Introduction
Focal adhesion kinase (FAK) is expressed in
podocytes, where it affects the α-actin cytoskeleton thereby
regulating cell adhesion and migration. FAK may be activated by
glomerular injury, resulting in proteinuria and foot process
fusion. In animal models, the elevated expression levels of FAK may
lead to foot process fusion and increased levels of proteinuria,
processes which were significantly reduced following knock down of
FAK. Previous studies have demonstrated that the migration and
activation of podocytes was significantly reduced in the absence of
FAK (1,2). Activation of mitogen-activated
protein kinase (MAPK) signaling pathway is associated with podocyte
injury, foot process fusion and proteinuria (3). Furthermore, FAK affects podocyte
structure via the MAPK signaling pathway (4). The MAPK family is composed of
extracellular signal-regulated kinases (ERK), c-Jun N-terminal
kinases (JNK) and p38 (5).
Receptor activator of nuclear factor κB (RANK) and its ligand
(RANKL) are cytokines that are able to activate the nuclear factor
κB (NF-κB) or MAPK signaling pathways following binding (6). The MAPK and NF-κB signaling pathways
regulate numerous biological cellular processes, including cell
proliferation, transduction and apoptosis. Liu et al
(7) demonstrated that RANKL
inhibits the apoptosis of podocytes, and the expression levels of
RANKL increased following podocyte injury. Prednisone is the
preferred drug for the treatment of nephrotic syndrome; however,
the association between RANKL and kidney proteinuria remains to be
elucidated.
The aim of the present study was to investigate the
possible mechanisms underlying the therapeutic effects of
prednisone, including the decreased protein levels in kidney tissue
samples via the FAK/RANKL/MAPK signaling pathway in an
adriamycin-induced nephritic rat model. The rats with
adriamycin-induced nephropathy were treated with prednisone.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) was used to quantify the mRNA expression levels of FAK,
RANKL, p38, ERK, JNK and nephrin; electron microscopy was used to
observe renal pathology; immunohistochemistry was used to detect
the levels of nephrin in the kidney; and western blot analysis was
used to quantify the protein expression levels of FAK,
phosphorylated (p)-FAK, RANKL, p38, p-p38, ERK, p-ERK, JNK, p-JNK,
and nephrin in the kidney tissue samples.
Materials and methods
Reagents and instruments
The materials for the present study were purchased
from the following suppliers: SYBR Premix Ex Taq™ II kit
(cat. no. DRR820A; Takara Biotechnology Co., Ltd., Dalian, China);
RNAiso Plus (cat. no. D9108A; Takara Biotechnology Co., Ltd.);
ExScript™ RT Reagent kit (cat. no. DRR037A; Takara Biotechnology
Co., Ltd.); rat glyceraldehyde 3-phosphate dehydrogenase GAPDH
primer (cat. no. D379212; Takara Biotechnology Co., Ltd.);
oxorubicin hydrochloride (cat. no. H31020675; Zhejiang Hisun
Chemical Co., Ltd., Taizhou, China); RT-qPCR primers (Takara
Biotechnology Co., Ltd.); RT-qPCR kits (Beyotime Institute of
Biotechnology, Haimen, China); western blotting-associated reagents
(Beyotime Institute of Biotechnology), including SDS-PAGE kit,
radioimmunoprecipitation (RIPA) lysis buffer, BeyoECL Plus A,
BeyoECL Plus B, nitrocellulose membranes (cat. no. FFN09), and
phenylmethanesulfonyl fluoride (cat. no. ST506); secondary antibody
dilution buffer (cat. no. P0023D; Beyotime Institute of
Biotechnology); primary antibody dilution buffer (cat. no. P0023A;
Beyotime Institute of Biotechnology); anti-GAPDH monoclonal
antibody (cat. no. ab8245; Abcam, Cambridge, UK); anti-FAK (cat.
no. 3285), p38 (cat. no. 6381), ERK (cat. no. 2265) and JNK (cat.
no. 3630) total protein polyclonal antibodies (Bioworld Technology,
Inc., St. Louis Park, MN, USA); anti-RANKL monoclonal antibody
(cat. no. ab12125; Abcam); anti-nephrin monoclonal antibody (cat.
no. 377246; Santa Cruz Biotechnology, Inc., Dallas, TX, USA);
anti-β actin monoclonal antibody (cat. no. ab8226; Abcam); PV-6001
Two-Step Detection kit (OriGene Technologies, Inc., Beijing,
China); Polink-2 Plus® Polymer horseradish peroxidase
(HRP) Detection system for rabbit primary antibody (GBI Labs,
Bothell, WA, USA); osteoprotegerin (OPG) ELISA kit (Nanjing
Senbeijia Biotechnology Co., Ltd., Nanjing, China); RANKL ELISA kit
(Nanjing Senbeijia Biotechnology Co., Ltd.); 7500 Fast Real-Time
PCR system (Applied Biosystems Life Technologies, Foster City, CA,
USA); EL-311S enzyme standard instrument (BioTek Instruments, Inc.,
Winooski, VT, USA); western blotting kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA); gel image analyzer with Genesnap and
Genetool (Syngene, Frederick, MD, USA).
