Introduction
Curcumin, a polyphenol isolated from the rhizomes of
the turmeric plant (Curcuma longa), is known to exhibit
potent anti-neoplastic activity against a variety of human cancer
types, including pancreatic, colon, ovarian and breast cancer, by
modulating multiple signaling pathways (1–3).
Curcumin has been reported to activate p53 signaling pathways,
which upregulates the transcription of downstream genes, including
B-cell lymphoma-2 (Bcl-2)-associated X protein (Bax) and Bcl-2
homologous antagonist killer (Bak), and downregulates pro-survival
genes, including Bcl-2 and cyclin D1, to induce apoptosis in a
variety of cancer cells (4,5).
However, the tumor suppressor gene p53 is mutated or deficient in a
large percentage of human malignancies, and whether or not curcumin
has anti-cancer effects on these p53 deficient/mutant tumors has
remained elusive.
Programmed cell death (PCD) has an important
biological function in development and homeostasis in mammals. A
common type of PCD is apoptosis, which may occur in multicellular
organisms in response to endogenous or exogenous cellular injuries
and molecular stimuli, including death ligands such as tumor
necrosis factor-alpha (TNF-α), Fas ligand/CD95/apolipoprotein 1 and
TNF-related apoptosis-inducing ligand, as well as DNA damaging
agents, including benzo[a]pyrene, etoposide and 5-fluorouracil
(6). In addition, induction of
apoptosis is a key mechanism of action of cytotoxic anti-cancer
drugs (7,8). Cell necrosis is an alternative form
of PCD, which may, in contrast to apoptosis, proceed without
activation of caspase family members. Programmed cell necrosis may
participate in vital processes when caspase members are inhibited
by pharmacological or genetic means, i.e. when interdigital webbing
is removed during embryogenesis in apoptotic protease activating
factor 1 (Apaf1)-deficient embryos (9,10).
Similar to apoptosis, programmed cell necrosis can be activated by
several key mediators, which have been identified through a
genome-wide RNA interference screening; these include
receptor-interacting protein kinase-1 (RIP1), RIP3, mitochondrial
phosphoglycerate mutase family member 5 and mixed lineage kinase
domain-like proteins (11). Cells
may undergo programmed necrosis as an alternative mode of cell
death when the caspase machinery fails or is inactivated (e.g.
after viral infection or in apoptosis-resistant tumor cells)
(12,13). Variable doses of stress may induce
certain molecular events which are involved in apoptosis as well as
necrosis signaling; therefore, cross-talk between the two cell
death mechanisms exists (14,15).
This suggests that necrosis is an intrinsic pathway, which may be
equally important to apoptosis; however, the underlying molecular
mechanisms of programmed necrosis have remained to be fully
elucidated.
The tumor suppressor p53 has crucial roles in
apoptosis, genomic stability and inhibition of angiogenesis
(16). Upon activation, p53 can
halt cell cycle progression by inhibiting G0/G1 phase transition,
allowing for DNA repair factors to fix the DNA damage. If the
extent of DNA damage proves to be irreparable, p53 initiates
apoptosis through transcriptional activation of a series of target
proteins, including MDM2 and the pro-apoptotic proteins Bax and Bak
(17); or through transcriptional
inhibition of pro-survival protein Bcl-xl and Bcl-2 (18). Previous studies have also
demonstrated important roles for p53 in curcumin-induced apoptosis.
For instance, curcumin can induce human HT-29 colon adenocarcinoma
cell apoptosis by activating p53 and regulating
apoptosis-associated protein expression (19). In another study, curcumin treatment
was shown to improve the general health of patients with colorectal
cancer via increasing p53 expression in tumor cells and to
consequently accelerate tumor cell apoptosis (4). Loss of p53 function and the resulting
evasion of apoptosis is the most common event during tumorigenesis
in diverse types of human cancer, and the efficacy of
apoptosis-inducing anti-cancer drugs is therefore decreased
(20). To date, little is known
regarding the efficacy of curcumin on p53-mutant/deficient cancer
cells as well as the underlying mechanisms.
The present study sought to explore the
p53-independent mechanisms of action of curcumin in lung cancer
cells. Using a previous study as a guideline (21), curcumin at the concentration of 10
µM was selected for treating p53-proficient A549 cells and
p53-deficient H1299 cells. The effects of curcumin on the
proliferation, cytosolic and mitochondrial expression as well as
homo-oligomerization of Bax and Bak, cytochrome c release
and cell death in A549 and H1299 cells was assessed using an MTT
assay, western blot analysis and flow cytometry. Furthermore, the
caspase-dependency of the curcumin-induced cytotoxicity was tested
using a fluorometric assay in the presence of caspase inhibitors.
In addition, the effects of small interfering (si)RNA-mediated Bax
and Bak knockdown as well as Bcl-2 overexpression on the necrotic
death of H1299 cells were assessed. The present study suggested
that curcumin induces necrosis in p53-deficient H1299 cells through
mitochondrial-associated molecular pathways.
Materials and methods
Drugs
Curcumin (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at 10 mM as a
stock solution. The dilutions of all of the reagents were freshly
prepared prior to each experiment.
Cell lines
The p53-proficient lung cancer cell line A549 and
the p53-deficient cell line H1299 were supplied by the Cell Bank of
Type Culture Collection of the Chinese Academy of Science
(Shanghai, China). A549 and H1299 cells were grown in Dulbecco's
modified Eagle's medium (DMEM; Gibco, Thermo Fisher Scientific,
Waltham, MA, USA) and in RPMI 1640 medium (Gibco) supplemented with
10% (v/v) fetal bovine serum (FBS; Gibco). The two cell lines were
incubated at 37°C in a humidified atmosphere containing 5%
CO2.
Reagents and antibodies
Rabbit polyclonal anti-p53 (cat. no. BS1278), rabbit
polyclonal anti-BAX (cat. no. BS1725), rabbit polyclonal anti-BAK
(cat. no. BS6477) and rabbit polyclonal MDM2 (cat. no. BS6818)
primary antibodies were purchased from Bioworld Technology (St.
Louis Park, MA, USA); primary antibody against Bcl-2 (cat. no.
sc-492) was obtained from Santa Cruz Biotechnology (Dallas, TX,
USA); rabbit polyclonal anti-cytochrome c (cat. no. BA0781)
was supplied by Boster Bio-Engineering (Wuhan, China); rabbit
monoclonal anti-cyclooxygenase (Cox)IV (cat. no. 18-7379) was
purchased from Molecular Probes (Thermo Fisher Scientific); and all
peroxidase-conjugated goat anti-rabbit secondary antibodies (cat.
no. 00008-2) were obtained from Bioworld Technology. Chemical
cross-linking agent bisma-leimidohexane (BMH), was supplied by
Pierce Biotechnology, Inc. (Rockford, IL,USA). The
carbobenzoxy-Val-Ala-Asp fluoromethyl ketone (Z-VAD-FMK),
Asp-Glu-Val-Asp (DEVD)-7-amino-4-trifluoromethyl coumarin (AFC)
were obtained from Selleck Chemicals (Houston, TX, USA); and
propidium iodide (PI) and Annexin V fluorescein isothio-cyanate
(FITC) double staining apoptosis detection kit was a product of
Biotech (Hangzhou, China). The mitochondria/cytosol protein
isolation kit was obtained from KeyGen (Nanjing, China) and
Lipofectamine 2000 transfection reagent was from Invitrogen (Thermo
Fisher Scientific).
