Introduction
Pancreatic cancer is the fourth leading cause of
cancer-associated mortality, and is amongst the most
life-threatening types of human cancer. Its 5-year-survival rate is
<5% due to its aggressive growth, metastasis and resistance to
the majority of chemotherapies (1). Systemic chemotherapy remains reliant
on a few drugs and has not significantly increased overall patient
survival rates (2). Therefore,
more effective therapeutic approaches are necessary for improving
the outcome of pancreatic cancer treatment.
The signal transducer and activator of transcription
3 (STAT3) transcription factor is constitutively activated in
several human cancer cells, and aberrant STAT3 signaling is
implicated as an important process in malignant progression
(3). When STAT3 is activated upon
phosphorylation of the tyrosine 705 residue, monomeric STAT3 forms
dimers through their SH2 domains and translocates from the
cytoplasm into the nucleus. Once in the nucleus, STAT3
transcriptionally regulates the expression of its target genes,
including cyclin D1 and survivin (4). The activation of the STAT3 pathway
can stimulate cell proliferation, promote angiogenesis, mediate
immune evasion and confer increased resistance to apoptosis in
cancer cells (5,6). STAT3 is found to be constitutively
activated in pancreatic ductal adenocarcinoma (PDAC) through the
phosphorylation of Tyr705, which has been reported in human tumor
specimens and in various PDAC cell lines. Aberrant activation of
STAT3 in the pancreas accelerates pancreatic intraepithelial
neoplasia progression and PDAC development (7), and activated STAT3 signaling prevents
cell apoptosis and confers resistance to chemotherapeutic agents in
pancreatic carcinogenesis (8).
Furthermore, STAT3 knockdown reduces pancreatic cancer cell
invasiveness and expression of matrix metalloproteinase-7 in nude
mice (9). Inhibition of STAT3
activity by sorafenib also enhances TNF-related apoptosis-inducing
ligand-mediated apoptosis in human pancreatic cancer cells
(10). Thus, STAT3 signaling has
been considered as an attractive chemotherapeutic target for the
treatment of pancreatic cancer.
Cryptotanshinone (CPT) is one of the major
tanshinones isolated from Salvia miltiorrhiza Bunge, a
well-known Chinese herb, and is widely used in traditional and
modern medicine (11). Extensive
investigations have indicated that CPT may inhibit the
proliferation of cancer cells in vitro and reduce the growth
and metastases of tumors in vivo (11–13).
CPT inhibits the function of STAT3 though inhibiting dimerization
in DU145 prostate cancer cells (14). In addition, CPT significantly
decreases the activity of the STAT3 signaling pathway in human
glioma and chronic myeloid leukemia cells, indicating that CPT is a
potent STAT3 inhibitor (15,16).
However, the effect and mechanism of CPT on human pancreatic cancer
remains to be elucidated. In the present study, the effects of CPT
on the proliferation and apoptosis of BXPC-3 human pancreatic
cancer cells were evaluated, and the underlying molecular mechanism
was investigated. Elucidating of the effects of CPT on the
apoptosis of BXPC-3 pancreatic cancer cells and on the STAT3
pathway aimed to provide evidence for CPT as a potential
therapeutic agent for pancreatic cancer.
Materials and methods
Cell culture
The human BxPC-3 pancreatic cancer cell line was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were grown in RPMI-1640
containing 10% fetal bovine serum, 100 µg/ml streptomycin
and 100 U/ml of penicillin at 37°C in a humidified atmosphere of 5%
CO2.
Drugs and reagents
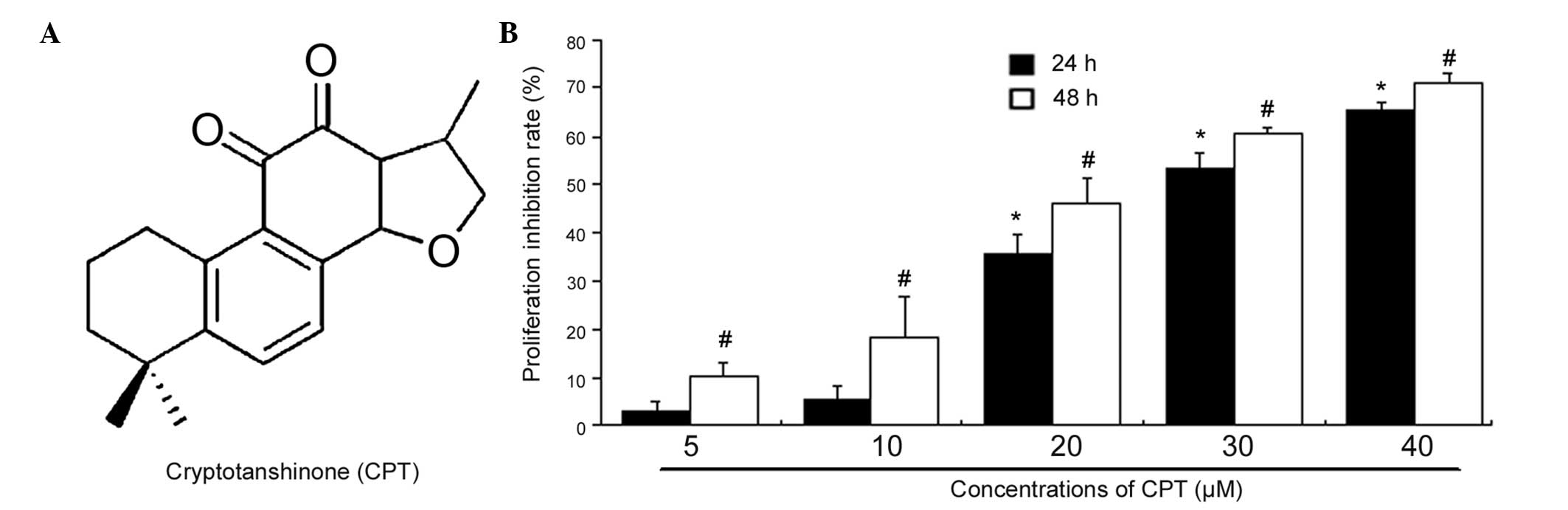
CPT (Fig. 1A) was
purchased from the National Institutes for Food and Drug Control
(Beijing, China), and was diluted using DMSO in a stock
concentration of 20 mM at −20°C. The methyl thiazolyl tetrazolium
(MTT) and DMSO were obtained from Sigma-Aldrich (St. Louis, MO,