Model establishment and group
assignment
Experimental procedures were conducted in conformity
with the institutional guidelines for the care and use of
laboratory animals of the Fujian University of Traditional Chinese
Medicine (Fujian, China), and conformed to the Laboratory Animal
Management Regulations. A total of 30 healthy male Sprague-Dawley
rats (age, 6–8 weeks; weight, 180–220 g) were obtained from the
Medical Experimental Animal Center of Guangdong Province
(certificate no. 70092411). Animals were fed on a standard
laboratory diet and provided with ad libitum access to
water. The medical experimental animals were housed in the
environmental facilities of the Mouse Laboratory of Animal
Experimental Center in the Fujian University of Traditional Chinese
Medicine in a pathogen-free environment. All the animal
experimental procedures were conducted in accordance with the
management rules of the Fujian Province Medical Laboratory Animal
Management Committee. Rats were kept at 23°C and 60% humidity in a
12 h light-dark cycle. The rats were randomly divided into the
normal, model and prednisone groups (n=10 per group). The rats in
the model group and prednisone groups were treated with a single
intravenous injection of 6.5 mg/kg adriamycin into the tail
(8). The normal rats were injected
with an equal quantity of saline. A total of 7 days after treatment
with adriamycin, samples of urine were collected over the course of
24 h, and the urinary protein levels were measured, indicating the
successful establishment of a nephritic rat model. Following model
establishment, the rats in the prednisone group were treated with a
daily dose of 10 mg/kg/day pred-nisone (cat. no. H31020675;
Shanghai Sine Pharmaceutical Laboratories Co., Ltd., Shanghai,
China) by gastric gavage. The rats in the normal and model groups
were treated daily with an equal amount of normal saline. At days
21 and 35 after model establishment, the rats were anesthetized by
intraperitoneal injection with chloralic hydras (BIO BASIC Int.,
Markham, ON, Canada) at a dose of 0.3 ml/100 g body weight. A
midline incision was made in the abdomen and blood samples were
obtained from the aorta. The kidneys were removed immediately and
weighed, and renal cortex tissue was removed using a small blade.
The renal cortex tissue were stored in 10% formalin subsequent to
pathological examination and immunohistochemical studies. The
remaining renal tissues were immediately snap-frozen in liquid
nitrogen and stored at −80°C for later analysis. Pre-experimental
observations determined that 7 days after model establishment, the
adriamycin-induced nephritic rats exhibited symptoms of
proteinuria, and 21 and 35 days after model establishment
proteinuria symptoms significantly increased (P<0.05).
Analysis of 24 h urinary protein
levels
The rats were placed in individual metabolic cages
for 24 h during which time samples of urine were collected, and the
24 h urinary protein levels were measured by biuret colorimetry
(Shanghai Yucan Biological Technology, Co., Ltd.) as described
previously (9).
Transmission electron microscopy
The renal cortex samples were fixed using 3%
glutaraldehyde, 0.22 mmol/l sucrose phosphate buffer (pH 7.2),
post-fixed in 1% osmium tetroxide, progressively dehydrated in
ethanol, and embedded in epoxy resin. The tissue samples were then
examined for kidney pathology using a HU-12A transmission electron
microscope (Hitachi, Ltd., Tokyo, Japan).
Serum OPG and RANKL expression in each
group
A total of 21 and 35 days after the first adriamycin
injection, 3 ml blood was collected from the abdominal aorta, and
serum was obtained via centrifugation (1,478 × g at 4°C for 5 min).
Serum RANKL and OPG levels were analyzed by ELISA. Nephrin
expression was detected by immunohistochemical staining (10).
RT-qPCR
The rat renal cortex tissue samples were packed with
tinfoil, frozen in liquid nitrogen, and preserved at −80°C until
further experimentation. Total renal cortex RNA was extracted using
TRIzol® Reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer's instructions. RNA was
reverse transcribed to cDNA using an ExScript™ RT kit and SYBR
Premix Ex Taq II Reagent kit in order to conduct the
fluorescence amplification. RT-qPCR was carried out on a Thermal
Cycler Dice™ Real Time system (Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions, and the target
primers were synthesized by Takara Biotechnology Co., Ltd.
(Table I). The PCR cycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 30 sec, and 60°C for 60 sec. The mRNA levels were
normalized to GAPDH. The mRNA expression levels in the normal group
were used to calculate the relative mRNA levels in the other
groups.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer sequences | Size (bp) |
|---|
| FAK | Forward
5′-GGACCTTACTGGCAACTGTGGA-3′ | 138 |
| Reverse
5′-CATACTGCTGCGCCAGCTTC-3′ | |
| RANKL | Forward
5′-GCAGCATCGCTCTGTTCCTGTA-3′ | 164 |
| Reverse
5′-GCATGAGTCAGGTAGTGCTTCTGTG-3′ | |
| p38MAPK | Forward
5′-TTACCGATGACCACGTTCAGTTTC-3′ | 107 |
| Reverse
5′-AGCGAGGTTGCTGGGCTTTA-3′ | |
| ERK | Forward
5′-CTGGACCAGCTCAACCACATTC-3′ | 97 |
| Reverse
5′-ACTGTAGGTAGTTTCGGGCCTTCA-3′ | |
| JNK | Forward
5′-ACCACCAAAGATCCCTGACAA-3′ | 104 |
| Reverse
5′-TAGTTCGCTCCTCCAAATCCA-3′ | |
| Nephrin | Forward
5′-GACACCTGCATGATGAAGTGGAG-3′ | 102 |
| Reverse
5′-CAGGCCAGCGAAGGTCATAG-3′ | |
Protein expression levels of FAK, RANKL,
p38, ERK, and JNK, as determined by western blot analysis
A total of 200 mg renal tissue samples, stored at
−80°C, were obtained from each group. The renal tissue samples were
lysed in 0.4 ml RIPA lysis buffer and homogenized. The homogenates
were placed into a 1.5 ml EP tube and lysed on ice for 30 min. The
homogenates were then centrifuged at 23,663 × g at 4°C for 60 min.
