Introduction
The major cause of hepatocellular cancer-associated
mortality is not only the growth of the primary tumor, buy also the
invasive spread of cancer cells to a secondary site (1,2).
Tumor metastasis is a complex process involving numerous key steps,
one of which is tumor cell migration, which is responsible for the
entry of tumor cells into blood vessels and lymph nodes (3). Various molecules and signaling
cascades are involved in the proliferation- and
metastasis-associated signaling pathways, including the
phosphatidylinositol 3-kinase (PI3K)/Akt, Ras/Raf/mitogen-activated
protein kinase (MAPK) and phospholipase-C (PLC)γ/protein kinase
C/(PKC) signaling pathways, as well as matrix metalloproteinases
(MMPs) and endogenous CXC chemokine receptor-4 (CXCR4) (4,5). The
deterioration of the extracellular matrix (ECM) is an important
step in tumor metastasis, in which MMPs, an important family of
proteinases, have an active role. Among the MMPs that have been
identified, increasing evidence showed that MMP2 and MMP9 are able
to efficiently degrade native collagen types IV and V, fibronectin,
entactin and elastin, and their overexpression is closely
associated with poor prognosis in patients (6–8).
MMP2 and -9 are therefore considered to be crucial for tumor cell
migration and invasion, leading to metastasis.
Hepatocellular carcinoma (HCC), the fifth most
common solid tumor type, is one of the leading causes for
cancer-associated mortality worldwide (9). Despite significant advances in early
detection and therapy, tumor recurrence in HCC patients can occur
as metastases, whereas >90% of HCC-associated mortalities are
the result of secondary local or distant disease. In the majority
of patients diagnosed with HCC, the tumor can be surgically
removed; however, the cancer is able to metastasize to distant
organ sites. The best treatment option available for patients with
HCC is systemic pharmacotherapy (9).
Taspine is a small molecular compound that exhibits
various biological properties, including bacteriostatic,
anti-biotic, antiviral, anti-inflammatory, anti-ulcer and
anti-cancer effects (10–15). TAS9 (Fig. 1) is a modified taspine derivative
with increased activity and solubility (16). In the present study, the effects of
TAS9 on tumor growth and migration of SMMC-7721 human liver cancer
cells as well as the associated signaling pathways were
investigated to elucidate the mechanisms underlying the
anti-tumorigenic effects of TAS9.
Materials and methods
Reagents
RPMI-1640, dimethyl sulfoxide (DMSO) and MTT were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine
serum (FBS) was purchased from GE Healthcare Life Sciences (Logan,
UT, USA). Trypsin was obtained from Amresco, LLC (Solon, OH, USA).
Penicillin was purchased from the Harbin Pharmaceutical Group Co.,
Ltd. (Harbin, China) and streptomycin was purchased from North
China Pharmaceutical Co., Ltd. (Shijiazhuang, China). The 96-, 12-
and six-well plates were purchased from Corning Inc. (Corning, NY,
USA). Crystal violet was purchased from Beijing Chemical Plant
(Beijing, China). The 24-well polycarbonate millicell membranes
were purchased from EMD Millipore (Billerica, MA, USA). The
radioimmunoprecipitation assay (RIPA) lysis buffer was purchased
from Shaanxi Pioneer Biotech Co., Ltd. (Xi'an, China). The
bicinchoninic acid (BCA) Protein Assay Reagent kit and the enhanced
Chemiluminescence (ECL) Plus Reagent kit were obtained from Pierce
Biotechnology, Inc. (Rockford, IL, USA). The protease inhibitor and
phosphatase inhibitor cocktail were purchased from Roche
Diagnostics (Basel, Switzerland, USA). The polyvinylidene fluoride
membranes were purchased from General Electric Company (Fairfield,
CT, USA). The RNAfast200 kit was purchased from Shanghai Fastagen
Biotechnology Co., Ltd. (Shanghai, China) and
Lipofectamine® 2000 reagent was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). The PrimeScript
RT Master mix Perfect Real Time kit and SYBR® Premix Ex
TaqTM II were purchased from Takara Biotechnology Co., Ltd.
(Dalian, China). The non-fat milk was purchased from Wandashan
(Heilongjiang, China). Tween-20 was purchased from Amresco, LLC.
Monoclonal anti-rabbit Akt (cat. no. 4685), monoclonal anti-rabbit
mammalian target of rapamycin (mTOR; cat. no. 2972), monoclonal
anti-p53 (cat. no. 2527) rabbit, and monoclonal anti-rabbit p38
(cat. no. 8690) antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Monoclonal anti-rabbit MMP-2
(cat. no. 1948-1), monoclonal anti-rabbit MMP-9 (cat. no. 2551-1)
and monoclonal anti-rabbit RAF (cat. no. 1560-1) rabbit antibodies
were purchased from Epitomics (Burlingame, CA, USA). Polyclonal
anti-rabbit p65 (cat. no. 10745-1-AP), polyclonal anti-rabbit PKCβ
(cat. no. 12919-1-AP), monoclonal anti-mouse CXCR4 (cat. no.
60042-1-IG) and monoclonal horseradish peroxidase-conjugated GAPDH
(cat. no. 60042-1-IG) antibodies were purchased from ProteinTech
Group, Inc. (Chicago, IL, USA). The corresponding secondary
antibodies used were anti-mouse IgG (H+L; cat. no. 14709) and
anti-rabbit IgG (cat. no. 14708) purchased from Cell Signaling
Technology, Inc. The RNA oligo was purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China).
Cell culture conditions
The SMMC-7721 human hepatocellular carcinoma cell
line was obtained from Shanghai Institute of Cell Biology of the
Chinese Academy of Sciences (Shanghai, China) and was cultured in
RPMI-1640 medium supplemented with 10% FBS and 0.1%
penicillin/streptomycin. The cultures were maintained at 37°C in a
CO2 incubator (Panasonic, Osaka, Japan) with a
controlled humidified atmosphere containing 5% CO2.