USA). Rabbit anti-human cleaved caspase-3 (Asp175/5A1E) monoclonal
antibody (cat. no. 9664), rabbit anti-human cleaved caspase-9
(Asp330) polyclonal antibody (cat. no. 9501), rabbit-anti human
cleaved poly ADP ribose polymerase (PARP; Asp214/D64E10) monoclonal
antibody (cat. no. 5625), rabbit anti-human phosphorylated
(p-)STAT3 (Tyr705) polyclonal antibody (cat. no. 9131), rabbit
anti-human p-Akt (Ser473) polyclonal antibody (cat. no. 9271),
rabbit anti-human p-p44/42 extracellular signal-regulated kinase
(ERK)1/2; Thr202/Tyr204) polyclonal antibody (cat. no. 9101),
rabbit anti-human p-mammalian target of rapamycin (mTOR; Ser2448)
polyclonal antibody (cat. no. 2971) and rabbit anti-human β-actin
polyclonal antibody (cat. no. 4967) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Rabbit anti-human
survivin (FL-142) polyclonal antibody (cat. no. sc-10811) and
rabbit anti-human Bcl-2 polyclonal antibody (cat. no. sc-783) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Mouse anti-human c-Myc monoclonal antibody (cat. no. 551101) and
mouse anti-human Cyclin D1 monoclonal antibody (cat. no. 556470)
were purchased from BD Biosciences, (Franklin Lakes, NJ, USA).
Rabbit anti-human phospho-Jak2 (Y1007/Y1008) monoclonal antibody
(cat. no. ab32101) was purchased from Abcam (Cambridge, MA, USA).
Secondary antibodies of goat anti-mouse IgG-horesradish peroxidase
(HRP; cat. no. sc-2005) and goat anti-Rabbit IgG-HRP (cat. no.
sc-2004) were obtained from Santa Cruz Biotechnology, Inc.). The
cell cycle detection kit (cat. no. 558662) and fluorescein
isothiocyanate-conjugated Annexin V Apoptosis Detection kit (cat.
no. 556547) were purchased from BD Biosciences.
MTT assay
Exponentially growing BxPC-3 cells were seeded into
96-well plates, at a density of 1×104 cells per well,
for viability measurements, and were incubated for 12 h at 37°C. To
examine the proliferation inhibitory effect, different
concentrations of CPT (0, 5, 10, 20, 30 and 40 µM) were
added to the wells, with a total volume of 200 µl in RPMI
1640, and incubated for 24 or 48 h at 37°C. Cells treated with
medium served as a control. Finally, 20 µl MTT was added
into each well and the absorbance at 570 nm was measured using a
microplate spectrophotometer (SpectraMax; Molecular Devices,
Sunnyvale, CA, USA). The viabilities of the cells in each well were
indicated by the measured values. Treatment effects were determined
as the percentage of viable cells, compared with the untreated
control cells. Three independent sets of experiments were
performed.
Apoptosis assay
To determine effects of CPT on apoptosis,
1×106 BxPC-3 cells were plated in 6-well plates with
medium for 12 h. The cells were then treated for a further 24 h
with 0, 10, 20, 30 or 40 µM CPT. Subsequently, the floating
and adherent cells were collected together for analysis. The cells
were washed twice with cold phosphate-buffered saline (PBS), and
resuspended in Annexin-Binding buffer (1X; BD Biosciences) at a
concentration of 1×106 cells per ml. Subsequently, 100
µl of the cell solution was stained with 5 µl Annexin
V and 5 µl propidium iodide (PI) at room temperature for 20
min in the dark. The data were collected and analyzed using a
FACScalibur flow cytometer with CellQuest Software (Version 5.1.1;
BD Biosciences). Three independent sets of experiments were
performed.
DAPI staining assay
The BxPC-3 cells were seeded within chamber dishes
and treated with or without 30 µM CPT for 24 h at 37°C.
Following treatment, the cells were washed with twice ice-cold PBS
and then fixed with ethanol for 30 min at −20°C. The fixed cells
were then washed with PBS and stained with 10 µg/ml DAPI
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) for 15 min at
room temperature. Following washing three times with PBS, the cells
were observed under a fluorescence microscope (IX2-ILL 100; Olympus
Corporation, Tokyo, Japan).