The supernatants were collected from the EP tube (Axygen, Union
City, CA, USA). Following extraction of total protein, the protein
concentrations were measured using a Bicinchoninic Acid (BCA)
Protein Concentration Assay kit (Beyotime Institute of
Biotechnology). The protein extracts were separated by 12% SDS-PAGE
for 124 min at 80 V, and transferred onto nitrocellulose membranes
for 1 h at 100 V. The nitrocellulose membranes were then blocked
with 5% non-fat dry milk in Tris-buffered saline with Tween 20
(TBST; Beyotime Institute of Biotechnology) for 2 h at room
temperature. The membranes were exposed to rat anti-FAK,
anti-p-FAK, anti-p38, anti-p-p38, anti-RANKL, and anti-GAPDH
(1:1,000), overnight at 4°C. The membranes were washed three times
in TBST for 5–10 min. The membranes were then incubated with
HRP-labeled goat anti-rabbit secondary antibody (1:1,000; Beyotime
Institute of Biotechnology; cat. no. A0208).or goat anti-rat IgG
(H+L) (1:1,000; Bioworld Technology, Inc., cat. no. BS10010).
Following extensive washing in TBST, the bands were detected by
chemiluminescence and analyzed by a gel image analyzer, and the
relative density of each band was calculated and normalized to that
of GAPDH (10).
Immunohistochemical analysis
Immunohistochemical staining was conducted in a
two-step method: The paraffin was removed from the sections using
xylene and rehydrated in graded ethanol as follows: 100% ethanol
for 5 min twice, 95% ethanol for 5 min twice, 90% for 5 min and 80%
for 5 min. For antigen retrieval, the sections were placed in a
microwave following treatment with blocking goat serum (Pingrui
Biotechnology Co.) for 45 min, the sections were incubated
overnight at 4°C with primary antibodies targeting nephrin. The
sections were then incubated with the appropriate Streptavidin-HRP
(Beyotime Institute of Biotechnology; cat. no. A0303) secondary
antibodies for 30 min at 37°C. The sections were stained with
diamino-benzidene, and counterstained with hematoxylin (Shanghai
YANYU Information Technology Co. Ltd., Shanghai, China). The
sections were subsequently observed under a microscope (EM-208;
Philips, Amsterdam, Holland), and the positive integral optical
density was calculated.
Statistical analysis
Data were presented as the mean ± standard
deviation. Groups of data were tested for normality using a
Shapiro-Wilk test, and tested for variance homogeneity. The results
were analyzed using a one-way analysis of variance, and a least
significant difference analysis method was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant result.
Results
Urinary protein levels
As compared with the normal group, the 24 h urinary
protein levels were significantly increased in the model and
prednisone groups (P<0.05). As compared with the model group,
the urinary protein levels in the prednisone group were
significantly decreased at day 21 (P<0.05) and further decreased
at day 35 (P<0.01). As compared with the urinary protein levels
at day 21, the urinary protein levels in the model group were
significantly increased at day 35, and those of the prednisone
group significantly decreased at day 35 (P<0.05; Table II).
 | Table IIUrinary protein levels over the course
of 24 h. |
Table II
Urinary protein levels over the course
of 24 h.
| Time | Group (n=10)
|
|---|
| Normal (mg) | Model (mg) | Prednisone (mg) |
|---|
| Day 7 | 3.19±1.88 | 10.94±6.91b | 18.27±16.65b |
| Day 21 | 5.59±1.67 | 40.98±15.37c |
19.96±5.32b,d |
| Day 35 | 4.58±2.22 |
61.04±10.55a,c |
12.49±7.35a,e |
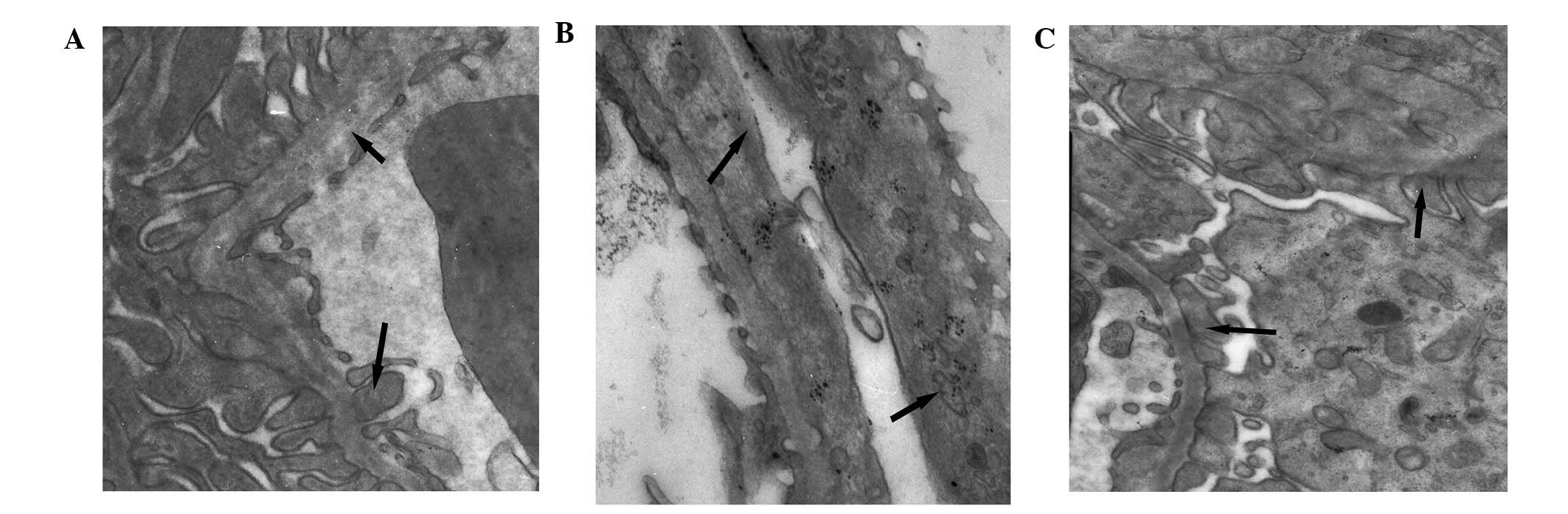
Pathomorphology of the tissue samples of
each group, as determined by electron microscopy
The foot processes in the normal group were clearly
defined, with no observed fusion or microvilli degeneration. The
podocytes of the model group exhibited swelling, and the foot
processes were abnormally broadened and exhibited diffused fusion.