Growth and cell proliferation
analysis
A549 and H1299 cells were seeded in 96-well
microplates and treated with 10 µM curcumin. The controls
were treated with an equal volume of the vehicle (DMSO; final
concentration, ≤0.1%). Following incubation, the supernatant in
each well was replaced with 90 µl FBS-free medium and 10
µl MTT solution (final concentration, 5 mg/ml). The plates
were then further incubated at 37°C for 4 h. Subsequently, the
supernatant in each well was carefully aspirated and 150 µl
DMSO was added to dissolve the MTT formazan. Following 15 min of
incubation, the absorbance of each well was read using a microplate
reader (model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
at 570 nm wavelength. Three independent experiments using six
parallel wells were performed.
Flow cytometric analysis
H1299 cells were seeded into six-well plates at
density of 5×105 cells/well. The cells were treated with
10 µM curcumin. After incubation for 0, 8, 16 and 24 h,
H1299 cells were collected and incubated with 5 µl Annexin
V-FITC and 10 µl PI per 100 µl binding buffer in the
dark for 20 min at room temperature. Finally, cell death mode
analysis was performed using a FACSort flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). For each group, 10,000
events were collected and analyzed using Cell Quest software.
Measurement of caspase activity
H1299 cells were plated in six-well plates at a
density of 8×105 cells/well and subse quently treated
with 10 µM curcumin. After incubation for 0, 8, 16 and 24 h,
the cells were collected and extracted with 1% Triton X-100
(Sigma-Aldrich). A total of 25 µg whole-cell lysate was
subjected to an enzymatic reaction in the presence of 50 M DEVD-AFC
at 37°C for 1 h. The fluorescent emission (excitation at 360 nm and
emission at 530 nm) was measured. In addition, a standard curve was
plotted for each measurement using free AFC. Using the standard
curve, the fluorescence intensity of each enzymatic reaction was
determined. The caspase activity was expressed in pmol/min/mg
protein.
Bax/Bak siRNA transfection
To inhibit the expression of Bax or Bak in H1299
cells, gene silencing was performed using the Lipofectamine 2000
transfection reagent according to the manufacturer's instructions.
The target siRNA and non-specific control siRNA sequences were as
follows: Bak (beginning at nt 310), 5′-P-UGCCUACGAACUCUUCACCdTdT-3′
(sense) and 5′-P-GGUGAAGAGUUCGUAGGCAdTdT-3′ (anti-sense); Bax
(beginning at nt 217), 5′-P-UAUGGAGCUGCAGAGGAUGdTdT-3′ (sense) and
5′-P-CAUCCUCUGCAGCUCCAUAdTdT-3′ (anti-sense); non-specific control
siRNA, 5′-NNATTGTATGCGATCGCAGAC-3′. All of the above siRNA
sequences were designed and synthesized by GenePharma (Shanghai,
China). For transfection, H1299 cells were seeded into six-well
plates at 3×105 cells per well. After incubation for 24
h, the cells were transfected with the specific Bax or Bak siRNA
sequence using Lipfectamine 2000 trans-fection agent following the
manufacturer's instructions. After 36 h of transfection, the cells
were harvested for further study.
Western blot analysis
Western blotting was performed to determine the
expression levels of apoptotic signaling molecules and their
associated mitochondrial molecules in H1299 cells. Briefly, after
incubation with 10 µM curcumin, 1×107 H1299 cells
were harvested for protein extraction. Mitochondrial and
mitochondria-free proteins were separated according to the
instruction manual of the kit (KeyGen). The whole-cell lysate was
extracted using radioimmunoprecipitation assay buffer (1 M
Tris-HCl, 5 M NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.05%
SDS and 1 mM phenylmethyl sulfonyl fluoride). Western blot analysis
was then performed as previously described using SDS buffer (25 mM
Tris, 192 mM glycine, 0.1% SDS) (22). The blots were subsequently
incubated with the specific primary antibodies (1:400 dilution for
all) at 4°C overnight, and with the corresponding IR dye-conjugated
secondary antibodies (1:5,000 dilution for all) at room temperature
for 1 h. Membranes were visualized using the Odyssey Infrared
Imaging System and Odyssey v1.2 (LI-COR Biosciences, Lincoln, NE,
USA). The relative densities of the protein bands were analyzed
using Quantity One software (Bio-Rad Laboratories, Inc.).
Bax/Bak homo-oligomerization
The membrane-permeable cross-linking reagent BMH was
used to analyze Bax/Bak oligomerization. Briefly, after incubation
with indicated concentrations of curcumin, 1×107 of
H1299 cells were collected for protein extraction. The
mitochondrial-membrane fractions were separated by
ultracentrifugation at 100,000 × g for 60 min at 2–8°C and
re-suspended in 250 µl phosphate-buffered saline (PBS). The
collected mitochondrial-membrane fraction was then incubated with
0.2 mM BMH in PBS for 1 h at room temperature with agitation at 12
× g/min. Finally, the above mixtures were dissolved by 4X SDS
buffer, subjected to 12% SDS-PAGE and analyzed by immunoblot
analysis using the corresponding primary antibodies.
Bcl-2 overexpression in H1299 cells
To establish Bcl-2-overexpressing H1299 cells, a
Bcl-2 expression plasmid, pcDNA3-Bcl-2, was constructed (23). For transfection of the plasmid,
H1299 cells were plated into six-well plates at 3×105
cells per well. The transfection procedure was performed as
previously described with minimal revision (22).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Data were analyzed using a t-test. All statistical
analyses were performed using SPSS, version 13.0 for Windows (SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Curcumin inhibits A549 and H1299 cell
proliferation
The effects of 10 µM curcumin on A549 and
H1299 cell viability were assessed using an MTT assay at 8, 16 and
24 h post-treatment. The deficiency of p53 in H1299 cells was
confirmed by western blot analysis of the expression of p53 and
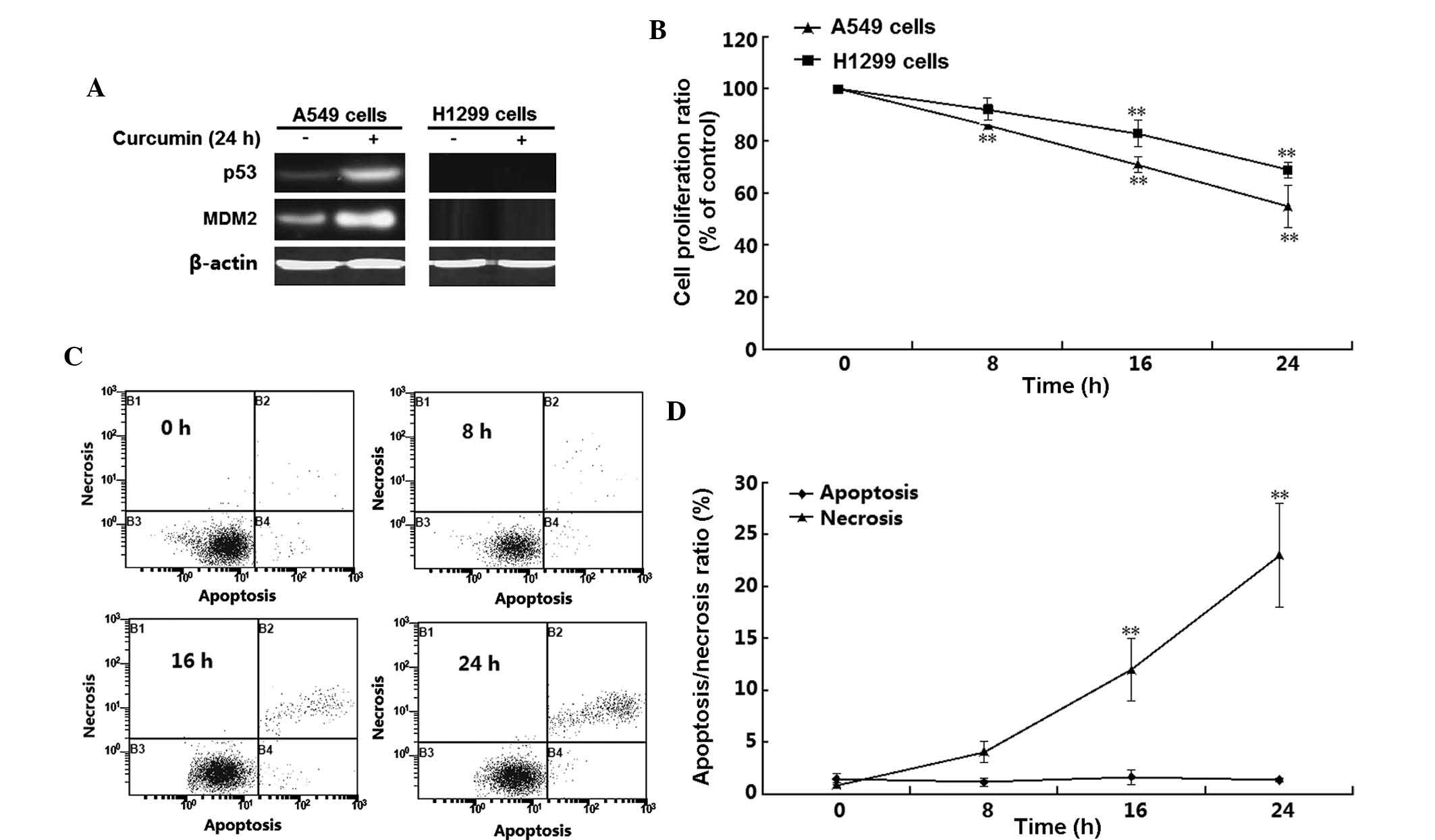
p53-target gene MDM2 (Fig. 1A).
