aldrich) was added to each well.
Absorbance at 570 nm was measured using a fluorescence plate reader
(3001; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The data
were expressed as the percentage cell viability compared with the
control (DMSO). The inhibition rate was quantified using the
following formula:
Morphological assessment
To investigate whether LC induces apoptosis in T24
cells, the cells were plated in a 4-well chamber slide at
2×104 cells/slide, and treated with increasing
concentrations of LC (0, 25, 30, 35, 40 and 45 µg/ml) for 24
h to examine the apoptosis of T24 cells. The cells were fixed in
formaldehyde (40 g/l; Sigma-Aldrich) in phosphate-buffered saline
(PBS) for 20 min followed by Hoechst 33258 (10 mg/l; Sigma-Aldrich)
staining for 30 min in the dark at 37°C. Cell nuclei were then
analyzed under a computer-assisted microscope (459330; Carl Zeiss
AG, Oberkochen, Germany) by fluorescence microscopy. Apoptotic
cells were characterized by chromatin condensation and multiple
chromatin fragments (21).
Detection of cell apoptotic rates by flow
cytometry
Apoptotic rates were determined by staining cells
with annexin V fluorescein isothiocyanate (FITC) and propidium
iodide (PI) labeling (22). The
Annexin V/PI Apoptosis kit was purchased from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China). Cells (1.5×105
cells/ml) were incubated with LC for 24 h following which they were
washed twice with ice-cold PBS, and 5 µl annexin V-FITC and
5 µl PI (1 mg/ml) were added to stain the cells. The cell
staining was analyzed using the FACStar Flow Cytometer (BD
Biosciences, San Jose, CA, USA). Viable cells were regarded to be
negative for PI and annexin V-FITC, apoptotic cells were positive
for annexin V-FITC and negative for PI, whereas late apoptotic dead
cells displayed clear annexin V-FITC and PI labeling. Non-viable
cells, which underwent necrosis, were positive for PI however were
negative for annexin V-FITC.
RNA extraction and semi-quantitative
reverse transcription-polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Sangon Biotech
Co., Ltd., Shanghai, China) according to the manufacturer's
instructions. RNA quality was assessed using the A260/A280 ratio
with a Nanodrop Spectrophotometer (ND-2000; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA) and 1.5% agarose gel
electrophoresis (Biodee Biotechnology Co., Ltd., Beijing, China).
Following extraction, 3 µl total RNA was reverse-transcribed
to cDNA using a RevertAid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific) in a 20 µl reaction volume. The reaction
conditions of reverse transcription PCR were established using 12.5
µl 2X Taq PCR MasterMix (Tiangen Biotech Co., Ltd., Beijing,
China), 3 µl cDNA template and 0.5 µl of each primer
synthesized by Sangon Biotech. Thermocycling conditions were as
follows: Pre-denaturation at 94°C for 3 min, 30 cycles of
denaturation at 94°C for 30 sec, annealing at 58°C for 30 sec and
extension at 72°C, and a final extension at 72°C for 10 min. The
RT-qPCR products were quantified using a Bio-Rad gel imaging system
(Bio-Rad Laboratories, Inc.) with GelPro analysis software 4.0
(Media Cybernetics, Rockville, MD, USA). The primer sequences are
presented in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer | Forward | Reverse |
|---|
| Bax |
TGGAGCTGCAGAGGATGATTG |
GAAGTTGCCGTCAGAAAACATG |
| Bim |
CACATGAGCACATTTCCCTCT |
AAGGCACAAAACCTGCAGTAA |
| Bcl-w |
CGGAACATGGCTTGTAGCTC |
AATCCCATTCATCTAGTCGAG |
| Bcl-2 |
AGTACCTGAACCGGCATCTG |
GCTGAGCAGGGTCTTCAGAG |
| Bcl-XL |
ACATCCCAGCTCCACATCAC |
CGATCCGACTCACCAATACC |
| GAPDH |
GACATCAAGAAGGTGGTGAAGC |
GTCCACCACCCTGTTGCTGTAG |
Measurement of caspase-3 activity
The activity of caspase-3 was assessed using the
Caspase-3 Colorimetric Assay kit (R&D Systems, Inc.,
Minneapolis, MN, USA), which is based on the spectrophotometric
detection of the color reporter molecule p-nitroanaline (pNA)
following cleavage from the labeled substrate DEVD-pNA (caspase-3)
as an index. Cells were incubated with the designated
concentrations of LC (0, 25, 35 and 45 µg/ml). The cells
were washed with PBS and suspended in 5 volumes lysis buffer (20
mmol/l HEPES, pH 7.9, 20% glycerol, 200 mmol/l KCl, 0.5 mmol/l
EDTA, 0.5% NP40, 0.5 mmol/l DTT and 1% protease inhibitor cocktail;
Sigma-Aldrich). The lysates were collected and stored at −20°C
until use. The protein concentration was determined by the Bradford
method as per the manufacturer's instructions of the Caspase-3
Colorimetric Assay kit. Supernatant samples, containing 100
µg total protein, were added to 96-well plates with the
DEVD-pNA and LEHD-pNA at 37°C for 1–2 h to determine caspase-3
activity. The optical density of each well was measured at 405 nm
using a fluorescence plate reader (3001; Bio-Rad Laboratories,
Inc). Each plate contained three wells of a given experimental
condition and three control wells. The activity of caspase-3 was
expressed in arbitrary absorbance units (absorbance at a wavelength
of 405 nm).