In the prednisone group, the number of glomerular lesions
decreased, and only partial foot process fusion was observed, as
compared with the model group (Fig.
1).
mRNA expression levels of FAK, RANKL,
p38, ERK, and JNK
At day 21, the mRNA expression levels of FAK were
significantly higher in the model group, as compared with those of
the normal group (P<0.01). The mRNA expression levels of RANKL
and ERK were significantly higher in the model group, as compared
with those of the normal group (P<0.05). The mRNA expression
levels of JNK were significantly decreased in the prednisone group,
as compared with those of the normal group (P<0.05). The mRNA
expression levels of p38 were significantly lower in the prednisone
group, as compared with those of the model group (P<0.01), and
the mRNA expression levels of RANKL and ERK were significantly
lower in the prednisone group, as compared with those of the model
group (P<0.05). At day 35, the mRNA expression levels of FAK in
the model group were significantly higher, compared to the mRNA
expression levels on day 21 (P<0.05), and significantly higher,
as compared with the prednisone group (P<0.01). As compared with
the normal group, a signifi-cant decrease was observed in the mRNA
expression levels of p38 in the model and prednisone groups
(P<0.01). The mRNA expression levels of RANKL in the model group
were significantly increased, as compared with the normal
(P<0.05) and prednisone groups (P<0.01). At day 35, the mRNA
expression levels of ERK in the prednisone group were significantly
decreased (P<0.05), as compared with normal group. No
statistically significant differences were observed in the mRNA
expression levels of FAK, p38, and RANKL at day 21, as compared
with day 35 (Table III).
 | Table IIImRNA expression levels of FAK, RANKL,
p38, ERK, JNK, and nephrin. |
Table III
mRNA expression levels of FAK, RANKL,
p38, ERK, JNK, and nephrin.
| mRNA | Time | Group (n=5)
|
|---|
| Normal | Model | Prednisone |
|---|
| FAK | Day 21 | 1.00±0.00 | 1.76±0.48a | 1.34±0.50 |
| Day 35 | 1.00±0.00 |
2.25±0.08a,e | 1.00±0.03d |
| RANKL | Day 21 | 1.00±0.00 | 2.49±0.30b | 0.58±0.11c |
| Day 35 | 1.00±0.00 | 1.56±0.55b | 0.63±0.25d |
| p38 | Day 21 | 1.00±0.00 | 0.99±0.07 |
0.68±0.08b,d |
| Day 35 | 1.00±0.00 | 0.71±0.11a | 0.73±0.04a |
| ERK | Day 21 | 1.00±0.00 | 1.17±0.06b | 0.91±0.30c |
| Day 35 | 1.00±0.00 | 1.23±0.01b | 0.77±0.05c |
| JNK | Day 21 | 1.00±0.00 | 0.85±0.20 | w0.71±0.01b |
| Day 35 | 1.00±0.00 | 0.85±0.21 | 0.83±0.40 |
| Nephrin | Day 21 | 1.00±0.00 | 0.39±0.11a |
0.59±0.10a,d |
| Day 35 | 1.00±0.00 | 0.38±0.08a |
0.54±0.01a,c |
Serum protein expression levels of OPG
and RANKL
As compared with the normal group, the expression
levels of RANKL in the prednisone group were significantly higher
at day 21 and day 35 (P<0.01 and P<0.05, respectively). In
addition, at day 21 the expression levels of RANKL were
significantly higher in the prednisone group, as compared with the
model group (P<0.01). At day 35, the expression level of RANKL
was significantly higher in the prednisone group, as compared with
the model group (P<0.01). Conversely, at day 21 and day 35, the
expression levels of OPG were significantly lower in the prednisone
group, as compared with the model group (P<0.05; Table IV).
 | Table IVSerum protein expression levels of
OPG and RANKL. |
Table IV
Serum protein expression levels of
OPG and RANKL.