H1299 cells did not express p53 and MDM2 regardless of whether the
cells were incubated with curcumin. By contrast, p53 as well as
MDM2, were detected in A549 cells, and their expression was induced
by curcumin treatment. These results confirmed the absence of p53
in H1299 cells, which were used in the subsequent experiments. The
MTT assay showed that curcumin treatment time-dependently decreased
the viability of the two cell lines (P<0.01) (Fig. 1B). Of note, the viability of A549
cells was affected by curcumin to a greater extant than that of
H1299 cells; for instance, at the 16-h time-point, the
proliferation rates of A549 and H1299 cells were 54 and 75%,
respectively. To further determine the mode of H1299 cell death,
flow cytometric analysis was performed following double staining
with Annexin V-FITC and PI. As shown in Fig. 1C and D, a significant and
time-dependent induction of H1299-cell necrosis was detected
following 16–24 h of curcumin treatment (P<0.05). However,
apoptosis was not significantly induced in H1299 cells by treatment
with 10 µM curcumin for 8–24 h (P>0.05).
Curcumin-induced cell death is
mitochondria-dependent
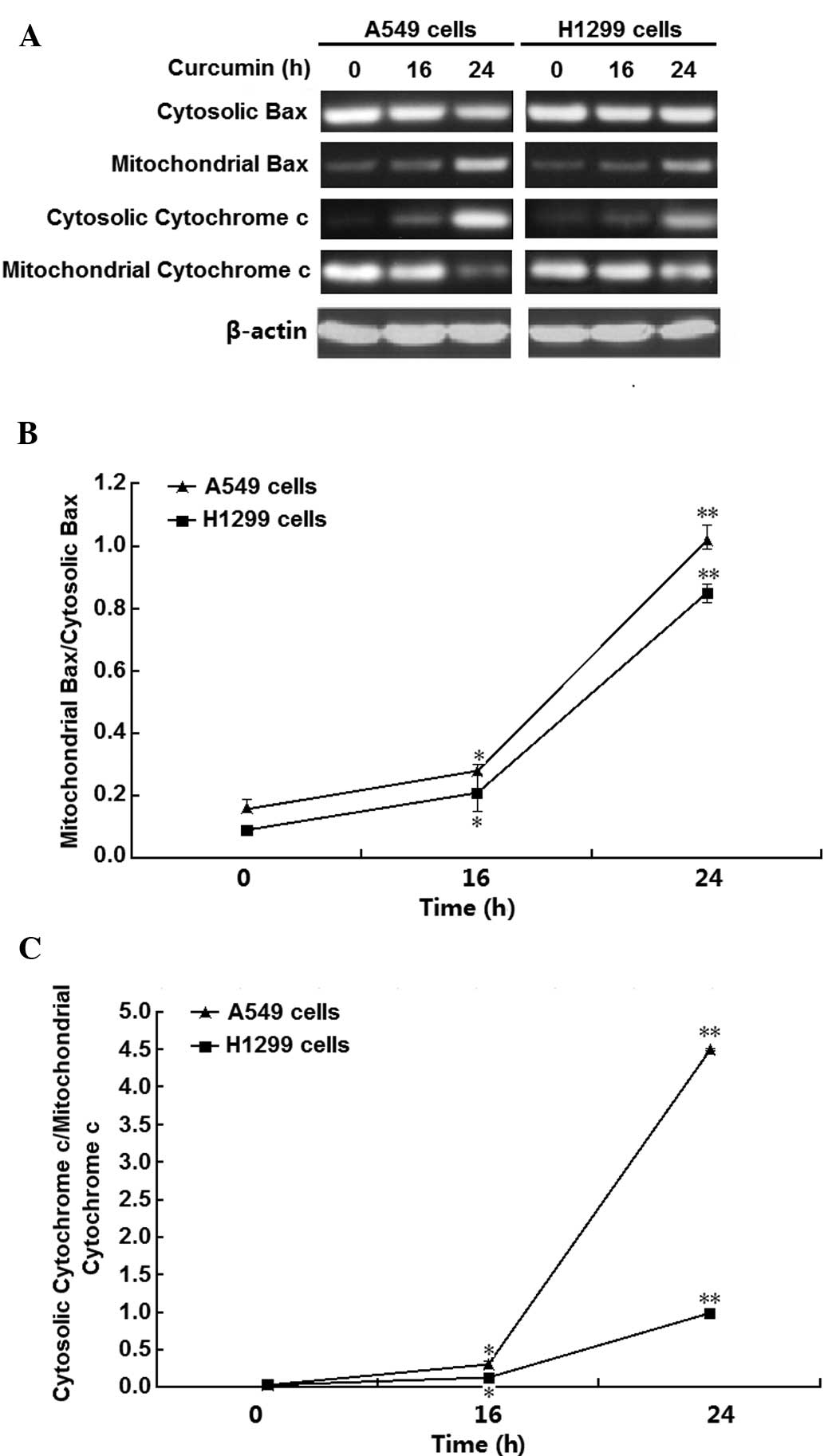
To investigate the underlying mechanisms of
curcumin-induced H1299-cell necrosis, Bax and cytochrome c
expression and distribution were examined. The results demonstrated
that curcumin induced Bax translocation to mitochondria and
cytochrome c release from the mitochondria in A549 and H1299
cells (Fig. 2A). The relative
expression of Bax and cytochrome c in mitochondria and
cytosol were then quantified by densito-metric analysis (Fig. 2B and C), showing that 10 µM
curcumin significantly and time-dependently enhanced Bax
translocation and cytochrome c release in the two cell lines
(P<0.01 and P<0.05, respectively), particularly at the
time-point of 24 h. These results suggested that mitochondria have
important roles in curcumin-induced necrosis of A549 and H1299
cells.