Western blot analysis
Cells at a density of 1.5×105 cells/ml
were incubated with LC for 24 h. The soluble lysates (15 µl
per lane) were subjected to 10% SDS-PAGE, then were transferred
onto the nitrocellulose membranes (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA) and blocked with 5% non-fat milk in
Tris-buffered saline with Tween-20 (TBST; Biodee Biotechnology Co.,
Ltd) for 2 h at room temperature. Membranes were incubated with the
respective primary antibody [anti-caspase-3 antibody (1:2,000; cat
no. sc-65496), anti-poly(adenosine diphosphate-ribose) polymerase
(PARP) antibody (1:2,000; cat no. sc-56196) or anti-β-actin
antibody (1:2,000, cat no. sc-47778), all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA] at 4°C overnight and then
incubated with horseradish peroxidase-conjugated bovine anti-mouse
immunoglobulin G (1:10,000; cat no. sc-2371; Santa Cruz
Biotechnology, Inc.) as the secondary antibody for 1 h at room
temperature. Western blots were developed using enhanced
chemiluminescence (Thermo Fisher Scientific) and were exposed on
Kodak radiographic film (Kodak, Rochester, NY, USA).
Statistical analysis
The data were presented as the mean ± standard
deviation from a minimum of three independent experiments and
evaluated through analysis of variance followed by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference. The analyses were performed using the
Origin software, version 8.0 (OriginLab, Northampton, MA, USA).
Results