Cell cycle analysis
The BxPC-3 cells were seeded into 6-well plates and
were treated with series of concentrations of CPT (0, 10, 20, 30
and 40 µM) for 24 h at 37°C. The cells were harvested and
fixed in 70% ethanol and stored at 4°C overnight. Subsequently, the
cell pellets were suspended in 0.5 ml of PI solution containing 5
µg/ml RNase (Sigma-Aldrich) and 20 µg/ml PI.
Following incubation in the dark for 30 min at 37°C, the
percentages of cells within each stage of the cell cycle (G0-G1, S
or G2-M) were determined using flow cytometry (FACSCalibur, BD
Biosciences). Cells treated with vehicle alone (100% ethanol) were
used as a control. At least 10,000 events were counted for each
sample.
Western blot analysis
Total proteins were extracted using
Radioimmunoprecipitation Assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China), according to the manufacturer's
instructions. The protein contents were determined using a Bradford
assay kit (Nanjing KeyGen Biotech Co., Ltd.). Subsequently, ~24
µg total protein was resolved on 10% SDS-polyacrylamide gels
on a Minigel apparatus (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), and were then transferred onto a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA) by semidry transfer
cell. The membranes were washed three times and blocked with
Tris-buffered saline with Tween 20 (0.5% Tween) containing 5%
nonfat milk for 60 min, followed by incubation of the membrane with
the appropriate primary antibodies (1:1,000) at 4°C overnight, the
membranes were washed in TBST and were subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies (1:2,000)
for 2 h at room temperature, immunoreactive bands were developed
using enhanced chemiluminescence (WBKLS0100; EMD Millipore).
β-actin was stained as a loading control. The density of the blot
was further analyzed by densitometry using a Gel-Pro Analyzer
(Universal Hood II; Bio-Rad Laboratories, Inc.). All western blot
experiments were performed at least three times. The blots depicted
in the figures are a single blot, representative of three
independent experiments, with density data collected from the
average values of the three.
Statistical analysis
All assays in the present study were repeated
independently three times. For analysis of experimental data,
comparison of categorical data was performed using Student's
t-test. Statistical analysis was performed using SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
CPT has an antiproliferative effect on
BXPC-3 cells
The effect of CPT on the proliferation of the BxPC-3
human pancreatic adenocarcinoma cell line was determined using an
MTT assay. As shown in Fig. 1B,
CPT inhibited the proliferation of BxPC-3 cells in a dose- and
time-dependent manner. Following culture with 20 or 30 µM
CPT for 24 h, the percentages of cell viability, relative to the
control were 74 and 46%, respectively. Following CPT treatment for
24 and 48 h, the half maximal inhibitory concentration values for