Caspase family members are activated in
curcumin-treated H1299 cells
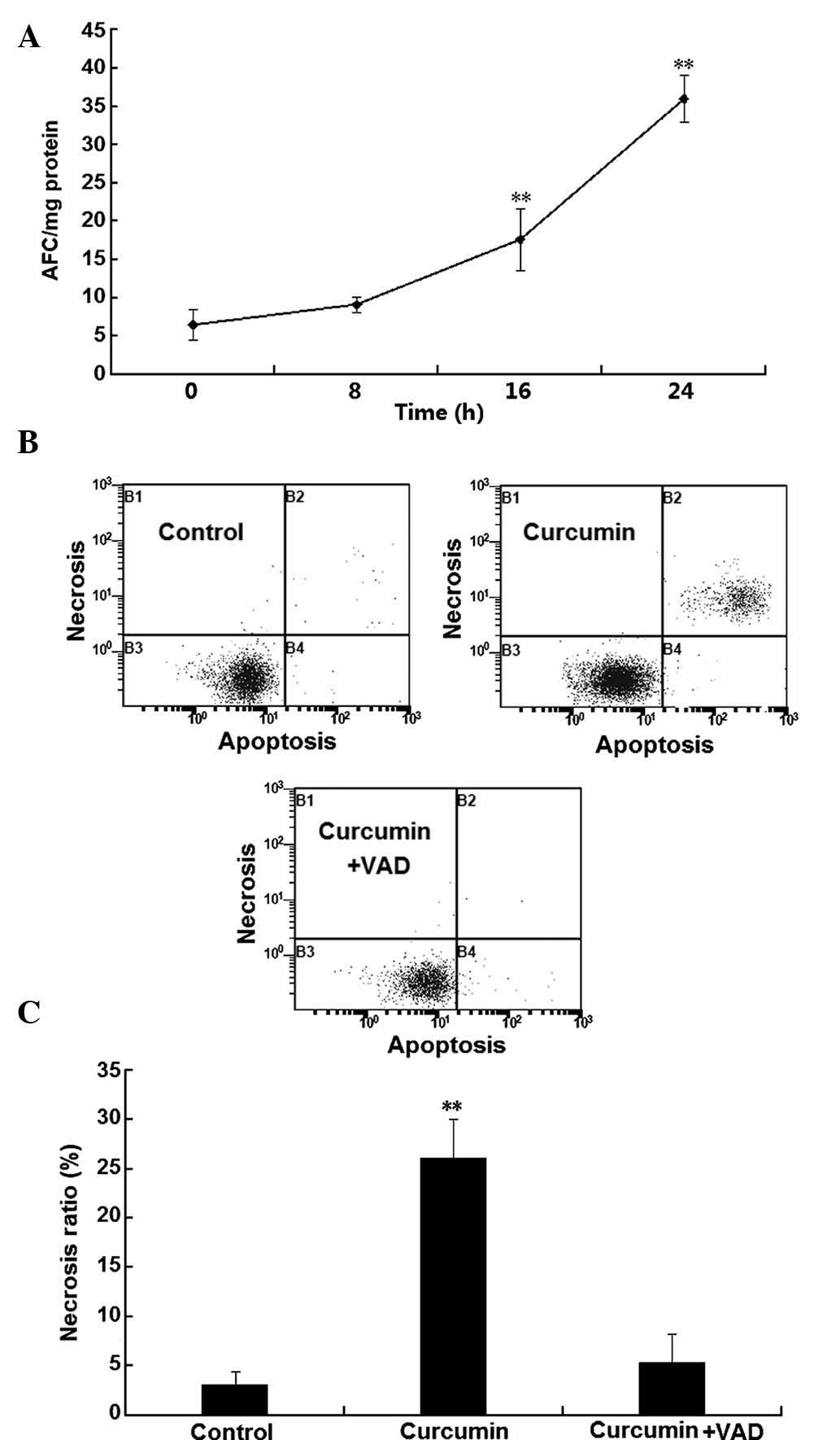
To investigate the involvement of caspases in the
programmed necrosis of H1299 cells, caspase activity was examined.
As shown in Fig. 3A, a significant
and time-dependent increase of caspase 3, 6 and 7 activity was
observed following incubation with curcumin for 16–24 h
(P<0.01). To confirm the involvement of caspases in the
curcumin-induced death of H1299 cells, the effects of the classical
caspase inhibitor z-VAD-FMK on H1299-cell necrosis were assessed
using flow cytometry. As shown in Fig.
3B and C, after treatment with curcumin for 24 h, the number of
necrotic cells was significantly increased, which was almost
completely abrogated in the presence of the caspase inhibitor. This
result suggested that curcumin-induced necrosis in H1299 cells was
caspase-dependent.
Oligomerization of Bax and Bak is
involved in curcumin-induced H1299 cell necrosis
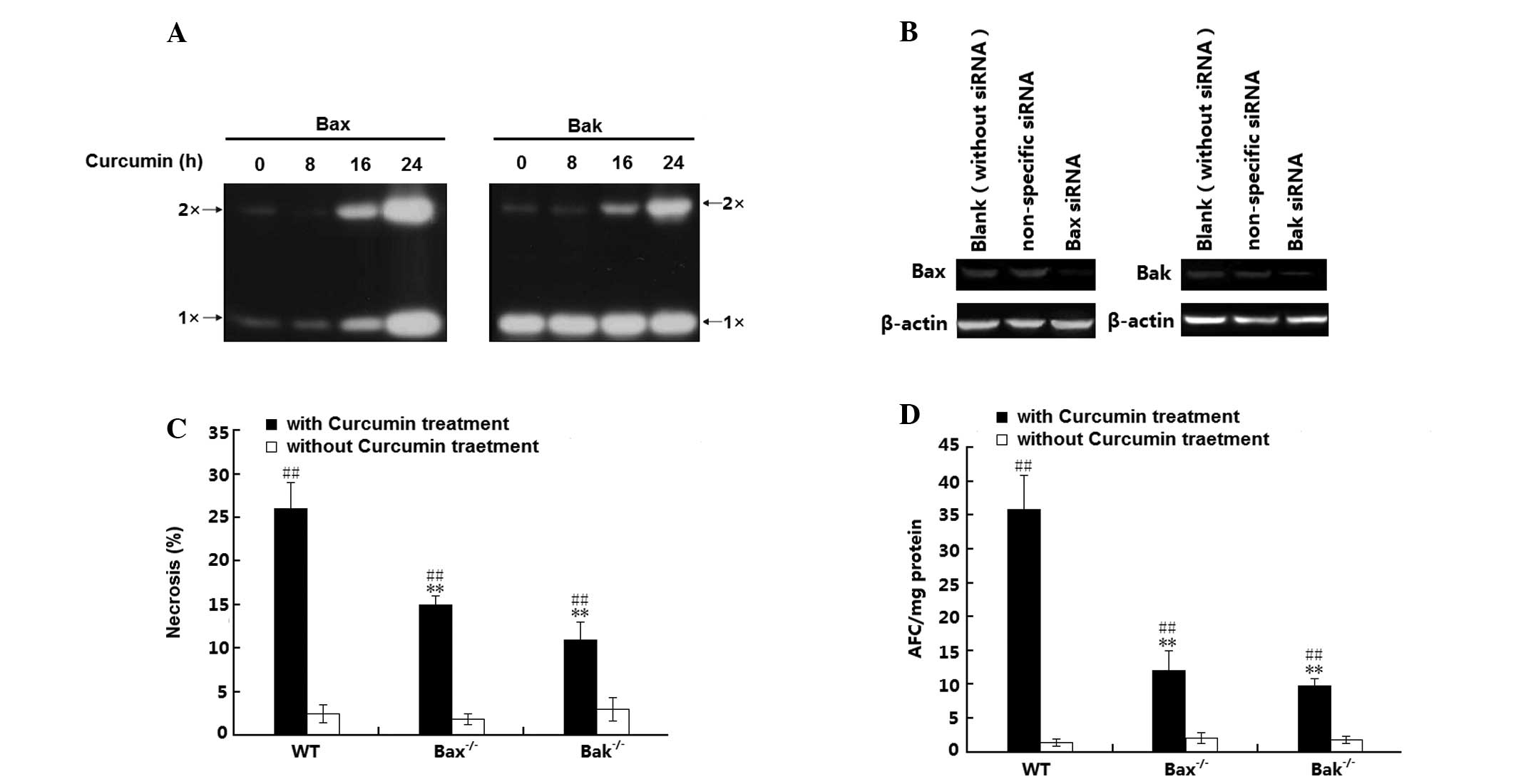
The present study further investigated whether
oligomerization between Bax and Bak is involved in curcumin-induced
H1299-cell necrosis. For this, mitochondrial lysates were reacted
with a chemical cross-linker to stabilize the oligomers for western
blot analysis. As shown in Fig.