Colony formation assay
The SMMC-7721 cells were plated at a density of
5×102 cells/well in 12-well plates and then incubated
for 24 h. The cells were subsequently treated with or without TAS9
at concentrations of 1.75, 3.5, 7 µM for 48 h or without
TAS9 as a control group. Colonies with cell numbers of >50 cells
per colony were counted following staining with crystal violet
solution. All the experiments were performed in triplicate wells in
three independent experiments.
Cell viability assay
The effects of TAS9 on cell viability were assessed
using an MTT assay. Briefly, exponentially growing SMMC-7721 or
small interfering (si)RNA-transfected cells were plated at a
density of 5×103 cells/well in 96-well plates and then
cultured for 24 h. The cells were subsequently treated with TAS9 at
1.75, 3.5, 7 µM for 10–15 days or without TAS9 as a control
group. Cell proliferation reagent MTT was added to each well, and
cells were incubated for a further 4 h at 37°C in an atmosphere
containing 5% CO2. The resulting formazan crystals were
dissolved in DMSO (150 ml/well) with constant agitation for 15 min.
Absorbance of the plates was read using a 550 microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490 nm.
Wound healing assay
The SMMC-7721 cells were seeded into 12-well plates
(6×105 cells/well) and cultured to 80% confluence
overnight. Wounds were generated on the following day by performing
line-shaped incisions of the monocell layers with pipette tips
(100–200 µl). The SMMC-7721 cells were then treated with or
without TAS9 at concentrations of 1.75, 3.5 and 7 mM, while cells
were allowed to migrate into the scratched area. The migration of
the cells was visualized at 0 h (immediately following wound
scratching), 48 h and 72 h following treatment with TAS9 using a
DM505 microscope (Nikon Corporation, Tokyo, Japan).
Migration assay
The cell migration assay was performed using a
Transwell system, which allows the cells to migrate through an 8
mm-pore millicell polycarbonate membrane (Baihao, Tianjin, China).
Briefly, the SMMC-7721 cells were serum-starved for 24 h and
subsequently plated (1×104 cells/well) in serum-free
medium containing TAS9 at concentrations of 1.75, 3.5 and 7
µM in the upper chamber of a 12-well plate. The lower
chamber was filled with 1.5 ml medium supplemented with 10% FBS.
After 48 h, the cells remaining on the upper surface of the
membranes were gently removed using a cotton swab, and the cells on
the lower surface of the membranes were fixed with cold methanol
for 15 min and stained with 0.2% crystal violet. The cells that had
migrated to the bottom of the membranes were visualized using a
DM505 inverted microscope, and then counted in random fields. For
each repetition, the cells in four randomly selected fields were
counted and averaged. The data were expressed as a ratio of the
untreated group.
Western blot analysis
The protein of the SMMC-7721 cells treated with or
without TAS9 for 48 h was extracted using RIPA lysis buffer
containing 10% protease inhibitor and phosphatase inhibitor
cocktail (Roche Diagnostics) on ice for 30 min. The insoluble
protein lysate was removed by centrifugation at 13,500 × g for 10
min at 4°C. The protein concentration was determined using a BCA
Protein Quantification kit according to manufacturer's
instructions. The cell lysates were denatured by boiling with a 5X
reducing sample buffer (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) for 5 min, and separated by 10% SDS-PAGE (Shaanxi Pioneer
Biotech Co., Ltd.). Following electrophoresis, the separated
proteins were transferred to polyvinylidene fluoride membranes
(Hangzhou Microna Membrane Technology Co., Ltd., Hangzhou, China)
and blocked with 5% non-fat milk in tris-buffered saline (Baihao)
containing Tween-20 (TBST; Shaanxi Pioneer Biotech Co., Ltd.) for 2
h at room temperature with continuous agitation. The membranes were
then incubated with specific primary antibodies, including
anti-MMP-2 (1:500), anti-MMP-9, anti-mTOR, anti-Akt, anti-PKCβ,
anti-mitogen-activated protein kinase kinase (MEK)-2, anti-RAF,
anti-c-Jun N-terminal kinase-1 (JNK)-1, anti-CXCR4, anti-nuclear
factor (NF)-κB, anti-p38, anti-p53 (1:1,000) and anti-GAPDH
(1:2,000) antibodies overnight at 4°C, followed by three washes
with TBST every 10 min, and incubation with secondary antibodies at
a dilution of 1:40,000 in TBST for 1 h at 37°C. The membranes were
then washed three times with TBST for 10 min and developed using
the ECL kit. A Lane 1D™ transilluminator (Beijing Creation Science
Co., Ltd., Beijing, China) was used to capture images of the blots.
Image-Pro Plus 5.1 (Media Cybernetics, Inc., Rockville, MD, USA)
was used to quantify the protein levels.
RNA interference
Specific knockdown was achieved using small
interfering (si)RNAs targeting PKCβ or a control siRNA. A smart
pool of double-stranded siRNAs targeting PKCβ as well as
non-specific siRNAs were obtained from Shanghai GenePharma Co.,
Ltd. The siRNA sequences were as follows: Forward,
5′-GCGACCUCAUGUAUCACAUTT-3′, and reverse,
5′-AUGUGAUACAUGAGGUCGCTT-3′ for PKCβ; and forward,
5′-UUCUCCGAACGUGUCACGUTT-3′, and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′ for the control. For transfection,
siRNA was delivered at a final concentration of 80 nM using
Lipofectamine® 2000 reagent according to the
manufacturer's instructions. The cells were incubated for 24 h to
allow knockdown of PKCβ. These cells were then used for the
proliferation assays.