the BxPC-3 cells were 29.4 and 22.8 µM, respectively. These
results indicated that CPT may function as a tumor suppressor in
human pancreatic cancer.
CPT induces apoptosis and cell cycle
arrest in BxPC-3 cells
To determine whether the CPT-mediated growth
inhibition in the BxPC-3 is associated with apoptosis, the
percentage of apoptotic cells were determined using flow cytometric
analysis with combined Annexin-V/PI staining following treatment
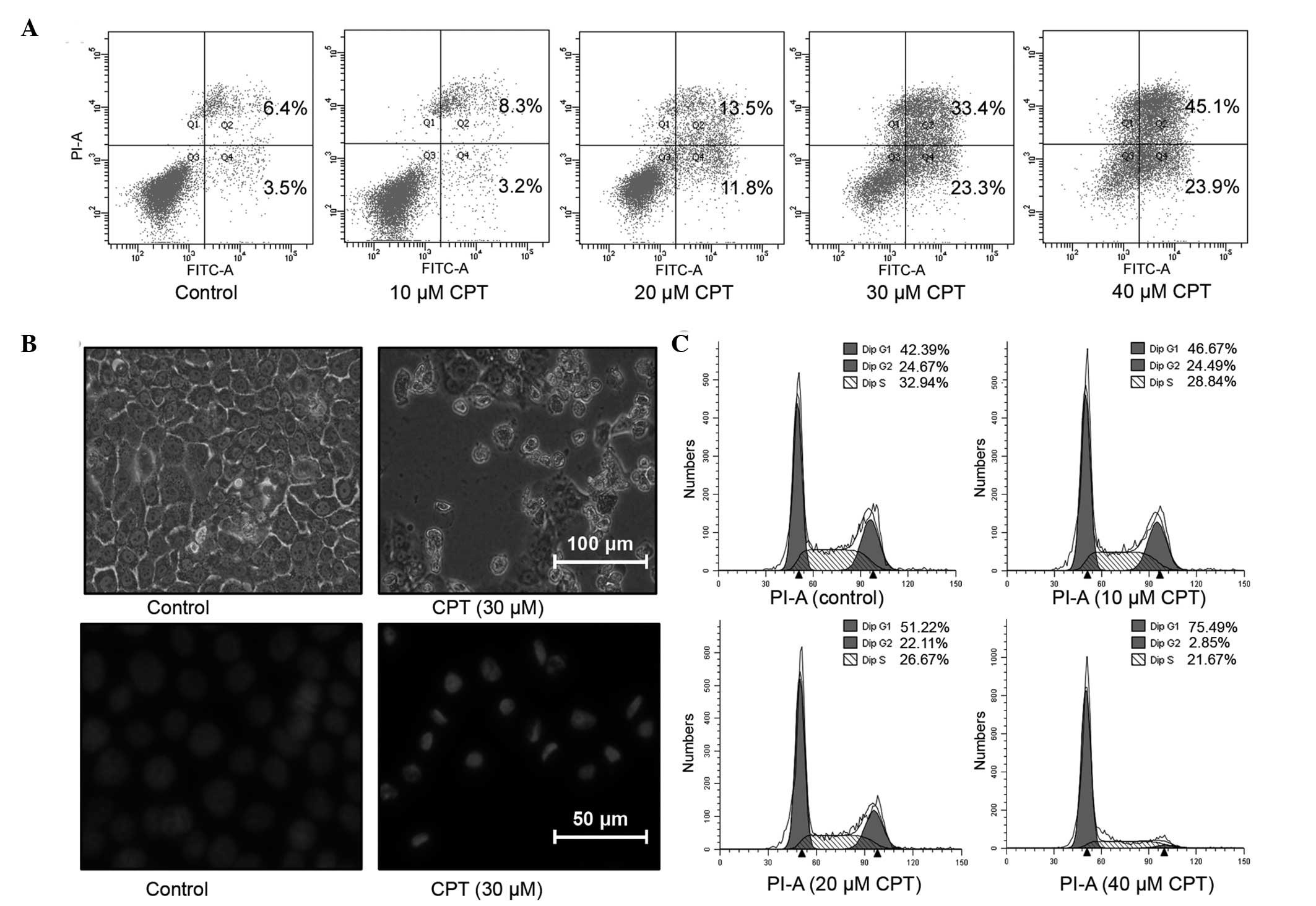
with CPT for 24 h. Compared with the control group, the apoptotic
cell fractions were significantly increased following treatment
with CPT. In the untreated control group, the percentage of
apoptotic cell fractions was only 9.9%. The addition of 20 and 30
µM CPT to the BxPC-3 cells resulted in apoptotic cell
fractions of 25.3 and 56.7%, which were 2.56- and 5.73-fold higher
than those of the control group, respectively (Fig. 2A). The observations performed under
a light microscopic also suggested the proliferation inhibiting and
apoptosis inducting effects of CPT (Fig. 2B). To further confirm the induction
of apoptosis by CPT, the cells were stained with DAPI and observed
under fluorescence microscopy. As shown in Fig. 2B, a normal homogeneous distribution
of chromatin was observed in the untreated control cells, whereas
the cells treated with CPT exhibited morphological features of
apoptotic cells, including chromatin condensation and
marginalization or DNA fragmentation.
To determine the effect of CPT on the cell cycle,
the BxPC-3 cells were treated with increasing concentrations of CPT
for 24 h, stained with PI and assayed using flow cytometry
(Fig. 2C). The results indicated
that CPT treatment arrested the BxPC-3 cells in the G1-G0 phase of
the cell cycle, in a dose-dependent manner. As shown in Fig. 2C, the BxPC-3 cells without CPT
exposure presented with 42.39% of the population in the G1-G0
phase. However, the G1-G0 phase fraction increased to 51.22 and
75.49%, following treatment with 30 and 40 µM CPT,
respectively. This increase in the G1-G0 cell population was
accompanied by a concomitant decrease in the number of cells in the
S-phase and G2-M phase of the cell cycle (Fig. 2C). These results demonstrated that
the inhibition of cell proliferation in BxPC-3 cells by CPT was
associated with the induction of apoptosis and arrest of the cell
cycle in BxPC-3 cells.
CPT regulates the expression levels of
apoptosis- and cell cycle-associated proteins in BxPC-3 cells
To further elucidate the molecular mechanisms
underlying the effects of CPT in human pancreatic cancer, the
preset study investigated the expression levels of protein markers,
which are associated with apoptosis and the cell cycle, following
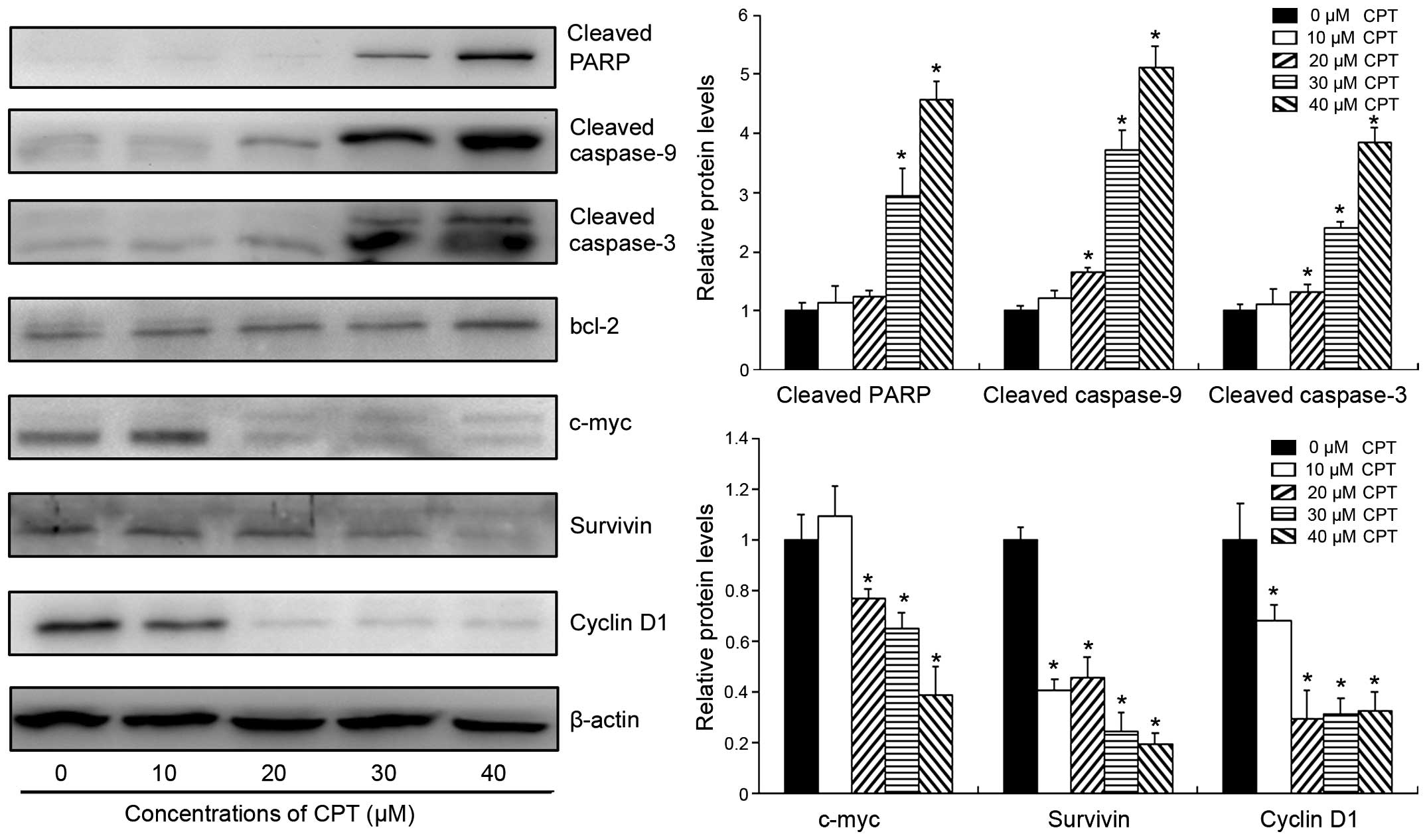
CPT treatment. As shown in Fig. 3,
CPT treatment markedly increased the activities of pro-apoptotic
proteins in BxPC-3 cells, including PARP, caspase-3 and caspase-9.