4A, almost no Bax oligomer was induced during 0–8 h of curcumin
treatment; however, after incubation for 16–24 h, curcumin induced
an increase of mitochondrial Bax levels and Bax oligomerization.
Furthermore, although no change in mitochondrial Bak levels was
detected, Bak oligomerization was also induced. To further
elucidate effects of this sub-cellular distribution of Bax and Bak
in curcumin-induced H1299 cell necrosis, the present study examined
the cell death mode of wild-type (wt) H1299 cells as well as H1299
(Bax−/−) and H1299 (Bak−/−) cells, in which Bax and Bak had been
silenced with specific siRNAs. The Bax and Bak knockdown efficiency
was confirmed by western blot analysis (Fig. 4B). Following treatment with
curcumin, a significant increase in the number of necrotic cells
was observed in the H1299 (wt), H1299 (Bax−/−) and H1299 (Bak−/−)
cells (P<0.01); of note, the amount of necrotic H1299 cells (wt)
was significantly greater than that in the Bax- or Bak knockdown
H1299 cell groups (Fig. 4C). In
addition, these effects were confirmed by caspase activation
analysis, revealing a significant increase of caspase activity in
all of the three H1299-cell groups (P<0.01). In parallel to the
necrotic rates, curcumin-increased caspase activity in (Bax−/−) and
in (Bak−/−) H1299 cells was lower than that in H1299 cells (wt)
(P<0.01). All of these results suggested that Bax and Bak as
well as their oligomerization are involved in the curcumin-induced
necrosis of H1299 cells.
Inhibition of Bax and Bak attenuates
cytochrome c release in H1299 cells
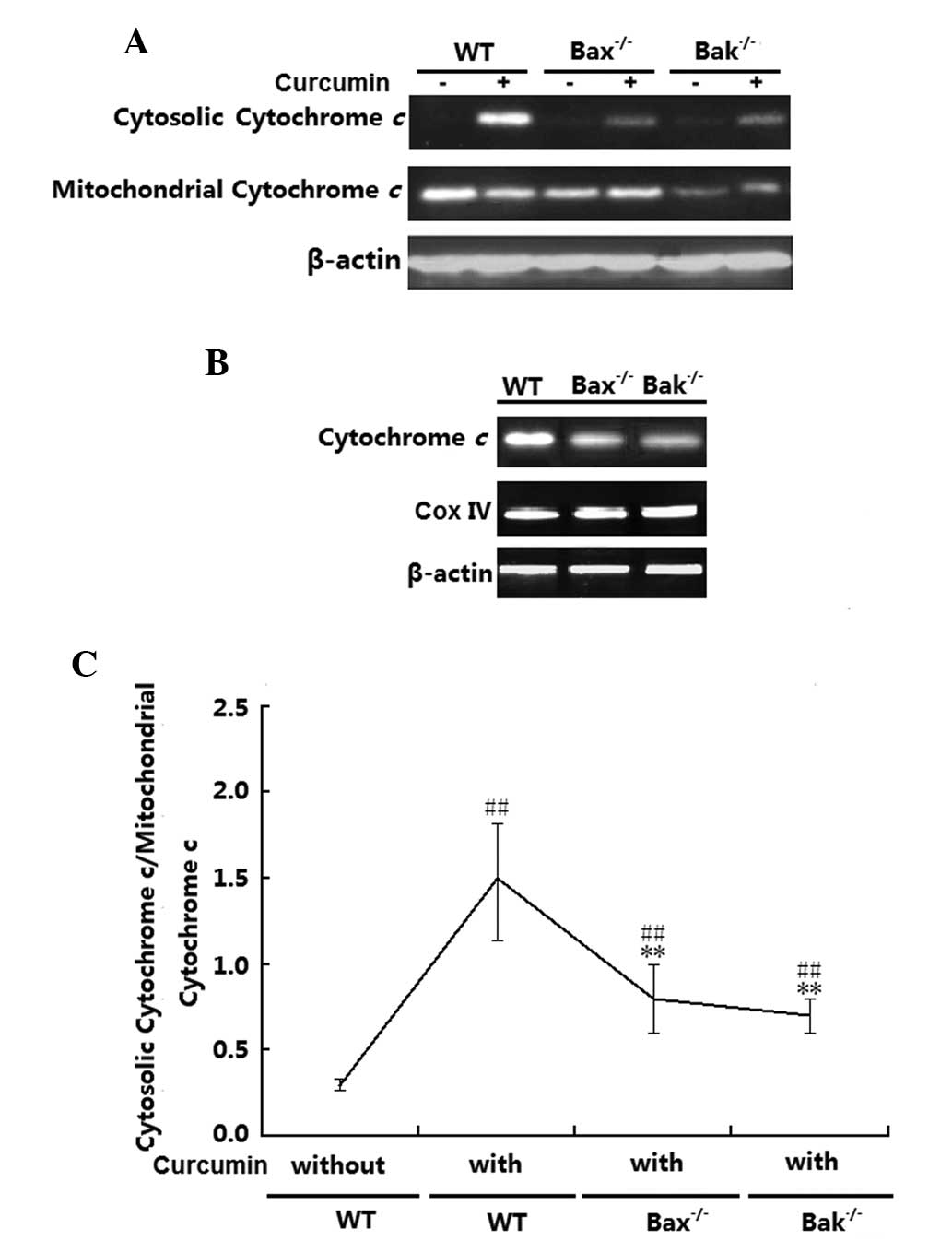
Cytochrome c release levels in the H1299
(wt), H1299 (Bax−/−) and H1299 (Bak−/−) cells were examined.
Fig. 5A and B shows that in the
absence of curcumin, cytochrome c was mainly localized to
the mitochondria irrespective of the cells' Bax or Bak status.