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from the SMMC-7721 cells was isolated
using a Total RNA Extraction kit (Takara Biotechnology Co.). Total
RNA was then reverse-transcribed in 20 ml reaction solution
(containing 4 µl total RNA and 16 µl PCR mixture)
using a Revert AID™ First Strand cDNA Synthesis kit (Takara
Biotechnology Co.). The cDNA was synthesized using Bio-Rad iScript
Reverse Transcriptase (Bio-RAD Laboratories, Inc.) and the PCR
reactions were performed using a Thermal Cycler Dice Real Time
system (Takara Biotechnology Co.). The primer sequences were as
follows: GAPDH forward, 5′-CACCCACTCCTCCACCTTTG-3′ and reverse,
5′-CCACCACCCTGTTGCTGTAG-3′; PKCβ forward,
5′-TTGGGATTTGACCAGCAGGAA-3′ and reverse, 5′-GGTGGCACAGGCACATTGA-3′,
synthesised by Shanghai GenePharma Co., Ltd.. The thermocycling
conditions were as follows: 95°C for 2 min and then 95°C for 10 s,
and 45 cycles of 60°C for 20 sec. The relative levels of mRNA for
each gene were normalized and represented as the ratio of the mRNA
value of a target gene to that of the GAPDH gene.
Statistical analysis
Values are expressed as the mean ± standard
deviation of data from several repetitions. Statistical analyses of
differences between groups were performed using GraphPad Prism v5.0
(GraphPad Software, Inc., La Jolla, CA, USA) and Student's
t-test was used to analyze statistical differences between
groups under various conditions. P<0.05 was considered to
indicated a statistically significant difference.
Results
TAS9 inhibits SMMC-7721-cell
proliferation
To assess the effects of TAS9 on cell proliferation,
SMMC-7721 cells were treated with TAS9 at concentrations of 0, 0.4,
2, 10 and 50 µM. The results showed that TAS9 inhibited the
growth of SMMC-7721 cells in a dose-dependent manner and the
IC50 value of TAS9 on SMMC-7721 cells was 7.57
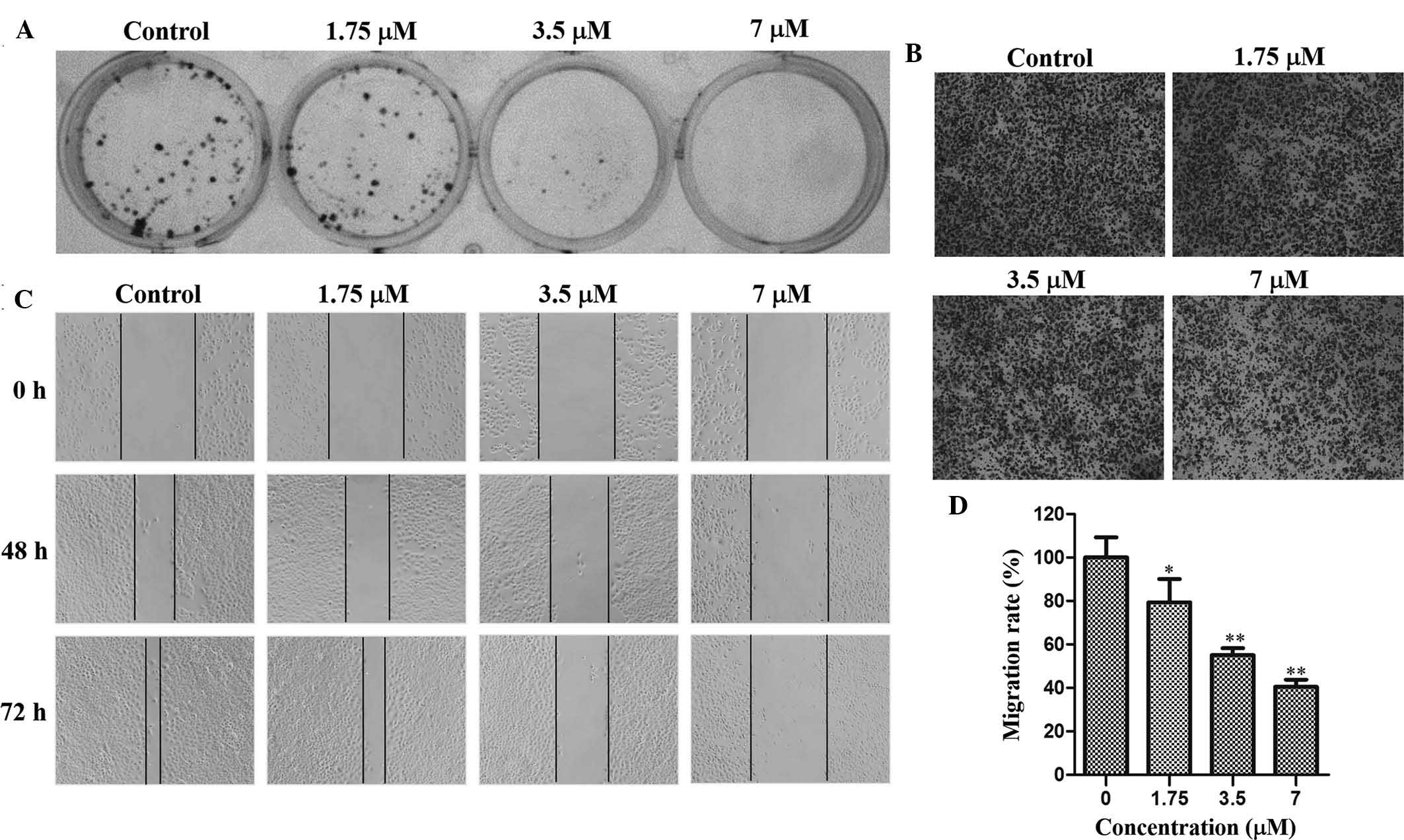
µM. Furthermore, TAS9 suppressed colony formation of
SMMC-7721 cells following 10–15 days of continuous culture. TAS9
evidently decreased the number of colonies formed by SMMC-7721
cells at concentrations of 1.75, 3.5 and 7 µM (Fig. 2A). The MTT as well as the colony
formation assay indicated that TAS9 significantly inhibited the
proliferation and clonogenicity of SMMC-7721 cells.