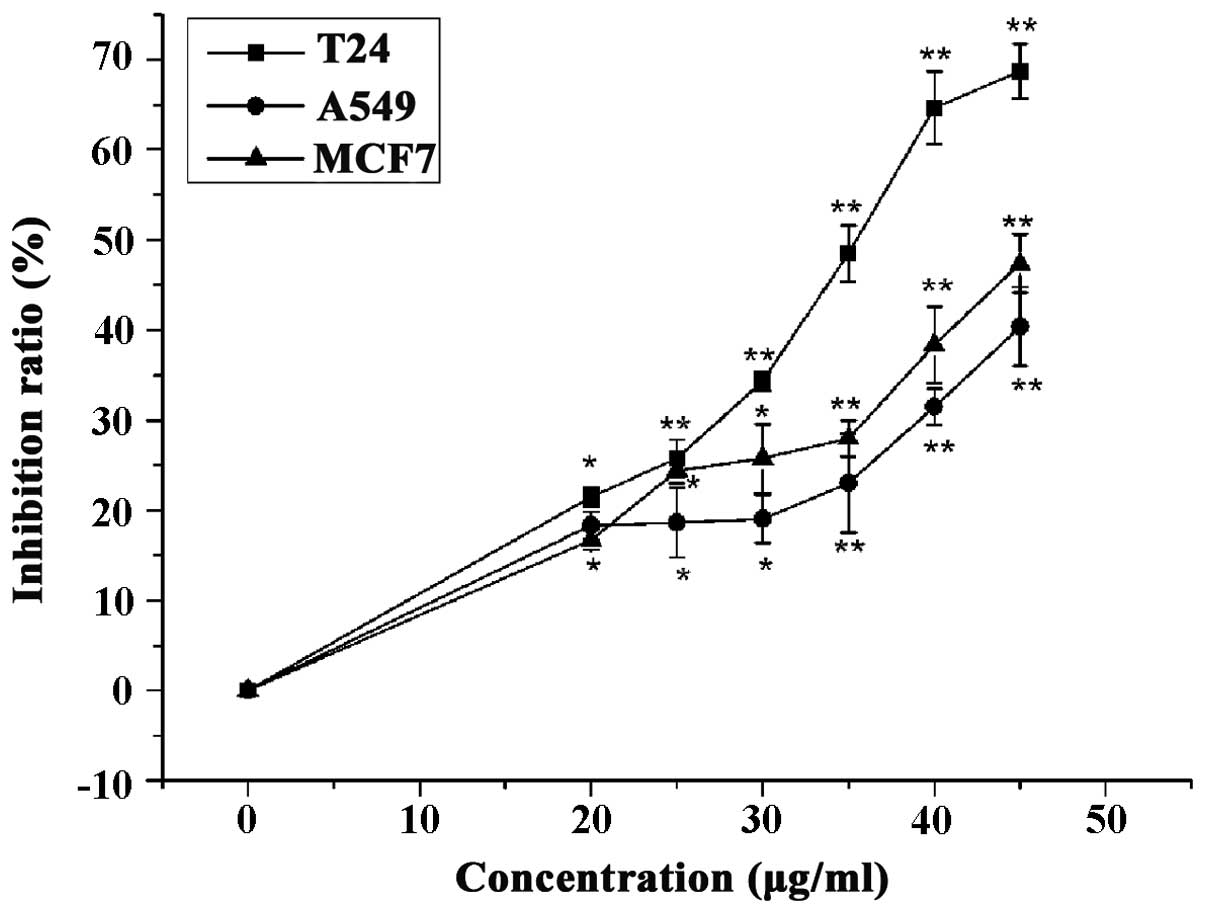
LC inhibited proliferation of T24, MCF7
and A549 cells
Breast, lung and bladder cancer are frequently
malignant, thus have a clear effect on health due to high incidence
and recurrence rates (23,24). The current study examined the
proliferation inhibition of LC (0, 25, 30, 35, 40 and 45
µg/ml) against T24 (bladder cancer), MCF7 (breast cancer)
and A549 (lung cancer) cells. Subsequent to treatment with LC (45
µg/ml) for 24 h, the rates of proliferation inhibition of
T24, MCF7 and A549 cells were 68, 47 and 40% respectively (Fig. 2). As T24 cells were observed to
exhibit a greater sensitivity to LC than MCF7 and A549 cells, T24
cells were selected for use in the subsequent experiments.
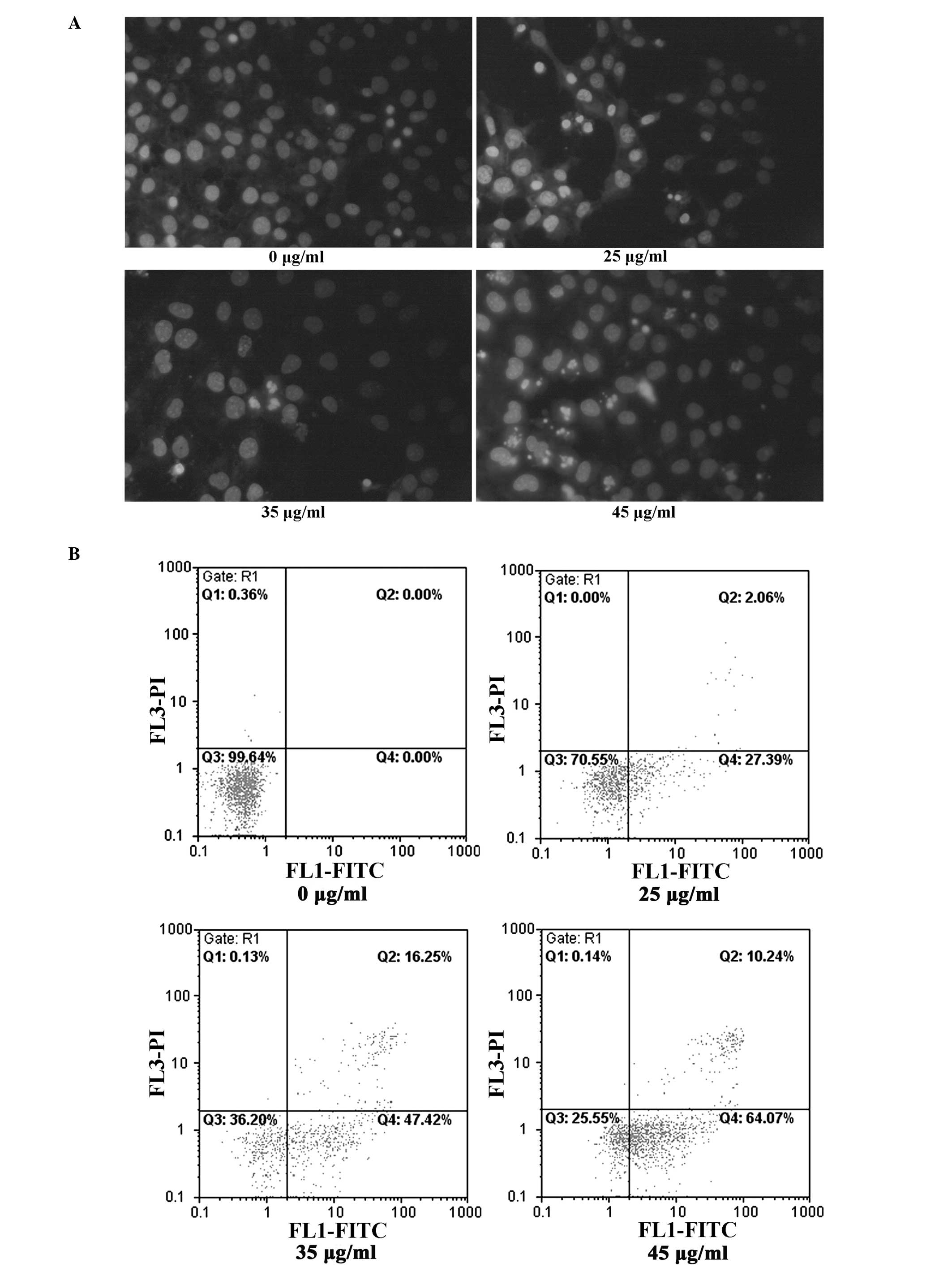
LC induces apoptotic cell death and
caspase activation in T24 cells
Morphological assessment with Hoechst staining
verified the fact that LC induces T24 cell apoptosis, with
LC-treated cells exhibiting typical morphological features of
apoptosis, such as nuclear condensation and fragmentation (Fig. 3A). Annexin V-FITC-PI
double-staining was used to detect phosphatidyl serine
externalization, a hallmark of early apoptosis, to indicate whether
LC-induced apoptosis occurred (25). Treatment of T24 cells with LC (0,
25, 35 and 45 µg/ml) for 24 h led to a significant increase
in the percentage of apoptotic cells, from 0.6% in control cells to
30, 64 and 74% respectively (Fig.
3B). In addition, measurement of a key factor in apoptosis,