For example, following treatment with 30 µM CPT for 24 h,
the cleavage of PARP and caspase-3 were 3.6 and 2.9-folds higher,
respectively, compared with the levels in the control group
(Fig. 3). Although CPT did not
inhibit the protein expression of anti-apoptotic bcl-2, it
significantly decreased the expression levels of c-myc and
survivin, which are two important regulators for cell growth and
apoptosis in cancer cells (17,18).
Following treatment with 30 µM CPT for 24 h, the expression
levels of c-myc and survivin reduced by 35 and 76%, respectively.
In addition, the expression of cyclin D1 was also significantly
downregulated by CPT in a dose-dependent manner, suggesting that
CPT arrested cells at the G1-G0 phase by inhibiting the expression
of cyclin D1 in human pancreatic cancer. These results further
confirmed the apoptosis induction and cell cycle arrest induced by
CPT in the human pancreatic cancer.
CPT inhibits the STAT3 signaling pathway
in BxPC-3 cells
As the pivotal role of STAT3 signaling pathway is in
tumor growth and apoptosis, the present study examined whether CPT
had an effect on the constitutively active STAT3 in BxPC-3 cells.
As shown in Fig. 4A, CPT inhibited
the phosphorylation of STAT3 at Tyr705 in a dose-dependent manner,
without marked effects on the total protein level of STAT3.
Following exposure to 20 µM CPT for 24 h, the expression of
p-STAT3 was almost undetectable in the BxPC-3 cells. To clarify the
mechanism underlying the inhibition of STAT3 phosphorylation by
CPT, the activities of STAT3 upstream regulators were also
determined following CPT exposure (Fig. 4A). The results of the western blot
analysis indicated that CPT decreased the phosphorylation of the
JAK2, Akt, mTOR and ERK proteins only when the concentrations of
CPT increased to 20 µM. The phosphorylation of STAT3 was
inhibited ~70% by 10 µM CPT, whereas the phosphorylation
levels of the other upstream proteins were not altered or were
increased only marginally by CPT at the same concentration.
Furthermore, the inhibition of the upstream kinases appeared later
than the inhibition of the STAT3 phosphorylation, according to the
time course analysis (Fig. 4B). As
shown in Fig. 4B, the suppression
of STAT3 phosphorylation occurred with 30 min in the BxPC-3 cells.
However, CPT had no effect on the activities of JAK2 or mTOR
following 6 h treatment with 30 µM CPT, and the
phosphorylation level of Akt increased only marginally. In
addition, the expression of p-ERK was decreased only when the
treatment duration was increased to 2 h. These results indicated
that the inhibition of the STAT3 signaling pathway by CPT occurred
through a mechanism independent of the above-mentioned upstream
regulators, suggesting the possibility that CPT directly inhibited
the phosphorylation of STAT3 in the BxPC-3 cells. The suppression
of other signaling pathways following 24 h treatment appeared to be
involved the secondary effects of CPT in human pancreatic
cancer.