| Protein | Time | Group (n=10)
|
|---|
| Normal | Model | Prednisone |
|---|
| OPG | Day 21 | 1.21±0.43 | 1.28±0.45 | 0.71±0.17b |
| Day 35 | 1.33±0.59 | 1.24±0.49 | 0.65±0.22b |
| RANKL | Day 21 | 19.22±1.02 | 21.27±2.63 | 52.65±3.58c |
| Day 35 | 18.74±3.03 | 21.58±2.18 |
66.90±5.55a,c |
Expression levels of FAK, p-FAK, p38,
RANKL, p-p38, ERK, p-ERK, JNK and p-JNK in the kidney tissue
samples of each group
As compared with the normal group, the protein
expression levels of FAK, p38 and ERK, and their phosphorylated
counterparts were significantly higher in the model group
(P<0.01). The protein expression levels of FAK, p-FAK, p-ERK and
p-p38 were significantly decreased in the prednisone group
(P<0.01). As compared with the model group, the expression
levels of p-ERK in prednisone group were decreased (P<0.05) at
day 21 and significantly decreased at day 35 (P<0.01). As
compared with the normal group, at days 21 and 35, the protein
expression levels of RANKL were significantly increased in the
prednisone group (P<0.01). However, no statistically changes in
the protein expression levels of JNK and p-JNK were observed
(Fig. 2).
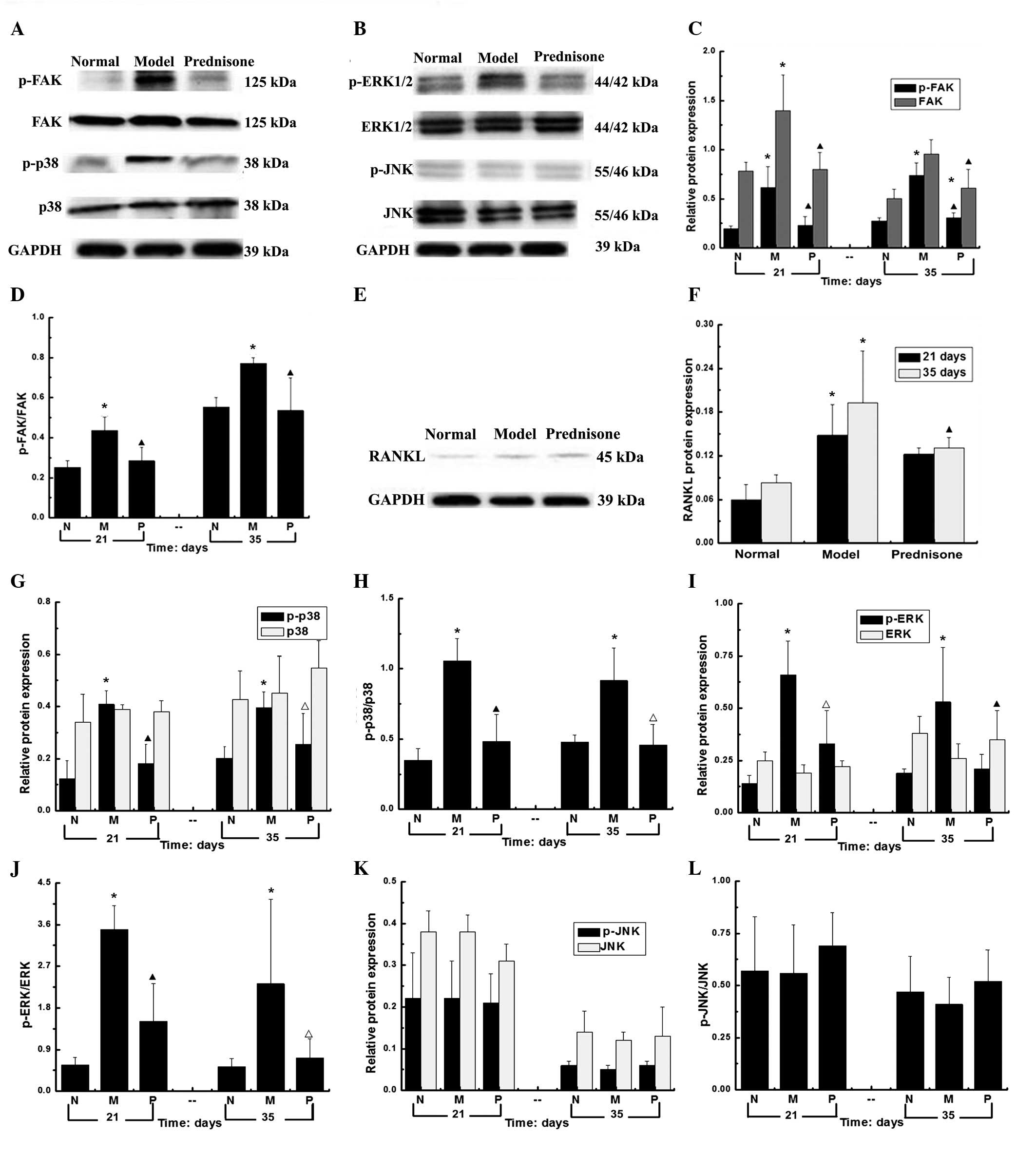 | Figure 2Protein expression levels of FAK,
p-FAK, p38, p-p38, ERK, p-ERK, JNK, p-JNK, and RANKL at days 21 and
35. (A) The expression levels of FAK, p-FAK, p38 and p-p38; (B) the
expression levels of ERK, p-ERK, JNK and p-JNK; (C) the relative
protein expression levels of FAK and p-FAK; (D) The protein
expression levels of FAK/p-FAK l; (E) The protein expression levels
of RANKL; (F) The protein expression levels of RANKL; (G) The
relative protein expression levels of p38 and p-p38; (H) The
protein expression levels of p-p38/p38; (I) The relative protein
expression levels of ERK and p-ERK; (J) The protein expression
levels of p-ERK/ERKl; (K) The relative protein expression levels of
JNK and p-JNK; (L) The protein expression levels of p-JNK/JNK.