caspase-3 activity, provided further support for the LC-induced
apoptotic response (Fig. 3C).
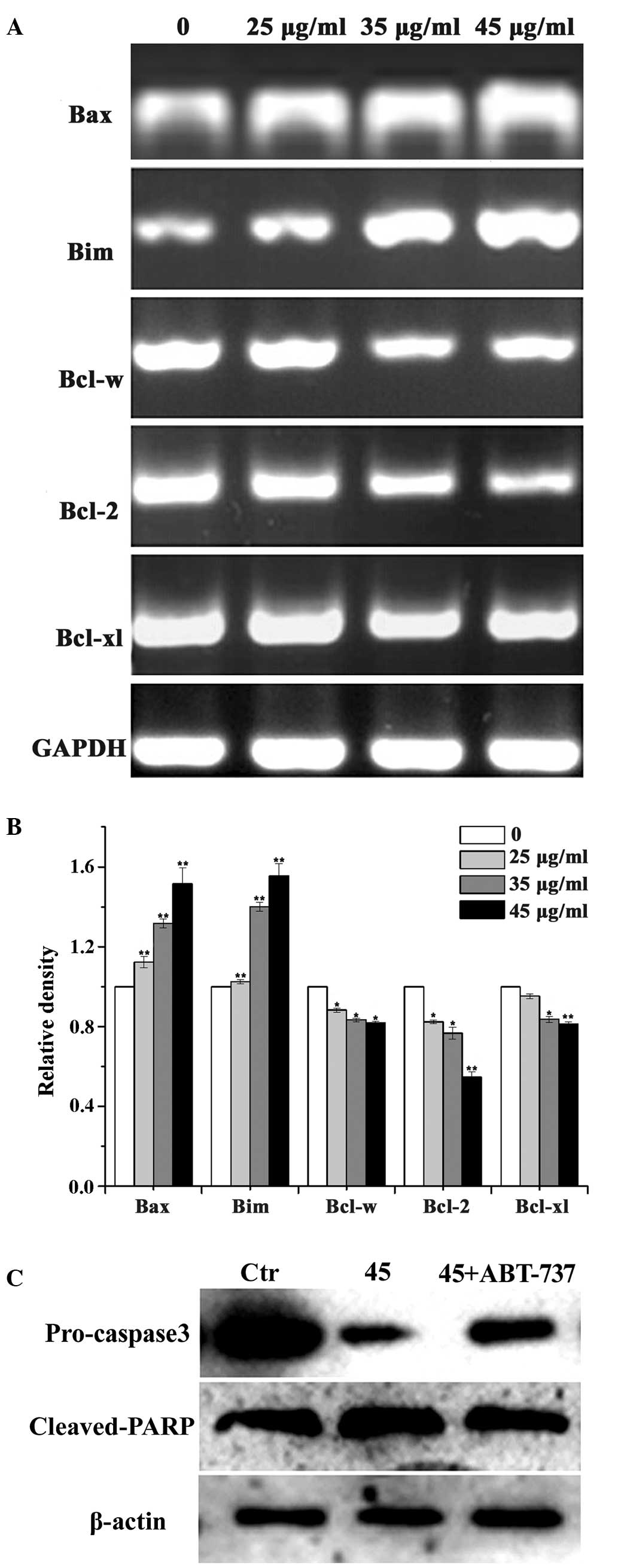
Bcl-2 family members served a crucial
role in LC-induced T24 cell apoptosis
As Bcl-2 family members serve a critical role in
inducing caspase-3 activation, regulating apoptosis and
irreversible cellular damage, they are suggested to be important in
the determination of cell fate (25). LC-induced caspase-3 activation and
apoptosis were observed in the present study. To investigate
whether Bcl-2 family members are involved in the apoptosis of T24
cells induced by LC, the expression levels of Bcl-2 family members
(Bax, Bim, Bcl-w, Bcl-2 and Bcl-XL) in T24 cells treated with LC
were analyzed (Fig. 4A). Compared
with the control group, exposure of T24 cells to LC (25, 35 and 45
µg/ml) resulted in a concentration-dependent reduction in
the mRNA level of Bcl-2, Bcl-w and Bcl-XL, with a concomitant
increase observed in the levels of Bax and Bim. Based on the
importance of Bcl-2 family members in inducing apoptosis, and the
alterations in the levels of Bcl-2 mRNA observed in the present
study, an inhibitor of the Bcl-2 family (ABT-737) was used to
confirm the role of Bcl-2 family members in LC-treated T24 cell
apoptosis. As presented in Figure
4B, the Bcl-2 family inhibitor ABT-737 effectively blocked
LC-induced apoptosis associated proteins (pro-caspase-3 and cleaved
PARP).
Discussion
Bladder cancer is a common, however serious, health