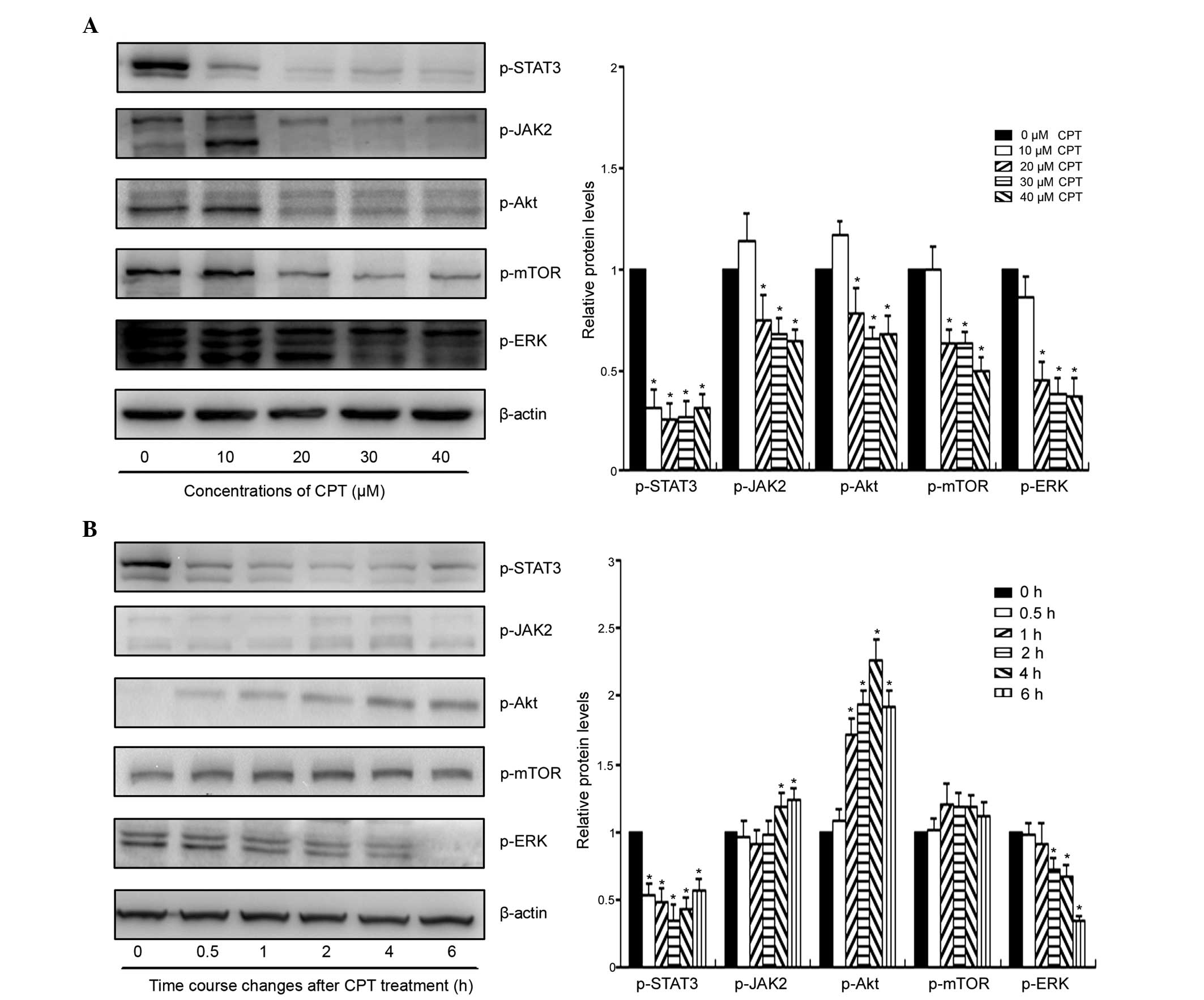 | Figure 4Effect of CPT on the activities of
STAT3 and other survival-signaling pathways in the BxPC-3 cells.
(A) Cells were treated with the indicated concentrations of CPT for
24 h, following which the cells were subjected to western blot
analysis to determine the expression levels of p-STAT3, p-JAK2,
p-Akt, p-mTOR and p-ERK. (B) Time course analysis of the survival
pathways following CPT treatment in the BxPC-3 cells. Cells were
treated with 30 µM CPT for the indicate durations, following
which the cells were collected and subjected to western blot
analysis. *P<0.05, vs. control group (0 h).
p-phosphorylated; STAT3, signal transducer and activator of
transcription 3; JAK2, Janus kinase 2; mTOR, mammalian target of
rapamycin; ERK, extracellular signal-regulated kinase. |
Discussion
In the present study, it was demonstrated that CPT
inhibited the viability of BxPC-3 human pancreatic cancer cells by
arresting cell cycle at the G1-G0 phase and inducing cell
apoptosis. The anticancer effect of CPT is associated with
inhibition of the activity of the STAT3 signaling pathway. The
results of the present study demonstrated that CPT may be used a
potential drug for adjuvant therapy or a complementary alternative
medicine for the management of pancreatic cancer.
Despite significant advances in modern medicine
during the last two decades, pancreatic cancer remains associated
with a high rate of mortality (1).