*P<0.01, vs. the normal group; ▲P<0.01,
vs. the model group; and △P<0.05, vs. the model
group.. No statistically significant changes were observed in the
protein expression levels of JNK and p-JNK. N, normal group; M,
model group; P, prednisone group; p, phosphorylated; FAK, focal
adhesion kinase; RANKL, receptor activator of nuclear factor-κB
ligand; ERK, extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase. |
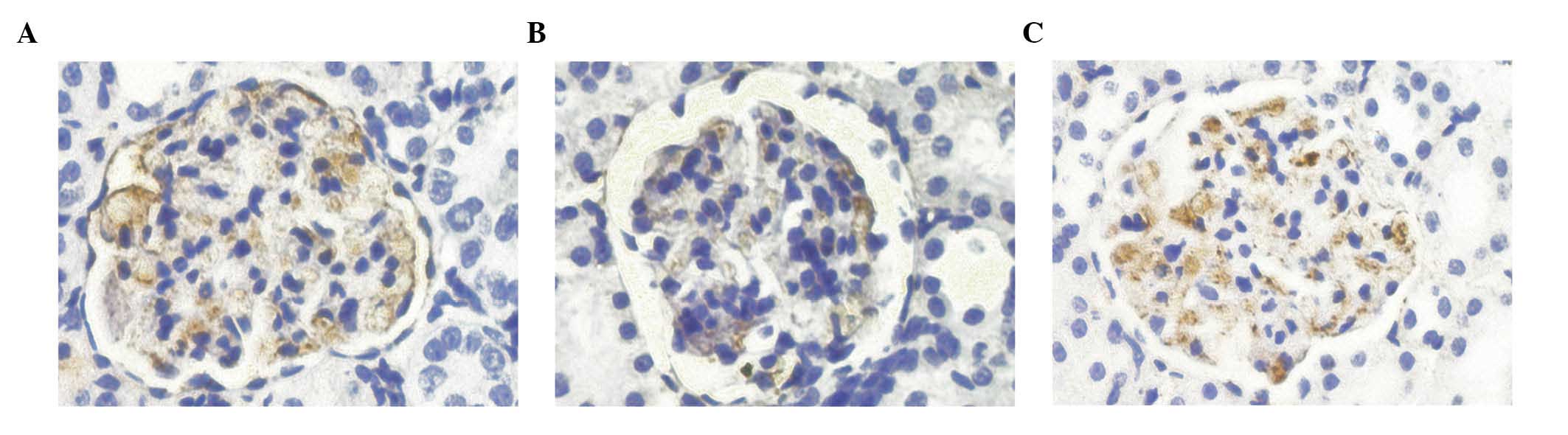
Protein expression levels of nephrin, as
determined by immunohistochemistry
On day 21, the nephrin proteins of the model group
exhibited uneven distribution, as compared with the normal group,
suggesting the presence of focal enhancements. In addition, the
protein expression levels of nephrin were significantly decreased
(P<0.01). On day 35, the protein expression levels of nephrin in
the model group were significantly lower, as compared with those of
the normal group (P<0.01). As compared with the model group, the
protein expression level of nephrin in the prednisone group
increased (P<0.01; Table V;
Fig. 3).
 | Table VProtein expression levels of
nephrin. |
Table V
Protein expression levels of
nephrin.
| Groups (n=3) | Integral optical
density
|
|---|
| Day 21 | Day 35 |
|---|
| Normal | 135.69±14.83 | 132.66±6.72 |
| Model | 35.21±4.87a | 40.57±4.20a |
| Prednisone | 82.81±10.03b | 78.90±6.83b |
Discussion
FAK is a non-receptor tyrosine kinase that localizes
to focal adhesions in adherent cells, and binds with the
cytoplasmic tails of β1 integrins (11). A previous study demonstrated that
phosphorylation of FAK regulates podocyte actin cyto-skeletal
formation and cell adhesion via the Ras/MAPK and
phosphoinositide-3-kinase signaling pathways (12). Podocyte structure is regulated via
the MAPK signaling pathway, composed of p38, ERK and JNK (5). Yang et al (13) demonstrated that the activation of
ERK and p38/MAPK is able to decrease the expression levels of
nephrin and podocin in podocytes. Therefore, the present study
hypothesized that foot process fusion and proteinuria may be
regulated by the FAK/MAPK signaling pathway. Podocyte migration and
activity were significantly decreased in the absence of FAK, and
glomerular injury occurred following FAK activation. In animal
models, the levels of proteinuria was significantly reduced
following FAK knockout (14). The
present study demonstrated that adriamycin increased both the mRNA
and protein expression levels of FAK, p38, ERK and their
phosphorylated proteins in kidney tissue samples; increased the
mRNA and protein expression levels of RANKL; increased the levels
of proteinuria; and decreased the expression levels of nephrin in
the model group. In addition, foot process fusion was observed
under light microscopy. Following treatment with prednisone, the
mRNA and protein expression levels of FAK, p38, ERK and their
phosphorylated proteins decreased in the kidney tissue samples; the
mRNA and protein expression levels of RANKL decreased; and the
expression levels of nephrin increased. In addition, the podocyte
lesions were less severe and proteinuria was markedly reduced in
the prednisone group, as compared with the model group. Following
treatment with prednisone, the expression levels of of RANKL
significantly decreased. However, the role of RANKL in kidney
tissues remains to be elucidated.