TAS9 inhibits SMMC-7721-cell
metastasis
To investigate the effects of TAS9 on SMMC-7721-cell
migration and invasion, wound healing and Transwell assays were
performed. The Transwell assay indicated that after 48 h of
incubation, cells migrated to the lower surface of the membrane,
which was inhibited by TAS9 in a dose-dependent manner (Fig. 2B and D). In the wound healing
assay, cells in the control group rapidly moved into the scratched
area and almost covered the wounds, while cells treated with TAS9
had migrated to a lesser extent than those in control group after
72 h (Fig. 2C). TAS9 decreased the
distance of cell migration in a dose-dependent manner, and fully
inhibited cell migration at the highest concentration of 7
µM. These results validated that TAS9 inhibited
SMMC-7721-cell invasion and migration.
TAS9 inhibits cell growth via PI3K/Akt
and MAPK signaling pathways
In order to elucidate the underlying mechanisms of
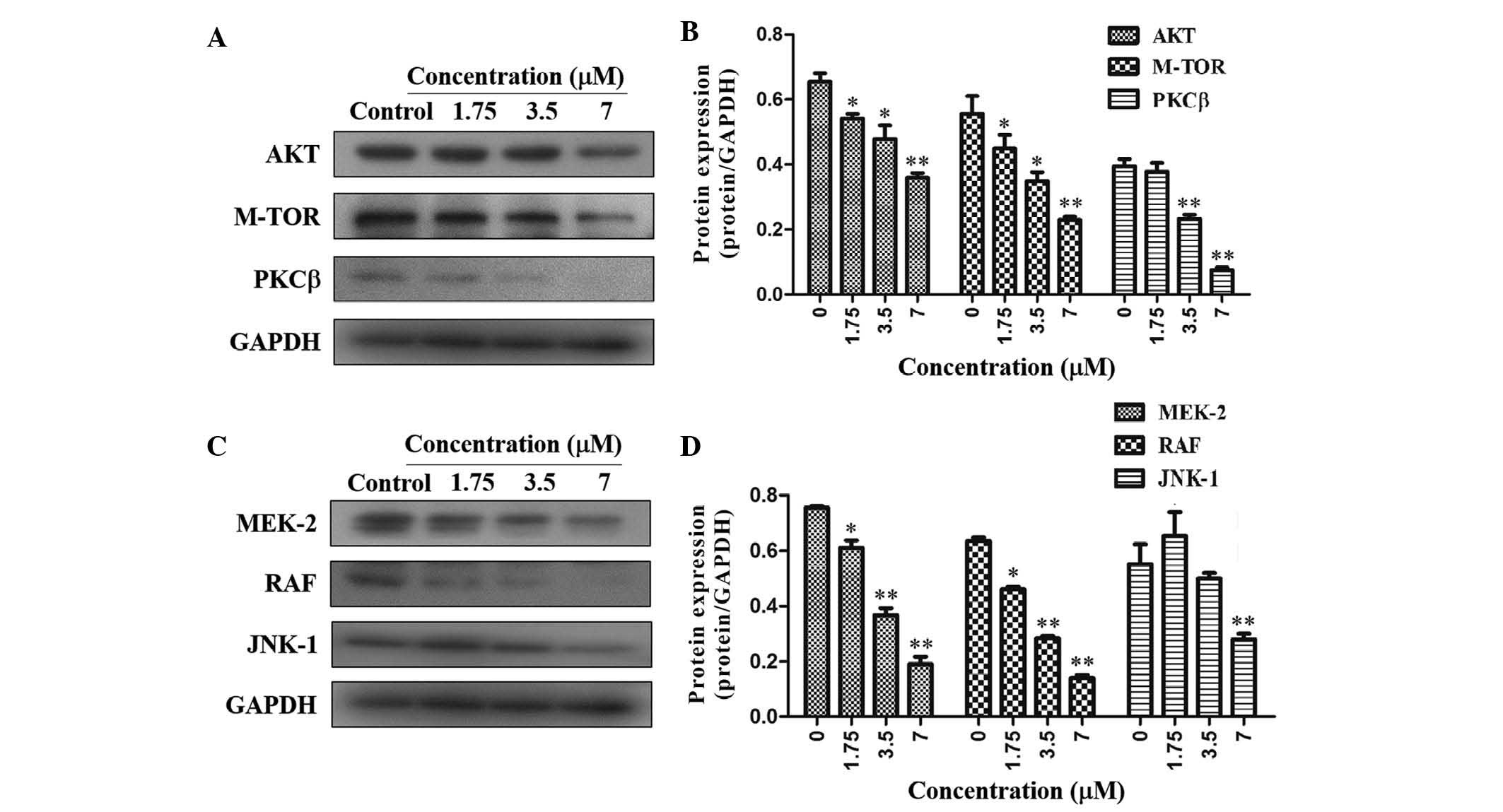
the anti-proliferative effects of TAS9, the expression levels of
PKCβ, Akt and mTOR, which are representative molecules of the
PI3K/Akt signaling pathways, were assessed. Of note, TAS9
significantly inhibited the expression of PKCβ, Akt and mTOR in
SMMC-7721 cells, suggesting that TAS9 may act via the PI3K/Akt
signaling pathway to inhibit cell proliferation (Fig. 3A and B). In addition, the
expression levels of MEK-2, RAF and JNK-1, which are representative
molecules of the MAPK signaling pathway, were assessed. The results
showed that the expression levels of MEK-2, RAF and JNK-1 were
significantly decreased in SMMC-7721 cells following treatment with
TAS9 (Fig. 3C and D). Therefore,
TAS-9 exerts its anti-proliferative effects by decreasing the
expression of PKCβ, Akt and mTOR in the PI3K/Akt signaling pathway
as well as decreasing the expression of MEK-2, RAF and JNK-1 in the
MAPK signaling pathway.