Cytotoxic chemotherapy based on the pyrimidine analogue,
gemcitabine, remains the standard approach in the adjuvant and
palliative setting, however, the resulting responses are minimal
(19). Resistance to cytotoxic
chemotherapy has remained a major factor contributing to the poor
outcomes observed in patients with pancreatic cancer (20). The activation of STAT3 regulates
oncogenic signaling in several types of tumor and leads to
increased cell survival, proliferation and tumor growth (21). Activated STAT3 has been considered
as a potential biomarker of chemotherapeutic resistance, and
inhibition of the STAT3 pathway as a promising treatment strategy
for pancreatic tumors (8). In the
present study, the effect of CPT, a potent STAT3 pathway inhibitor,
on BxPC-3 human pancreatic cells were investigated. The results
revealed that CPT inhibited proliferation through the induction of
apoptosis and cell cycle arrest in the BxPC-3 cells (Fig. 2). Furthermore, CPT decreased the
activity of the STAT3 pathway in a dose- and time-dependent manner.
Previous studies have indicated that CPT affects the STAT3
signaling pathway either directly or by alterations of certain
upstream regulators (14,22). The results of the present study
demonstrated that CPT significantly inhibited the activities of
JAK2, Akt, mTOR and ERK, when the duration of CPT exposure
increased to 24 h (Fig. 3).
However, CPT only affected the expression of p-STAT3 within 30 min,
and no significant effects on other proteins were observed,
suggesting that CPT regulated the STAT3 signaling pathway directly
in the BxPC-3 cells.
Phytochemicals from dietary plants and other plant
sources, including herbs are becoming increasingly important
sources of anticancer drugs or compounds for cancer chemopreventive
or adjuvant chemotherapy (23).
The use of traditional Chinese herbs has exhibit beneficial effects
in overcoming apoptosis resistance in pancreatic cancer (24). CPT, a biologically active compound
from the root of Salvia miltiorrhiza, has exhibited
significant anticancer effects in several tumor cells. The results
from a study by Chen et al suggested that CPT is a potential
drug for the treatment and prevention of human lung cancer
(25). The results of our previous
study indicated that CPT remained potent in tumor cells with an MDR
phenotype, suggesting its potential application in cancer
exhibiting a high level of chemotherapeutic resistance (12). In the present study, CPT
significantly decreased the viability of the BxPC-3 pancreatic
cancer cells via the induction of apoptosis and cell cycle arrest,
suggesting the possibility of CPT being developed as an agent for
pancreatic cancer therapy. Previous studies have indicated that
compounds from herbal medicines may augment the sensitivity of
pancreatic cancer cells to gemcitabine, and the combination therapy
with natural products and cytotoxic agents has achieved promising
results. Von Hoff et al demonstrated that the combination of
nab-paclitaxel and gemcitabine significantly improve overall
survival rates and response rates in patients with metastatic
pancreatic adenocarcinoma (26).
Enhanced antitumor efficacy has also been observed following
treatment with emodin and gemcitabine in human pancreatic cancer
cells (27,28). Of note, CPT can also act
synergistically with cytotoxic agents in other cancer cells
(16). Thus, a combination
therapeutic approach using CPT and gemcitabine in pancreatic cancer
is promising and requires further investigations in the future.
In conclusion, the results of the present study
provide the first evidence, to the best of our knowledge, that CPT
inhibited the proliferation of BxPC-3 cells by inducing apoptosis
and cell cycle arrest. The antitumor effect was found to be
associated with inhibition of the STAT3 pathway. Taking into
account the wide acceptance and low toxicity of plant extracts, the
results of the present study suggest the possibility of further
developing CPT as an alternative treatment option, or using it as
adjuvant chemotherapeutic agent in the treatment of pancreatic
cancer.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant. nos. 81202820 and 81303269),
the Zhejiang Provincial Natural Science Foundation of China (grant.
no. Q12H28005) and the Zhejiang Provincial Public Technology
Applied Research Project (grant. no. 2014C33211).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang BH, Cao WM, Yu J and Wang XL:
Gemcitabine-based concurrent chemoradiotherapy versus chemotherapy
alone in patients with locally advanced pancreatic cancer. Asian
Pac J Cancer Prev. 13:2129–2132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matthaios D, Zarogoulidis P, Balgouranidou
I, Chatzaki E and Kakolyris S: Molecular pathogenesis of pancreatic
cancer and clinical perspectives. Oncology. 81:259–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sehara Y, Sawicka K, Hwang JY,
Latuszek-Barrantes A, Etgen AM and Zukin RS: Survivin is a
transcriptional target of STAT3 critical to estradiol
neuroprotection in global ischemia. J Neurosci. 33:12364–12374.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takezawa K, Okamoto I, Nishio K, Jänne PA
and Nakagawa K: Role of ERK-BIM and STAT3-survivin signaling
pathways in ALK inhibitor-induced apoptosis in EML4-ALK-positive
lung cancer. Clin Cancer Res. 17:2140–2148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lesina M, Kurkowski MU, Ludes K, Rose-John
S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S,
et al: Stat3/Socs3 activation by IL-6 transsignaling promotes
progression of pancreatic intraepithelial neoplasia and development
of pancreatic cancer. Cancer Cell. 19:456–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagaraj NS, Washington MK and Merchant NB:
Combined blockade of Src kinase and epidermal growth factor
receptor with gemcitabine overcomes STAT3-mediated resistance of
inhibition of pancreatic tumor growth. Clin Cancer Res. 17:483–493.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HD, Huang C, Huang KJ, Wu WD, Jiang T,
Cao J, Feng ZZ and Qiu ZJ: STAT3 knockdown reduces pancreatic
cancer cell invasiveness and matrix metalloproteinase-7 expression
in nude mice. PLoS One. 6:e259412011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang S and Sinicrope FA: Sorafenib
inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in
human pancreatic cancer cells. Mol Cancer Ther. 9:742–750. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park IJ, Kim MJ, Park OJ, Choe W, Kang I,
Kim SS and Ha J: Cryptotanshinone induces ER stress-mediated
apoptosis in HepG2 and MCF7 cells. Apoptosis. 17:248–257. 2012.