problem worldwide. Approximately 70–80% of patients with bladder
cancer are diagnosed with non-muscle invasive bladder cancer
(NMIBC) and may be treated with endoscopic resection (5). Two main problems may occur in the the
patients undergoing resection: i) High intravesical recurrence
rates; ii) progression to muscle invasive cancer during repeated
intravesical recurrence (26).
Therefore, the next step is adjuvant intravesical therapy aimed at
reducing the risk of tumor recurrence and possibly progression
(3). Intravesical Bacillus
Calmette-Guérin (BCG) therapy is the most effective and widely used
immunotherapeutic method against bladder cancer. However, BCG
therapy is associated with clear side effects, and 60–80% patients
fail to tolerate the therapy due to the local symptoms of cystitis,
including dysuria, pollakisuria, low-grade fever and malaise
(27). In addition, mitomycin C,
thiotepa, doxorubicin and epirubicin are commonly used to prevent
recurrence, however they also have side effects (28). The strong systemic toxicity and
incomplete efficacy of the intravesical agents has contributed to
the search for novel drugs to reduce the rate of recurrence of
bladder cancer. In the present study, it was demonstrated that LC
inhibited the growth of several cancer cell lines (T24, MCF7 and
A549) with significant growth inhibition against T24 cells thus
suggesting that LC has potential to as novel therapeutic agent
against various types of human cancer, particularly bladder
cancer.