ETAS9 decreases cell migration-associated
signaling
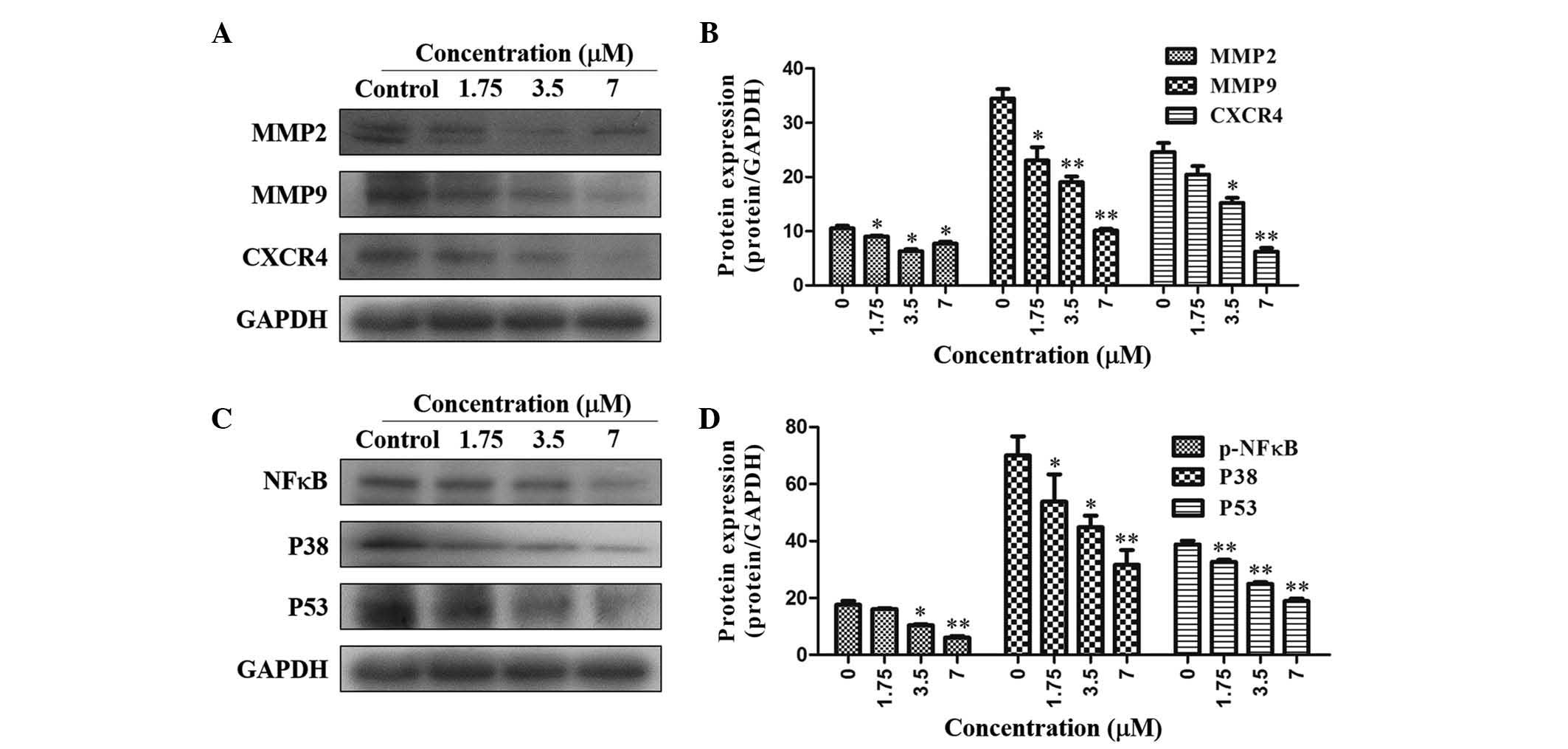
As shown in Fig. 4A
and B, TAS9 was able to significantly inhibit MMP-2 expression;
furthermore, MMP-9 expression was inhibited in a dose-dependent
manner, as compared with that in the control. Furthermore, the
expression of the cell migration-associated proteins CXCR4, NF-κB,
P38 and P53 was downregulated by treatment with TAS9 at the
concentrations of 1.75, 3.5 and 7 µM (Fig. 4C and D).