After treatment with curcumin, cytochrome c release was
significantly increased in the three groups (P<0.01); however,
the release in the H1299 (Bax−/−) and (Bak−/−) H1299 cells was
obviously lower than that in the H1299 (wt) cells (P<0.01).
Total cytochrome c expression levels in the three cell lines
were also detected by western blot analysis, revealing that
cytochrome c expression levels in Bax- or Bak-deficient
H1299 cells was lower than that in H1299 (wt) cells (Fig. 5C). However, no changes in the
expression of mitochondrial CoxIV were observed in the three
groups.
Overexpression of Bcl-2 attenuates
necrosis and cytochrome c release in curcumin-treated H1299
cells
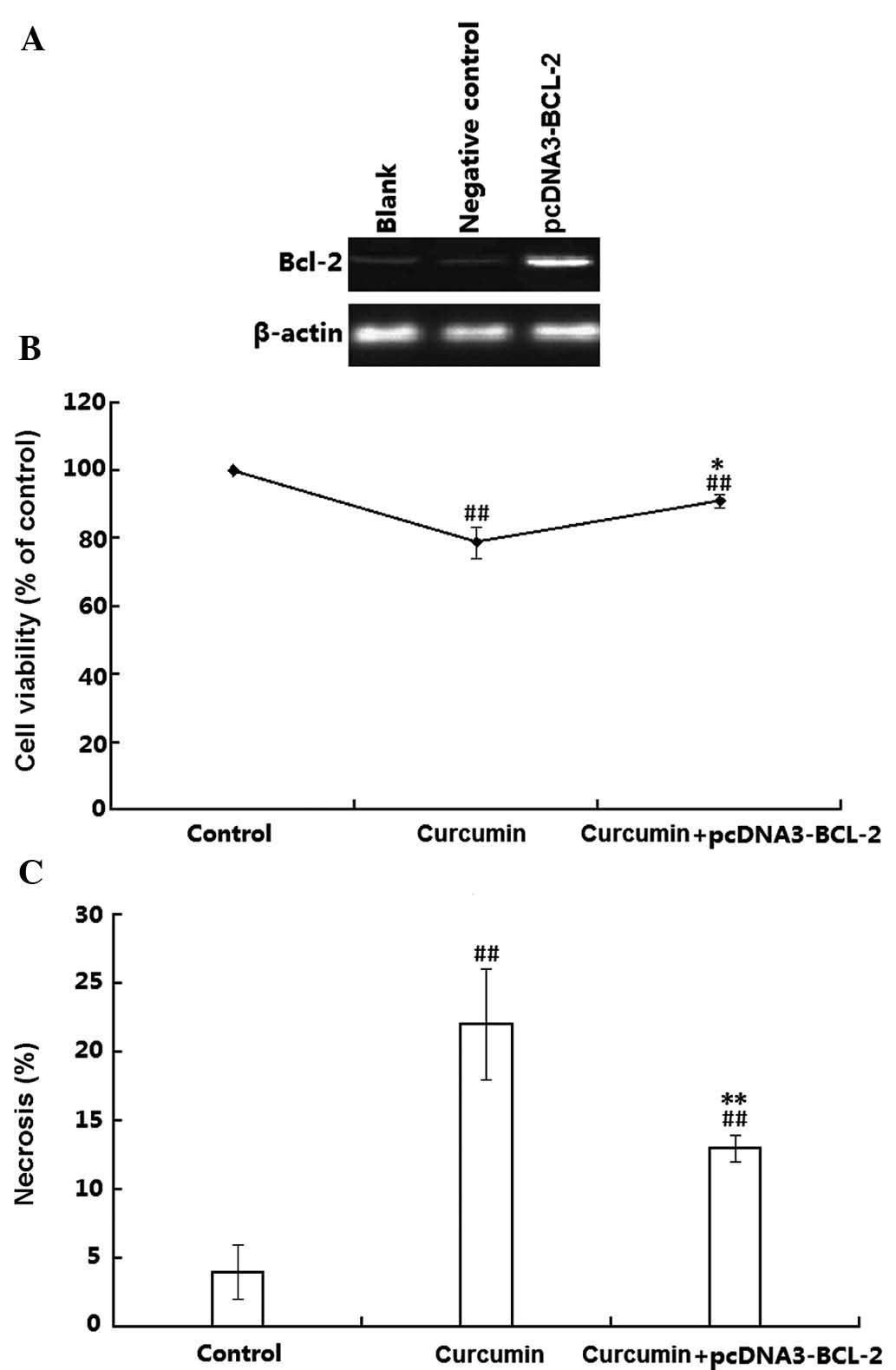
Finally, the present study examined the effects of
plasmid-mediated Bcl-2 overexpression on the toxicity of curcumin
on H1299 cells. Transfection efficiency was confirmed by western
blot analysis, which demonstrated that the Bcl-2 protein levels in
the transfected H1299 cells were obviously higher than those in the
untransfected H1299 cells (Fig.
6A). The transfected H1299 cells were then treated with
curcumin and the cell viability was assessed using an MTT assay. As
shown in Fig. 6B, curcumin
significantly decreased the cell viability in wt as well as in
Bcl-2-overexpressing H1299 cells (P<0.01). However, the cell
death rate in the Bcl-2 overexpressing cells was obviously lower
than that in the wild-type H1299 cells (P<0.05). The effects of
Bcl-2 on the cell viability were further proved by flow cytometry
using double staining with Annexin V-FITC and PI. As shown in
Fig. 6C, compared with the
controls, the necrotic rate of wt H1299 cells was 22%, which was
reduced to 13% in the Bcl-2 overexpressing H1299 cells. These
results suggested that Bcl-2 attenuated curcumin-induced necrosis
in H1299 cells.
Discussion
Curcumin, an active component of turmeric, which is
used as a dietary supplement and a herbal medicine in numerous
Asian countries, has been demonstrated to have potent
anti-neoplastic effects on a variety of cancer cell types in
vitro as well as chemo-preventive effects in murine carcinoma
models (5). Curcumin inhibits the
proliferation and induces apoptosis of various types of cancer cell
(24,25). In agreement with previous studies,
the present study revealed that after treatment for 16–24 h, 10
µM curcumin significantly decreased the viability of
p53-proficient A549 cells and p53-deficient H1299 cells. In
addition, the mode of cell death of H1299 cells was analyzed using
flow cytometry, which demonstrated that the curcumin treatment
resulted in necrosis, while only few apoptotic cells were present.
Furthermore, the present study revealed that mitochondria were
involved in the curcumin-induced H1299-cell necrosis. In addition,
Bax and Bak were activated, which resulted in cytochrome c
release caspase activation. Finally, the present study showed that
Bax/Bak inhibition and Bcl-2 overexpression attenuated
curcumin-induced H1299 cell necrosis. The present study indicated
that the p53-independent mechanism of curcumin-induced necrosis of
H1299 cells is associated with the mitochondria.
Apoptosis, an extensively studied and well-known
type of PCD, is a highly regulated process in all multicellular
organisms and is essential for embryonic development and adult
tissue homeostasis. It has been recognized that deregulation of
apoptosis contributes to the development of cancers (6). Conventional chemotherapeutic agents
were developed based on the fact that the proliferation of tumor
cells is upregulated. By targeting intracellular molecules
including the DNA or microtubules, chemotherapeutics induce various
degrees of damage or stress in rapidly dividing cells, leading to
apoptosis (26). p53 has important
roles in apoptosis-inducing cancer therapies through initiating the
DNA damage response (27,28). p53 regulates DNA damage-induced
apoptosis through a transcription-dependent pathway [involving MDM2
and p53-upregulated modulator of apoptosis (PUMA)], and/or a
transcription-independent pathway (involving Bax, Bak and Bcl-2)
(29,30). Of note, in the p53-deficient H1299
cells, Bax and Bak can be activated and form oligomers at
mitochondrial sites, which may be involved in the regulation of the
permeability of the mitochondrial outer membrane to induce
permeability transition and cytochrome c release, which can
in turn activate the caspase cascade (31). Results of previous studies and the
present study indicated that curcumin exerts its cytotoxic effects
via p53-dependent as well as p53-independent
mitochondria-associated cell death mechanisms.