Induction of apoptosis is considered as an important
strategy in the treatment of cancer (6), and numerous previous studies have
demonstrated the effect of natural products on cancer cell
apoptosis (29,30). The results obtained in the present
study provide evidence that LC induced significant apoptosis in T24
cells (Fig. 3A), however the
mechanism remains to be fully elucidated. Understanding the
mechanism by which LC induces apoptosis in T24 cells may aid in the
optimization of its anticancer activity. In the present study, LC
treatment was observed to result in a reduction of anti-apoptotic
mRNAs (Bcl-2, Bcl-w and Bcl-XL), and an increase in levels of
pro-apoptotic mRNAs (Bax and Bim). Notably, several small molecules
have been selected on the basis of their anti-Bcl-2 activity and
among them ABT-737 has been previously demonstrated to be a potent
inhibitor of Bcl-2/Bcl-w/Bcl-XL (31). The apoptotic response of LC-treated
T24 cells was attenuated by ABT-737, supporting a pivotal role of
Bcl-2 family members in LC-induced T24 cells apoptosis. Evidence
based on these observations supports an important therapeutic
effect of LC on bladder cancer. However, the specific role of Bcl-2
family members was only investigated in brief in the current study
and considering the fundamental role of Bcl-2 family members in the
integration of apoptotic cell stimuli, further investigation is
required to fully elucidate this. In conclusion, the evidence of
the current study demonstrates that LC led to a
concentration-dependent inhibition of bladder cancer cell
proliferation, and this antiproliferative effect appears to be due
to its ability to promote apoptotic cell death. LC results in
alterations in the expression of Bcl-2 family member genes, leading
to the cleavage of PARP and the activation of the caspase-mediated
cell death signaling pathway. Therefore, LC is suggested to be a
promising candidate for further development as a therapeutic agent
for bladder cancer.
Acknowledgments
Professor Qiusheng Zheng and Mr. Penglong Wang were
involved in the experimental design, acquisition of data, data
interpretation, in addition to manuscript preparation. The present
study was supported by the National Natural Science Foundation of
China (grant no. 81260338), the Xinjiang Production and
Construction Corps Funds for Distinguished Young Scientists (grant
no. 2011CD006), and International Cooperation Projects (grant no.
2012BC001) to Professor Qiusheng Zheng.
References
|
1
|
Zieger K: High throughput molecular
diagnostics in bladder cancer - on the brink of clinical utility.
Mol Oncol. 1:384–394. 2008. View Article : Google Scholar
|
|
2
|
Jacobs BL, Lee CT and Montie JE: Bladder
cancer in 2010: How far have we come? CA Cancer J Clin. 60:244–272.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian B, Wang Z, Zhao Y, Wang D, Li Y, Ma
L, Li X, Li J, Xiao N, Tian J, et al: Effects of curcumin on
bladder cancer cells and development of urothelial tumors in a rat
bladder carcinogenesis model. Cancer Lett. 264:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soloway MS, Sofer M and Vaidya A:
Contemporary management of stage T1 transitional cell carcinoma of
the bladder. J Urol. 167:1573–1583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan X, Li T, Xiao E, et al: Licochalcone
B inhibits growth of bladder cancer cells by arresting cell cycle
progression and inducing apoptosis. Food Chem Toxicol. an
international journal published for the British Industrial
Biological Research Association. 65:242–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Zhang S, Geng JX and Hu XY: Curcumin
inhibits human non-small cell lung cancer A549 cell proliferation
through regulation of Bcl-2/Bax and cytochrome C. Asian Pac J
Cancer Prev. 14:4599–4602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li PM, Li YL, Liu B, Wang WJ, Wang YZ and
Li Z: Curcumin inhibits MHCC97H liver cancer cells by activating
ROS/TLR-4/caspase signaling pathway. Asian Pac J Cancer Prev.
15:2329–2334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim H, Tu HC, Ren D, Takeuchi O, Jeffers
JR, Zambetti GP, Hsieh JJ and Cheng EH: Stepwise activation of BAX
and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis.