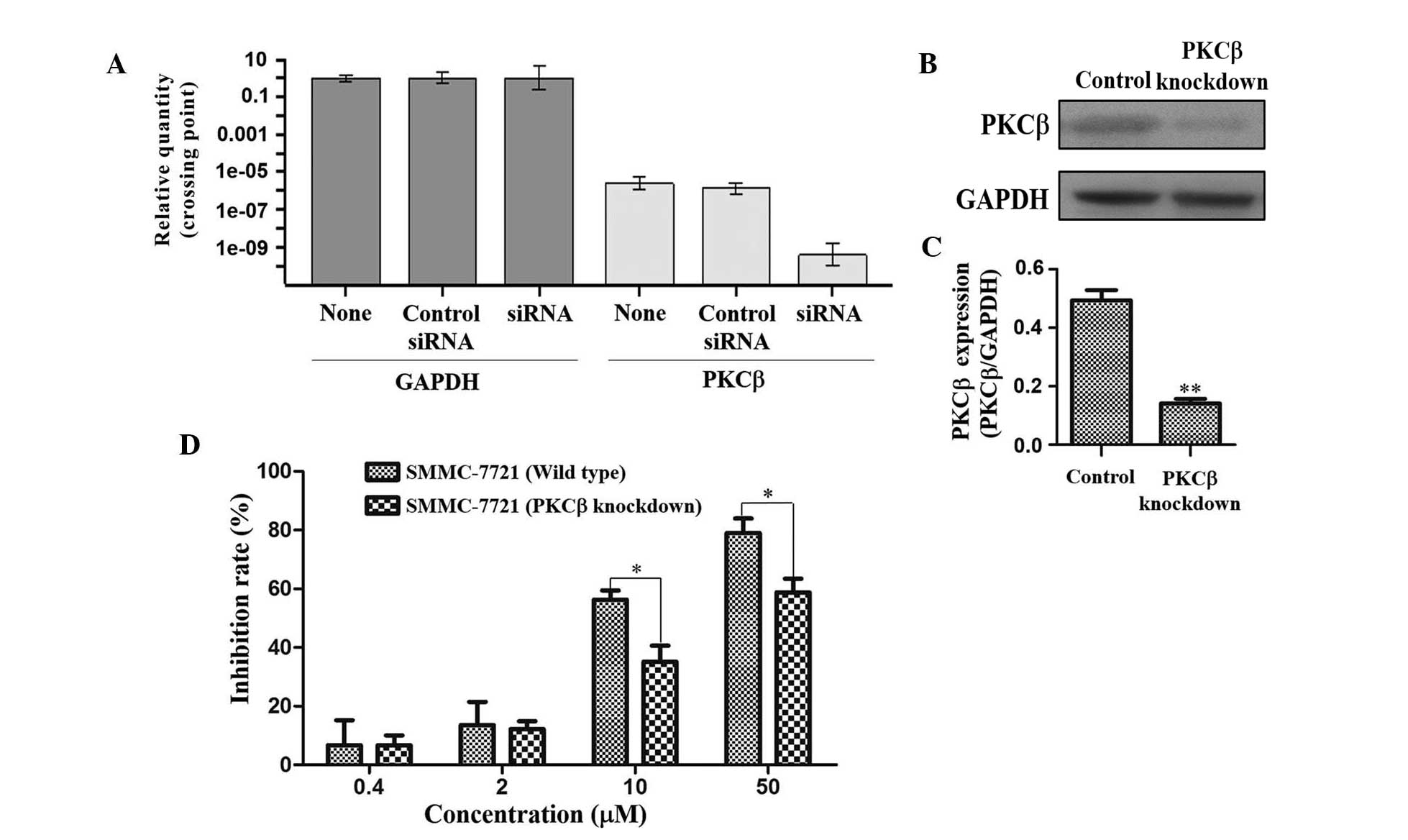
Knockdown of PKCβ
As PKCβ expression was most significantly
downregulated by TAS9 amongst all proteins assessed in the present
study (Fig. 3A and B),
siRNA-mediated knockdown of PKCβ was performed in SMMC-7721 cells
in order to further elucidate its role in the mechanism of action
of TAS9. The mRNA and protein expression levels of PKCβ were
quantified by RT-qPCR and western blot analyses in untreated,
control-transfected and PKCβ-knockdown SMMC-7721 cells. As shown in
Fig. 5A–C, PKCβ was selectively
knocked down in the SMMC-7721 cells. In order to validate the roles
of PKCβ in SMMC-7721 cells treated with TAS9, wild-type and
PKCβ-knockdown SMMC-7721 cells were treated with TAS9 at various
concentrations and subjected to an MTT assay. TAS9 inhibited the
growth of native as well as PKCβ-knockdown cells in a
dose-dependent manner; however, the inhibitory effects on the
SMMC-7721 PKCβ-knockdown cells were markedly reduced as compared
with those on the control cells (Fig.
5D). siRNA-mediated knockdown of PKCβ in SMMC-7721 cells
significantly attenuated the anti-proliferative effects of TAS9,
suggesting that PKCβ is involved in the mechanisms of action of
TAS9.
Discussion
In the present study, the anti-tumorigenic effects
of TAS9 on SMMC-7721 hepatocellular carcinoma cells were
investigated. The anti-neoplastic effects of TAS9 may be attributed
to the inhibition of cancer-associated biochemical mechanisms,
including inhibition of cell proliferation, migration and invasion,
as well as regulation of the corresponding signal transduction
pathways. The results of the present study demonstrated that TAS9
exerts its anti-tumorigenic effects via inhibiting cell
proliferation and migration in SMMC-7721 cells. TAS9 may have
important roles in reducing cell survival and proliferation, as
well as decreasing the migratory and invasive potential of
SMMC-7721 cells.
The molecular signaling pathways associated with
cellular growth are diverse and include the PI3K/AKT, MAPK and
PLCγ/PKC signaling pathways (17–19).
Upregulation of these pathways may cause aberrant cell
proliferation. Upon activation by calcium and diacylglycerol,
members of the PKC family can phosphorylate a diversity of protein
targets at serine and threonine moieties to activate molecular
signaling pathways (20,21). AKT regulates cell growth,
proliferation, migration and apoptosis by interacting with its
numerous downstream protein targets (17). mTOR belongs to the PI3K protein
family, and is an important kinase that can regulate cell growth
and proliferation (18). The
MAPK/ERK pathway is a junction point for numerous biochemical
signaling pathways, which regulates cell proliferation,
differentiation and development. JNK-1, MEK-2 and RAF are key
checkpoints in these pathways (21). Furthermore, in the MAPK/ERK
pathway, RAS binds to RAF and thereby activates to trigger a kinase
cascade involving the phosphorylation of MRK, followed by
phosphorylation of MAPK to affect cell growth and tumori-genesis
(23,24). The results of the present study
showed that TAS9 exerted its inhibitory effects by reducing the
expression of PKCβ, AKT, MEK-2, mTOR, JNK-1 and RAF, indicating
TAS9 may target the PI3K/AKT, PLCγ/PKC and MAPK signaling pathways
to suppress tumor progression.