View Article : Google Scholar
|
|
12
|
Ge Y, Cheng R, Zhou Y, Shen J, Peng L, Xu
X, Dai Q, Liu P, Wang H, Ma X, et al: Cryptotanshinone induces cell
cycle arrest and apoptosis of multidrug resistant human chronic
myeloid leukemia cells by inhibiting the activity of eukaryotic
initiation factor 4E. Mol Cell Biochem. 368:17–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen W, Luo Y, Liu L, Zhou H, Xu B, Han X,
Shen T, Liu Z, Lu Y and Huang S: Cryptotanshinone inhibits cancer
cell proliferation by suppressing Mammalian target of
rapamycin-mediated cyclin D1 expression and Rb phosphorylation.
Cancer Prev Res (Phila). 3:1015–1025. 2010. View Article : Google Scholar
|
|
14
|
Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ,
Han DC and Kwon BM: Cryptotanshinone inhibits constitutive signal
transducer and activator of transcription 3 function through
blocking the dimerization in DU145 prostate cancer cells. Cancer
Res. 69:193–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu L, Li C, Li D, Wang Y, Zhou C, Shao W,
Peng J, You Y, Zhang X and Shen X: Cryptotanshinone inhibits human
glioma cell proliferation by suppressing STAT3 signaling. Mol Cell
Biochem. 381:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ge Y, Yang B, Xu X, Dai Q, Chen Z and
Cheng R: Cryptotanshinone acts synergistically with imatinib to
induce apoptosis of human chronic myeloid leukemia cells. Leuk
Lymphoma. 56:730–738. 2015. View Article : Google Scholar
|
|
17
|
Papanikolaou V, Iliopoulos D, Dimou I,
Dubos S, Kappas C, Kitsiou-Tzeli S and Tsezou A: Survivin
regulation by HER2 through NF-κB and c-myc in irradiated breast
cancer cells. J Cell Mol Med. 15:1542–1550. 2011. View Article : Google Scholar
|
|
18
|
Sun M, Liu C, Nadiminty N, Lou W, Zhu Y,
Yang J, Evans CP, Zhou Q and Gao AC: Inhibition of Stat3 activation
by sanguinarine suppresses prostate cancer cell growth and
invasion. Prostate. 72:82–89. 2012. View Article : Google Scholar
|
|
19
|
Cao H, LE D and Yang LX: Current status in
chemotherapy for advanced pancreatic adenocarcinoma. Anticancer
Res. 33:1785–1791. 2013.PubMed/NCBI
|
|
20
|
Schüler S, Diersch S, Hamacher R, Schmid
RM, Saur D and Schneider G: SKP2 confers resistance of pancreatic
cancer cells towards TRAIL-induced apoptosis. Int J Oncol.
38:219–225. 2011.
|
|
21
|
Pandurangan AK and Esa NM: Signal
transducer and activator of transcription 3-a promising target in
colitis-associated cancer. Asian Pac J Cancer Prev. 15:551–560.
2014. View Article : Google Scholar
|
|
22
|
Don MJ, Liao JF, Lin LY and Chiou WF:
Cryptotanshinone inhibits chemotactic migration in macrophages
through negative regulation of the PI3K signaling pathway. Br J
Pharmacol. 151:638–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ge YQ, Xu XF, Yang B, Chen Z and Cheng RB:
Saponins from Rubus parvifolius L. induce apoptosis in human
chronic myeloid leukemia cells through AMPK activation and STAT3
inhibition. Asian Pac J Cancer Prev. 15:5455–5461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Wang P, Ouyang H, Yin J, Liu A,
Ma C and Liu L: Targeting cancer-related inflammation: Chinese
herbal medicine inhibits epithelial -to- mesenchymal transition in
pancreatic cancer. PLoS One. 8:e703342013. View Article : Google Scholar
|
|
25
|
Chen L, Wang HJ, Xie W, Yao Y, Zhang YS
and Wang H: Cryptotanshinone inhibits lung tumorigenesis and
induces apoptosis in cancer cells in vitro and in vivo. Mol Med
Rep. 9:2447–2452. 2014.PubMed/NCBI
|
|
26
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang ZH, Chen H, Guo HC, Tong HF, Liu JX,
Wei WT, Tan W, Ni ZL, Liu HB and Lin SZ: Enhanced antitumor
efficacy by the combination of emodin and gemcitabine against human
pancreatic cancer cells via downregulation of the expression of
XIAP in vitro and in vivo. Int J Oncol. 39:1123–1131.
2011.PubMed/NCBI
|
|
28
|
Liu A, Chen H, Tong H, Ye S, Qiu M, Wang
Z, Tan W, Liu J and Lin S: Emodin potentiates the antitumor effects
of gemcitabine in pancreatic cancer cells via inhibition of nuclear
factor-κB. Mol Med Rep. 4:221–227. 2011.PubMed/NCBI
|