Mol Cell. 36:487–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen X, Lin ZQ, Liu B and Wei YQ:
Caspase-mediated programmed cell death pathways as potential
therapeutic targets in cancer. Cell Prolif. 45:217–224. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diederich M: Natural Compounds and their
Role in Apoptotic Cell Signaling Pathways. 1171. Wiley-Blackwell;
Boston: pp. 1–660. 2009
|
|
13
|
Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni
SJ, Wang LS and Du X: Licochalcone A inhibits growth of gastric
cancer cells by arresting cell cycle progression and inducing
apoptosis. Cancer Lett. 302:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwon SJ, Park SY, Kwon GT, Lee KW, Kang
YH, Choi MS, Yun JW, Jeon JH, Jun JG and Park JH: Licochalcone E
present in licorice suppresses lung metastasis in the 4T1 mammary
orthotopic cancer model. Cancer Prev Res (Phila). 6:603–613. 2013.
View Article : Google Scholar
|
|
15
|
Yuan X, Li D, Zhao H, Jiang J, Wang P, Ma
X, Sun X and Zheng Q: Licochalcone A-induced human bladder cancer
T24 cells apoptosis triggered by mitochondria dysfunction and
endoplasmic reticulum stress. BioMed Res Int. 2013:4742722013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dao TT, Nguyen PH, Lee HS, Kim E, Park J,
Lim SI and Oh WK: Chalcones as novel influenza A (H1N1)
neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg Med Chem
Lett. 21:294–298. 2011. View Article : Google Scholar
|
|
17
|
Yoon G, Jung YD and Cheon SH: Cytotoxic
allyl retrochalcone from the roots of Glycyrrhiza inflata. Chem
Pharm Bull (Tokyo). 53:694–695. 2005. View Article : Google Scholar
|
|
18
|
Park EJ, Park HR, Lee JS and Kim J:
Licochalcone A: An inducer of cell differentiation and cytotoxic
agent from Pogostemon cablin. Planta Med. 64:464–466. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franceschelli S, Pesce M, Vinciguerra I,
Ferrone A, Riccioni G, Patruno A, Grilli A, Felaco M and Speranza
L: Licocalchone-C extracted from Glycyrrhiza glabra inhibits
lipopolysaccharide-interferon-γ inflammation by improving
antioxidant conditions and regulating inducible nitric oxide
synthase expression. Molecules. 16:5720–5734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung JI, Lim SS, Choi HJ, Cho HJ, Shin HK,
Kim EJ, Chung WY, Park KK and Park JH: Isoliquiritigenin induces
apoptosis by depolarizing mitochondrial membranes in prostate
cancer cells. J Nutr Biochem. 17:689–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawai K, Miyazaki J, Joraku A, Nishiyama H
and Akaza H: Bacillus Calmette-Guerin (BCG) immunotherapy for
bladder cancer: Current understanding and perspectives on
engineered BCG vaccine. Cancer Sci. 104:22–27. 2013. View Article : Google Scholar
|
|
24
|
Silva SC, Wilson C and Woll PJ:
Bone-targeted agents in the treatment of lung cancer. Ther Adv Med
Oncol. 7:219–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murray PJ, Wivell G and Denton E: Breast
cancer screening and diagnosis in the 21st century within the UK.
(Post Reprod Health). 23–Jul;2015.Epub ahead of print.
|
|
26
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Böhle A, Thanhäuser A, Ernst M, Flad HD,
Rüsch-Gerdes S, Jocham D and Ulmer AJ: Reduction of side effects of
intravesical therapy with bacille Calmette-Guérin by
pentoxifylline? - an in vitro approach. Clin Infect Dis. 31(Suppl
3): S101–S105. 2000. View
Article : Google Scholar
|
|
28
|
Hansel DE, McKenney JK, Stephenson AJ and
Chang SS: The Urinary Tract: A Comprehensive Guide to Patient
Diagnosis and Management. 32. Springer; New York: pp. 199–201.
2012
|
|
29
|
Kuno T, Tsukamoto T, Hara A and Tanaka T:
Cancer chemo-prevention through the induction of apoptosis by
natural compounds. J Biophys Chem. 03:156–173. 2012. View Article : Google Scholar
|
|
30
|
Safarzadeh E, Sandoghchian Shotorbani S
and Baradaran B: Herbal medicine as inducers of apoptosis in cancer
treatment. Advanced Pharml Bull. 4(Suppl 1): 421–427. 2014.
|
|
31
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|