The formation of metastasis mostly occurs at
advanced tumor stages and requires a series of sequential events
resulting in the translocation of cells from the primary tumor mass
to distant sites of the body to form secondary tumors; these steps
include the reduction of cell adhesion, as well as enhancement of
the invasive, proliferative and vessel-forming potential of the
cells (25,26). By contrast, interference with any
of these steps may prevent tumor migration and metastasis. In the
present study, TAS9 was demonstrated to exert inhibitory effects on
cell migration by downregulating the expression of proteins
associated with metastasis formation. MMP2 and MMP9 have key roles
in cancer-cell invasion and metastasis; hence, inhibiting the
expression of MMP2 and MMP9 is the most direct approach for
anti-metastatic therapies (2,27).
Furthermore, the expression of CXCR4 in cancer cells was linked to
metastasis, and its inhibition may therefore block tumor migration
(28). NF-κB has important roles
in numerous signaling pathways associated with cell proliferation
and migration, as well as the promotion and progression of cancer
(29,30). P53, which has anti-proliferative
and tumor-suppressive effects, is a frequently mutated gene in
human cancers; however, its overexpression may be correlated with
tumor metastasis, recurrence and poor prognosis (31). Inhibition of P38 was shown to block
the migration of epithelial cells (32). P53 as well as P38 are essential for
cell migration, fusion and proliferation. In the present study,
treatment with TAS9 reduced the protein expression levels of not
only MMP-2 and MMP-9, but also CXCR4, NF-κB, p53 and p38,
indicating that TAS9 inhibited cell migration.
PKCβ is important for cell survival and tumor
development in-vivo, and inhibition of PKCβ induces
apoptosis and stimulates tumor necrosis factor and NF-κB signaling
(33). The results of the present
study indicated a significant reduction in the protein expression
levels of PKCβ in SMMC-7721 cells following treatment with TAS9. To
further investigate the role of PKCβ, siRNA-mediated knockdown of
PKCβ in SMMC-7721 cells was performed, which significantly
attenuated the inhibitory effects of TAS9. The inhibitory effects
of TAS9 on the SMMC-7721 wild-type cells were markedly increased as
compared with those on the SMMC-7721 PKCβ-knockdown cells, which
suggested that PKCβ is an important target of TAS9.
In conclusion, the proliferative and migratory
potential of SMMC-7721 hepatocellular carcinoma cells was
suppressed by TAS9 via the MAPK and PI3K/AKT signaling pathways and
by downregulating MMP2, MMP9, CXCR4, NFκB, P38 and P53. In
addition, knocking down of PKCβ significantly decreased the
inhibitory effects of TAS9 on the proliferation of SMMC-7721 cells.
These results suggested that TAS9 is a potential anti-cancer agent,
which may be used for treating hepatocellular carcinoma.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant. nos. 81370088 and
81227802), the Fundamental Research Funds for the Central
Universities of Zhuizong, the Project of Shaanxi Star of Science
and Technology (grant. no. 2012KJXX-06) and the Supporting Plan of
Education Ministry's New Century Excellent Talents (grant. no.
NCET-13-0467).
References
|
1
|
Shen B, Chu ES, Zhao G, Man K, Wu CW,
Cheng JT, Li G, Nie Y, Lo CM, Teoh N, et al: PPARgamma inhibits
hepatocellular carcinoma metastases in-vivo and in mice. Br J
Cancer. 106:1486–1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yodkeeree S, Chaiwangyen W, Garbisa S and
Limtrakul P: Curcumin, demethoxycurcumin and bisdemethoxycurcumin
differentially inhibit cancer cell invasion through the
down-regulation of MMPs and uPA. J Nutr Biochem. 20:87–95. 2009.
View Article : Google Scholar
|
|
3
|
Zhen C, Chen L, Zhao Q, Liang B, Gu YX,
Bai ZF, Wang K, Xu X, Han QY, Fang DF, et al: Gankyrin promotes
breast cancer cell metastasis by regulating Rac1 activity.
Oncogene. 32:3452–3460. 2013. View Article : Google Scholar
|
|
4
|
Kim GD, Oh J, Park HJ, Bae K and Lee SK:
Magnolol inhibits angiogenesis by regulating ROS-mediated apoptosis
and the PI3K/AKT/mTOR signaling pathway in mES/EB-derived
endothelial-like cells. Int J Oncol. 43:600–610. 2013.PubMed/NCBI
|
|
5
|
Song MK, Kim YJ, Song M, Choi HS, Park YK
and Ryu JC: Polycyclic aromatic hydrocarbons induce migration in
human hepatocellular carcinoma cells (HepG2) through reactive
oxygen species-mediated p38 MAPK signal transduction. Cancer Sci.
102:1636–1644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hidalgo M and Eckhardt SG: Development of
matrix metal-loproteinase inhibitors in cancer therapy. J Natl
Cancer Inst. 93:178–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang XX, Fu ZY, Zhang Z, Miao C, Xu P, et
al: Microcystin-LR promotes melanoma cell invasion and enhances
matrix metal-loproteinase-2/-9 expression mediated by NFκB
activation. Environ Sci Technol. 46:11319–11326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Gao D, Guo K, Kang X, Jiang K, Sun
C, Li Y, Sun L, Shu H, Jin G, et al: Novel synergistic antitumor
effects of rapamycin with bortezomib on hepatocellular carcinoma
cells and orthotopic tumor model. BMC Cancer. 12:1662012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perdue G, Blomster RN, Blake DA and
Farnsworth NR: South-American plants II: Taspine isolation and
anti-inflammatory activity. J Pharm Sci. 68:124–126. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rollinger JM, Schuster D, Baier E,
Ellmerer EP, Langer T and Stuppner H: Taspine: Bioactivity-guided
isolation and molecular ligand-target insight of a potent
acetylcholinesterase inhibitor from Magnolia x soulangiana. J Nat
Prod. 69:1341–1346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porras-Reyes BH, Lewis WH, Roman J,
Simchowitz L and Mustoe TA: Enhancement of wound healing by the
alkaloid taspine defining mechanism of action. Proc Soc Exp Biol
Med. 203:18–25. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Itokawa H, Ichihara Y, Mochizuki M,
Enomori T, Morita H, Shirota O, Inamatsu M and Takeya K: A
cytotoxic substance from Sangre De Grado. Chem Pharm Bull.