A recent study demonstrated that RANKL and its
receptor RANK are cytokines (15).
RANK and RANKL are not only involved in lymphocyte development and
lymphoid organ formation; they are expressed in embryonic kidneys,
myofibroblasts, vascular endothelial cells, and participate in the
development of autoimmune diseases (16-19).
A previous study reported that RANKL mRNA and protein expression
was detected in the renal glomeruli, convoluted tubules, and
parenchyma of developing fetal kidneys, whereas RANKL was not
detected in adult kidneys (20).
The same study reported that RANKL was moderately expressed in
renal glomeruli, renal tubules and renal interstitia in rat
embryos, and was moderatly to highly expressed in neonatal rat
renal tubules. However, lower expression levels of RANKL were
present in the renal tissue samples of adult rats. Liu et al
(7) demonstrated that the
expression levels of RANKL in the kidneys of rats with puromycine
amino-nucleoside nephrosis were significantly increased, as
compared with the control. In addition, RANKL was able to activate
the endoplasmic reticulum Ca2+/ATPase, and was important
for the response to podocyte injury in vitro. RANKL
expression reduced intracellular calcium levels, and eventually led
to the KCa current suppression, thus reducing podocyte apoptosis.
RANKL and RANK activate the transcription factor NF-κB and MAPK
signaling pathways (21). In
addition, a previous study demonstrated that prednisone inhibited
NF-κB activation (22). In the
present study the protein expression and phosphorylation levels of
FAK, p38, ERK protein were significantly increased in the model
group, as well as the mRNA and protein expression levels of RANKL,
and the levels of proteinuria. After 21 and 35 days of prednisone
treatment, the protein expression and phosphorylation levels of
FAK, p38, and ERK were significantly reduced, as well as the
protein expression levels of RANKL; the expression levels of
nephrin were significantly increased, and the levels of proteinuria
were markedly reduced. The protein expression levels of RANK were
not detectable using various concentrations of antibodies. The
results suggested that RANKL exerts protective effects on
podocytes, and is able to reduce proteinuria. Following proteinuria
reduction, the expression levels of RANKL decreased. These results
suggested that the FAK/RANKL/MAPK and FAK/RANKL/NF-κB signaling
pathways may be present in kidney tissues, and prednisone may
reduce proteinuria by inhibiting the FAK/RANKL/MAPK and/or the
FAK/RANKL/NF-κB signaling pathway.
In the serum of adriamycin-induced nephrotic rats,
OPG and RANKL expression was unchanged in the model group, as
compared with the normal group. The expression levels of OPG
decreased significantly and those of RANKL increased significantly
following treatment with prednisone. OPG acts as an osteoblast
decoy receptor, whereas RANKL is the primary factor inducing
osteoclast (OC) differentiation (8). OPG specifically competes with RANKL,
and binds to RANK (the activation receptor of NF-κB) to suppress OC
activity (8). Bucay et al
(23) and Hofbauer et al
(24) reported that OPG, RANK and
RANKL were associated with osteoporosis and the occurrence of
vascular calcification. The present study demonstrated that ther
expression levels of OPG were markedly reduced and those of RANKL
were markedly increased in the prednisone group. These results
suggested that prednisone may activate the OPG/RANK/RANKL signaling
pathway in murine serum, thereby inducing abnormal bone metabolism,
and the observed upregulation of RANKL expression may be due to the
secretion of bone tissue in the blood.
In conclusion, the results showed that prednisone is
able to reduce proteinuria by inhibiting the FAK/RANKL/MAPK and
FAK/RANKL/NF-κB signaling pathways in kidney tissue samples, and
RANKL has a role in transduction. In our future studies of the
RANKL signaling pathway, we aim to observe the pathology of the
kidney and proteinuria in the rats following knocking out the RANKL
gene. These studies may result in a new direction for the treatment
of kidney diseases.
Acknowledgments
The present study was supported by a grant from the
Natural Science Foundation of the Fujian province in China (grant
no. 2012J01378).
References
|
1
|
Ma H, Togawa A, Soda K, Zhang J, Lee S, Ma
M, Yu Z, Ardito T, Czyzyk J, Diggs L, et al: Inhibition of podocyte
FAK protects against proteinuria and foot process effacement. J Am
Soc Nephrol. 21:1145–1156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanasaki K, Kanda Y, Palmsten K, Tanjore
H, Lee SB, Lebleu VS, Gattone VH Jr and Kalluri R: Integrin
beta1-mediated matrix assembly and signaling are critical for the
normal development and function of the kidney glomerulus. Dev Biol.