Bcl-2 family members are key regulators of
mitochondria-dependent cell death. The molecular basis of the
regulation of apoptosis through either pro-apoptotic members
(Bax/Bak) or anti-apoptotic Bcl-2 is well known. Bcl-2 family
proteins regulate mitochondrial apoptosis or/and necrosis through
controlling the permeability of the mitochondrial membrane
(32). However, with regard to the
mechanism of action of curcumin on the p53-deficient H1299 cell
line, it remains elusive how cell death signaling is initiated and
how Bax and Bak are upregulated. Several potential pathways of
p53-independent apoptosis have been previously reported. For
instance, in doxorubicin-treated Saos-2 cells, overexpression of
p73γ, a p53 family member, caused apoptosis and PUMA
transactivation, which in turn activated mitochondrial
translocation of Bax and cytochrome c release (33). Another study reported that in
p53-mutant HT-29 colon cancer cells, quercetin induced apoptosis by
decreasing the mitochondrial membrane potential, and increasing
reactive oxygen species production and sestrin 2 expression through
the adenosine monophosphate-activated protein kinase/p38 pathway
(34). These previous studies,
combined with the results of the present study, indicated that
p53-dependent and p53-independent regulatory mechanisms may
simultaneously contribute to the activation of Bax/Bak, which in
turn trigger apoptosis; however, further studies are required to
determine the exact underlying molecular mechanism.
Another noteworthy finding of the present study was
the inter-dependence between Bax and Bak with regard to their
activation in curcumin-treated H1299 cells. Bax and Bak were
activated in H1299 cells (wt), while Bax and Bak translocation and
oligomerization was partially inhibited in H1299 (Bak−/−) and H1299
(Bax−/−) cells. Furthermore, the anti-apoptotic protein Bcl-2 was
shown to suppress curcumin-induced H1299 cell necrosis through
abrogating the Bax - Bak interaction and inhibiting cytochrome
c release. It remains elusive whether this Bax - Bak
inter-dependence is linked to their function of permeabilizing the
outer mitochondrial membrane. Previous studies suggested several
possibilities; for instance, Bax and Bak contain a hydrophobic BH3
domain, which only becomes available for binding during activation
to facilitate hetero- and homo-oligomerization (35,36).
Furthermore, TNF-α-induced mitochondrial cytochrome c
release and apoptosis of HeLa cells was associated with
simultaneous homo-oligomerization and hetero-oligomerization of
Bax/Bak (37). The present study
indicated that Bax and Bak may function cooperatively and
interdependently to permeabilize the outer mitochondrial membrane,
resulting in the release of cytochrome c and activation of
the caspase cascade.
Two pathways have been confirmed to be involved in
apoptosis induction, including death receptor-dependent extrinsic
and mitochondria-dependent intrinsic pathways (17,38).
The intrinsic apoptotic pathway mediated by mitochondria is mainly
triggered by the collapse of the mitochondrial membrane potential
(39). The collapse prompts the
release of pro-apoptotic cytochrome c into the cytoplasm,
which has been proposed as a 'point of no return' in the
mitochondrial pathway (40,41).
Once mitochondria sense the cell death stimuli, cytochrome c
is released into the cytosol, where it binds to Apaf-1 and
caspase-9 to form a large complex termed the apoptosome, which in
turn initiates the activation of downstream caspases 3, -6 and -7,
as well as other apoptotic effectors; therefore, activation of the
caspase-cascade system is the key event of mitochondria-dependent
apoptosis (42,43). In addition, several previous
studies suggested that the execution of necrotic cell death is
caspase-independent (12,44). However, in the curcumin-treated
H1299 cell model used in the present study, caspase activation was
induced simultaneously with the appearance of cell necrosis. These
results strongly indicated that apoptosis and necrosis may share
common features, such as caspase activation.
In conclusion, the results of the present study
revealed that curcumin exerts cytotoxic effects by inducing
necrosis in the p53-deficient H1299 cell line through a
mitochondria-associated pathway. The p53-independent mechanism was
shown to include Bax/Bak translocation to mitochondria, where they
permeabilized the outer mitochondrial membrane to trigger a cell
death cascade. These results supported the potential of curcumin as
a promising agent for the treatment of p53-defi-cient lung
cancers.
Acknowledgments
The present study was supported by grants from the
Science Technology Study of Hubei Department of Education
Foundation (grant no. Q20141677).
Abbreviations:
|
DMSO
|
dimethyl sulfoxide
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
BAX
|
Bcl-2-associated X protein
|
|
Bak
|
Bcl-2 homologous antagonist killer
|
References
|
1
|
O'Sullivan-Coyne G, O'Sullivan GC,
O'Donovan TR, Piwocka K and McKenna SL: Curcumin induces
apoptosis-independent death in oesophageal cancer cells. Br J
Cancer. 101:1585–1595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman L, Lin L, Ball S, Bekaii-Saab T,
Fuchs J, Li PK, Li C and Lin J: Curcumin analogues exhibit enhanced
growth suppressive activity in human pancreatic cancer cells.
Anticancer Drugs. 20:444–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steward WP and Gescher AJ: Curcumin in
cancer management: Recent results of analogue design and clinical
studies and desirable future research. Mol Nutr Food Res.
52:1005–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shehzad A, Lee J, Huh TL and Lee YS:
Curcumin induces apoptosis in human colorectal carcinoma (HCT-15)
cells by regulating expression of Prp4 and p53. Mol Cells.
35:526–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choudhuri T, Pal S, Agwarwal ML, Das T and
Sa G: Curcumin induces apoptosis in human breast cancer cells
through p53-dependent Bax induction. FEBS Lett. 512:334–340. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alenzi FQ, Wyse RK and Altamimi WG:
Apoptosis as a tool for therapeutic agents in haematological
diseases. Expert Opin Biol Ther. 4:407–420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S, Shao K, Liu Y, Kuang Y, Li J, An
S, Guo Y, Ma H and Jiang C: Tumor-targeting and
microenvironment-responsive smart nanoparticles for combination
therapy of antiangiogenesis and apoptosis. ACS Nano. 7:2860–2871.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Griffith TS: Induction of tumor cell
apoptosis by TRAIL gene therapy. Methods Mol Biol. 542:315–334.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sosna J, Voigt S, Mathieu S, Lange A, Thon
L, Davarnia P, Herdegen T, Linkermann A, Rittger A, Chan FK, et al:
TNF-induced necroptosis and PARP-1-mediated necrosis represent
distinct routes to programmed necrotic cell death. Cell Mol Life
Sci. 71:331–348. 2014. View Article : Google Scholar :
|
|
10
|
Proskuryakov SY, Gabai VL and
Konoplyannikov AG: Necrosis is an active and controlled form of
programmed cell death. Biochemistry (Mosc). 67:387–408. 2002.