39:1041–1042. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YM, He LC, Meng L, Luo WJ and Xu XM:
Suppression of tumor-induced angiogenesis by taspine isolated from
Radix et Rhizoma Leonticis and its mechanism of action in vivo.
Cancer Lett. 262:103–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang YM, Jiang Q, Wang N, Dai B, Chen Y
and He L: Effects of taspine on proliferation and apoptosis by
regulating caspase-3 expression and the ratio of Bax/Bcl-2 in A431
Cells. Phytother Res. 25:357–364. 2011.
|
|
16
|
Gao HP, Su P, Shi YL, Shen X, Zhang Y,
Dong J and Zhang J: Discovery of novel VEGFR-2 inhibitors. Part II:
Biphenyl urea incorporated with salicylaldoxime. Eur J Med Chem.
90:232–240. 2015. View Article : Google Scholar
|
|
17
|
Zinda MJ, Johnson MA, Paul JD, Horn C,
Konicek BW, Lu ZH, Sandusky G, Thomas JE, Neubauer BL, Lai MT, et
al: AKT-1,-2, and-3 are expressed in both normal and tumor tissues
of the lung, breast, prostate, and colon. Clin Cancer Res.
7:2475–2479. 2001.PubMed/NCBI
|
|
18
|
Hay N: The Akt-mTOR tango and its
relevance to cancer. Cancer Cell. 8:179–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faivre S, Kroemer G and Raymond E: Current
development of mTOR inhibitors as anticancer agents. Nat Rev Drug
Discov. 5:671–688. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Philippi A, Roschmann E, Tores F,
Lindenbaum P, Benajou A, Germain-Leclerc L, Marcaillou C, Fontaine
K, Vanpeene M, Roy S, et al: Haplotypes in the gene encoding
protein kinase c-beta (PRKCB1) on chromosome 16 are associated with
autism. Mol Psychiatr. 10:950–960. 2005. View Article : Google Scholar
|
|
21
|
Lintas C, Sacco R, Garbett K, Mirnics K,
Militerni R, Bravaccio C, Curatolo P, Manzi B, Schneider C, Melmed
R, et al: Involvement of the PRKCB1 gene in autistic disorder:
Significant genetic association and reduced neocortical gene
expression. Mol Psychiatry. 14:705–718. 2009. View Article : Google Scholar
|
|
22
|
Chuang SM, Wang IC and Yang JL: Roles of
JNK, p38 and ERK mitogen-activated protein kinases in the growth
inhibition and apoptosis induced by cadmium. Carcinogenesis.
21:1423–1432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo W, Wang X, Zheng L, Zhan Y, Zhang D,
Zhang J and Zhang Y: Brucine suppresses colon cancer cells growth
via mediating KDR signalling pathway. J Cell Mol Med. 17:1316–1324.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balan V, Leicht DT, Zhu J, Balan K, Kaplun
A, Singh-Gupta V, Qin J, Ruan H, Comb MJ and Tzivion G:
Identification of novel in vivo Raf-1 phosphorylation sites
mediating positive feedback Raf-1 regulation by extracellular
signal-regulated kinase. Mol Biol Cell. 17:1141–1153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lou L, Ye W, Chen Y, Wu S, Jin L, He J,
Tao X, Zhu J, Chen X, Deng A, et al: Ardipusilloside inhibits
survival, invasion and metastasis of human hepatocellular carcinoma
cells. Phytomedicine. 19:603–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adams LS, Phung S, Yee N, Seeram NP, Li L
and Chen S: Blueberry phytochemicals inhibit growth and metastatic
potential of MDA-MB-231 breast cancer cells through modulation of
the phosphatidylinositol 3-kinase pathway. Cancer Res.
70:3594–3605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen QY, Zheng Y, Jiao DM, Chen FY, Hu HZ,
Wu YQ, Song J, Yan J, Wu LJ and Lv GY: Curcumin inhibits lung
cancer cell migration and invasion through Rac1-dependent signaling
pathway. J Nutr Biochem. 25:177–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Stamatoyannopoulos G and Song CZ:
Down-regulation of CXCR4 by inducible small interfering RNA
inhibits breast cancer cell invasion in vivo. Cancer Res.
63:4801–4804. 2003.PubMed/NCBI
|
|
29
|
Shen HM and Tergaonkar V: NF kappaB
signaling in carcinogenesis and as a potential molecular target for
cancer therapy. Apoptosis. 14:348–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Banerjee S, Li Y, Rahman KMW,
Zhang Y and Sarkar FH: Down-regulation of notch-1 inhibits invasion
by inactivation of nuclear factor-kappaB, vascular endothelial
growth factor, and matrix metalloproteinase-9 in pancreatic cancer
cells. Cancer Res. 66:2778–2784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Connor PM, Jackman J, Jondle D, Bhatia
K, Magrath I and Kohn KW: Role of the p53 tumor-suppressor gene in
cell-cycle arrest and radiosensitivity of Burkitt's lymphoma cell
lines. Cancer Res. 53:4776–4780. 1993.PubMed/NCBI
|
|
32
|
Sharma GD, He J and Bazan HE: p38 and
ERK1/2 coordinate cellular migration and proliferation in
epithelial wound healing: Evidence of cross-talk activation between
MAP kinase cascades. J Biol Chem. 278:21989–21997. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Surdez D, Benetkiewicz M, Perrin V, Han
ZY, Pierron G, Ballet S, Lamoureux F, Rédini F, Decouvelaere AV,
Daudigeos-Dubus E, et al: Targeting the EWSR1-FLI1 oncogene-induced
protein kinase PKCβ abolishes ewing sarcoma growth. Cancer Res.
72:4494–4503. 2012. View Article : Google Scholar : PubMed/NCBI
|