313:584–593. 2008. View Article : Google Scholar
|
|
3
|
Koshikawa M, Mukoyama M, Mori K, Suganami
T, Sawai K, Yoshioka T, Nagae T, Yokoi H, Kawachi H, Shimizu F, et
al: Role of p38 mitogen-activated protein kinase activation in
podocyte injury and proteinuria in experimental nephrotic syndrome.
J Am Soc Nephrol. 16:2690–2701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shuyu L and Zhigang W: The progress on
focal adhesion kinase (FAK) and its single pathways. Biotechnol
Inf. 12:6–10. 2009.In Chinese.
|
|
5
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy - from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rogers A and Eastell R: Circulating
osteoprotegerin and receptor activator for nuclear factor kappaB
ligand: Clinical utility in metabolic bone disease assessment. J
Clin Endocrinol Metab. 90:6323–6331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu S, Shi W, Xiao H, Liang X, Deng C, Ye
Z, Mei P, Wang S, Liu X, Shan Z, et al: Receptor activator of
NF-kappaB and podocytes: Towards a function of a novel
receptor-ligand pair in the survival response of podocyte injury.
PLoS One. 7:e413312012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papachroni KK, Karatzas DN, Papavassiliou
KA, Basdra EK and Papavassiliou AG: Mechanotransduction in
osteoblast regulation and bone disease. Trends Mol Med. 15:208–216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang DW, Huang XZ, Wu JH, Fan YP and Shi
H: Effects of intercellular adhesion molecule-1 on renal damage in
spontaneously hypertensive rats. Ren Fail. 34:915–920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun W, He Y, Yu J, Lin Y, Wang Y, Gao X,
Wang Y, Zhao Z and Liu X: Effect of yiqiyangyin recipe on
heparanase and nephrin in rats with adriamycin-induced nephropathy.
J Tradit Chin Med. 33:334–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Yoshida Y, Nameta M, Xu B,
Taguchi I, Ikeda T, Ceccarelli DF, Song HK, Poy F, Schaller MD and
Eck MJ: Crystal structure of the FERM domain of focal adhesion
kinase. J Biol Chem. 281:252–259. 2006. View Article : Google Scholar
|
|
12
|
Fujinaka H, Magdeldin S, Tsukaguchi H,
Harita Y, et al: Glomerular proteins related to slit diaphragm and
matrix adhesion in the foot processes are highly tyrosine phosphory
lated in the normal rat kidney. Nephrol Dial Transplant.
25:1785–1795. 2010. View Article : Google Scholar
|
|
13
|
Yang L, Liang M, Zhou Q, Xie D, Lou A,
Zhang X and Hou F: Advanced oxidation protein products decrease
expression of nephrin and podocin in podocytes via ROS-dependent
activation of p38 MAPK. Sci China Life Sci. 53:68–77. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Aouni C, Herbach N, Blattner SM, Henger
A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar
S, et al: Podocyte-specific deletion of integrin-linked kinase
results in severe glomerular basement membrane alterations and
progressive glomerulosclerosis. J Am Soc Nephrol. 17:1334–1344.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boyce BF, Yao Z and Xing L: Functions of
nuclear factor kappaB in bone. Ann N Y Acad Sci. 1192:367–375.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romas E, Sims NA, Hards DK, Lindsay M,
Quinn JW, Ryan PF, Dunstan CR, Martin TJ and Gillespie MT:
Osteoprotegerin reduces osteoclast numbers and prevents bone
erosion in collagen-induced arthritis. The American Journal of
Pathology. 161:1419–1427. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fata JE, Kong YY, Li J, Sasaki T,
Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey
DL, et al: The osteoclast differentiation factor
osteoprotegerin-ligand is essential for mammary gland development.
Cell. 103:41–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gonzalez-Suarez E, Jacob AP, Jones J,
Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D and
Dougall WC: RANK ligand mediates progestin-induced mammary
epithelial proliferation and carcinogenesis. Nature. 468:103–107.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beleut M, Rajaram RD, Caikovski M, Ayyanan
A, Germano D, Choi Y, Schneider P and Brisken C: Two distinct
mechanisms underlie progesterone-induced proliferation in the
mammary gland. Proc Natl Acad Sci USA. 107:2989–2994. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kartsogiannis V, Zhou H, Horwood NJ,
Thomas RJ, Hards DK, Quinn JM, Niforas P, Ng KW, Martin TJ and
Gillespie MT: Localization of RANKL (receptor activator of NF kappa
B ligand) mRNA and protein in skeletal and extraskeletal tissues.
Bone. 25:525–534. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rauner M, Sipos W, Thiele S and
Pietschmann P: Advances in osteoimmunology: Pathophysiologic
concepts and treatment opportunities. Int Arch Immunol.
160:114–125. 2013. View Article : Google Scholar
|
|
22
|
Reichardt H, Tuckermann J, Göttlicher M,
Vujic M, Weih F, Angel P, Herrlich P and Schütz G: Repression of
inflammatory responses in the absence of DNA binding by the
glucocorticoid receptor. EMBO J. 20:7168–7173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bucay N, Sarosi I, Dunstan CR, Morony S,
Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, et al:
Osteoprotegerin-deficient mice develop early onset osteoporosis and
arterial calcification. Genes Dev. 12:1260–1268. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hofbauer LC, Brueck CC, Shanahan CM,
Schoppet M and Dobnig H: Vascular calcification and osteoporosis -
from clinical observation towards molecular understanding.
Osteoporos Int. 18:251–259. 2007. View Article : Google Scholar
|