View Article : Google Scholar
|
|
11
|
Vandenabeele P, W Declercq F, Van
Herreweghe, et al: The role of the kinases RIP1 and RIP3 in
TNF-induced necrosis. Sci Signal. 2010.115:re4
|
|
12
|
Baritaud M, Boujrad H, Lorenzo HK, Krantic
S and Susin SA: Histone H2AX: The missing link in AIF-mediated
caspase-independent programmed necrosis. Cell Cycle. 9:3166–3173.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwasaki R, Ito K, Ishida T, Hamanoue M,
Adachi S, Watanabe T and Sato Y: Catechin, green tea component,
causes caspase-independent necrosis-like cell death in chronic
myelogenous leukemia. Cancer Sci. 100:349–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicotera P and Melino G: Regulation of the
apoptosis-necrosis switch. Oncogene. 23:2757–2765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin X, Sun T, Cai M and Shen P:
Cell-death-mode switch from necrosis to apoptosis in hydrogen
peroxide treated macrophages. Sci China Life Sci. 53:1196–1203.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bergeron L, Perez GI, Macdonald G, et al:
Defects in regulation of apoptosis in caspase-2-deficient mice.
Genes Dev. 12:1304–1314. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosseinimehr SJ, Azadbakht M, Tanha M,
Mahmodzadeh A and Mohammadifar S: Protective effect of hawthorn
extract against genotoxicity induced by methyl methanesulfonate in
human lymphocytes. Toxicol Ind Health. 27:363–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kosmider B, Wojcik I, Osiecka R,
Bartkowiak J, Zyner E, Ochocki J and Liberski P: Enhanced P53 and
BAX gene expression and apoptosis in A549 cells by cis-Pt(II)
complex of 3-aminoflavone in comparison with cis-DDP. Invest New
Drugs. 23:287–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song G, Mao YB, Cai QF, Yao LM, Ouyang GL
and Bao SD: Curcumin induces human HT-29 colon adenocarcinoma cell
apoptosis by activating p53 and regulating apoptosis-related
protein expression. Braz J Med Biol Res. 38:1791–1798. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu JL, Rak JW, Coomber BL, et al: Effect
of p53 status on tumor response to antiangiogenic therapy. Science.
295:1526–1528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian F, Song M, Xu PR, et al: Curcumin
promotes apoptosis of esophageal squamous carcinoma cell lines
through inhibition of NF-kappaB signaling pathway. Ai Zheng.
27:566–570. 2008.In Chinese. PubMed/NCBI
|
|
22
|
Zhang W, Liu N, Wang X, et al:
Benzo(a)pyrene-7,8-diol-9,10-epoxide induced p53-independent
necrosis via the mitochondria-associated pathway involving Bax and
Bak activation. Hum Exp Toxicol. 34:179–190. 2015. View Article : Google Scholar
|
|
23
|
Guo BC and Xu YH: Bcl-2 over-expression
and activation of protein kinase C suppress the trail-induced
apoptosis in Jurkat T cells. Cell Res. 11:101–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bar-Sela G, Epelbaum R and Schaffer M:
Curcumin as an anti-cancer agent: Review of the gap between basic
and clinical applications. Curr Med Chem. 17:190–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian C, Xing G, Xie P, et al: KRAB-type
zinc-finger protein Apak specifically regulates p53-dependent
apoptosis. Nat Cell Biol. 11:580–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blankenberg FG: Apoptosis imaging:
Anti-cancer agents in medicinal chemistry. Anticancer Agents Med
Chem. 9:944–951. 2009. View Article : Google Scholar
|
|
27
|
Meulmeester E and Jochemsen AG: p53: A
guide to apoptosis. Curr Cancer Drug Targets. 8:87–97. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y: Regulation of p53 responses by
post-translational modifications. Cell Death Differ. 10:400–403.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leibowitz BJ, Qiu W, Liu H, Cheng T, Zhang
L and Yu J: Uncoupling p53 functions in radiation-induced
intestinal damage via PUMA and p21. Mol Cancer Res. 9:616–625.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thakur VS, Ruhul Amin AR, Paul RK, Gupta
K, Hastak K, Agarwal MK, Jackson MW, Wald DN, Mukhtar H and Agarwal
ML: p53-Dependent p21-mediated growth arrest pre-empts and protects
HCT116 cells from PUMA-mediated apoptosis induced by EGCG. Cancer
Lett. 296:225–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morales-Cruz M, Figueroa CM,
Gonzalez-Robles T, et al: Activation of caspase-dependent apoptosis
by intracellular delivery of cytochrome c-based nanoparticles. J
Nanobiotechnology. 12:332014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Antignani A and Youle RJ: How do Bax and
Bak lead to permeabilization of the outer mitochondrial membrane?
Curr Opin Cell Biol. 18:685–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holcakova J, Ceskova P, Hrstka R, Muller
P, Dubska L, Coates PJ, Palecek E and Vojtesek B: The cell
type-specific effect of TAp73 isoforms on the cell cycle and
apoptosis. Cell Mol Biol Lett. 13:404–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim GT, Lee SH, Kim JI and Kim YM:
Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway
and induces apoptosis by increasing the generation of intracellular
ROS in a p53-independent manner. Int J Mol Med. 33:863–869.
2014.PubMed/NCBI
|
|
35
|
Kim H, Tu HC, Ren D, et al: Stepwise
activation of BAX and BAK by tBID, BIM, and PUMA initiates
mitochondrial apoptosis. Mol Cell. 36:487–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Antonsson B, Montessuit S, Lauper S, et
al: Bax oligomerization is required for channel-forming activity in
liposomes and to trigger cytochrome c release from mitochondria.
Biochem J. 345(Pt 2): 271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sundararajan R, Cuconati A, Nelson D and
White E: Tumor necrosis factor-alpha induces Bax-Bak interaction
and apoptosis, which is inhibited by adenovirus E1B 19K. J Biol
Chem. 276:45120–45127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wachter F, Grunert M, Blaj C, Weinstock
DM, Jeremias I and Ehrhardt H: Impact of the p53 status of tumor
cells on extrinsic and intrinsic apoptosis signaling. Cell Commun
Signal. 11:272013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mehmeti I, Gurgul-Convey E, Lenzen S and
Lortz S: Induction of the intrinsic apoptosis pathway in
insulin-secreting cells is dependent on oxidative damage of
mitochondria but independent of caspase-12 activation. Biochim
Biophys Acta. 1813:1827–1835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Buratta M, Castigli E, Sciaccaluga M,
Pellegrino RM, Spinozzi F, Roberti R and Corazzi L: Loss of
cardiolipin in palmitate-treated GL15 glioblastoma cells favors
cytochrome c release from mitochondria leading to apoptosis. J
Neurochem. 105:1019–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee HJ, Lee HJ, Lee EO, Ko SG, Bae HS, Kim
CH, Ahn KS, Lu J and Kim SH: Mitochondria-cytochrome C-caspase-9
cascade mediates isorhamnetin-induced apoptosis. Cancer Lett.
270:342–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakabayashi J and Sasaki A: A mathematical
model for apoptosome assembly: The optimal cytochrome c/Apaf-1
ratio. J Theor Biol. 242:280–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Adrain C and Martin SJ: The mitochondrial
apoptosome: A killer unleashed by the cytochrome seas. Trends
Biochem Sci. 26:390–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kihana T, Tsuda H, Teshima S, et al: High
incidence of p53 gene mutation in human ovarian cancer and its
association with nuclear accumulation of p53 protein and tumor DNA
aneuploidy. Jpn J Cancer Res. 83:978–984. 1992. View Article : Google Scholar : PubMed/NCBI
|