Introduction
Asthma is a complex chronic airway inflammatory
disease, which is characterized by reversible airflow obstruction,
bronchial hyperresponsiveness, airway inflammation and airway
remodeling. Among these, airway remodeling, comprising a range of
structural alterations, including increased deposition of
extracellular matrix proteins (ECMs), epithelial denudation with
goblet cell metaplasia, angiogenesis and increased airway smooth
muscle (ASM) mass, has been proposed to result in persistent
airflow limitation and a decreased baseline lung function (1–3).
A number of studies have demonstrated that ASM may
participate in multiple processes associated with asthma (4,5). ASM
cells are considered to be the main cell type involved in bronchial
hyperresponsiveness due to their contractibility. The ASM not only
serves as the major target of inflammatory mediators in the
asthmatic inflammatory process, but also exerts pro-inflammatory
and immunomodulatory functions through expressing a host of cell
adhesion molecules, responding and secreting a myriad of cytokines
and growth factors and upregulating the expression of toll-like
receptors in ASM cells (6).
Furthermore, increases in the ASM mass have a key role in asthmatic
bronchial remodeling, and hyperplasia and hypertrophy of airway
smooth muscle cells are considered to be the primary cause of
airway obstruction (1,4,5). The
mechanisms accounting for ASM hyperplasia include increased
proliferation, reduced apoptosis and enhanced migration of
myofibroblasts within the ASM layer (4). Proliferation of ASM cells can be
induced by a variety of mitogens, including growth factors,
cytokines, inflammatory mediators and enzymes, such as
platelet-derived growth factor (PDGF), epidermal growth factor
(EGF), endothelin-1 and tryptase (4,7).
Inhibiting ASM-cell proliferation can be an effective approach for
the treatment of asthma. However, ASM remodeling is insensitive to
currently used asthma medications, which are usually effective in
controlling acute asthma exacerbation and bronchial inflammation
(8).
Flavonoids, commonly present in vegetables, nuts,
fruits, beverages and herbal remedies, are health-promoting and
disease-preventing dietary supplements (9). Chrysin (5,7-dihy-droxyflavone) is a
natural flavonoid, which is contained in medicinal herbs (10,11).
Previous studies showed that chrysin exerts multiple biological
activities, including anti-inflammatory, anti-proliferative and
anti-oxidative effects (12–14).
Chrysin is beneficial for asthma in numerous aspects (15,16);
however, the target cells and the mechanisms involved have remained
to be identified. The proliferation of ASM cells is involved in
various aspects of the pathogenesis of asthma (8,17).
The present study aimed to investigate whether chrysin affects
basal and PDGF-induced proliferation of human ASM cells (HASMCs) as
well as the possible underlying mechanisms.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from (Thermo Fisher Scientific,
Waltham, MA, USA). Chrysin (purity, 97%) was obtained from
Invitrogen-Gibco (Paisley, UK). Recombinant human PDGF-BB was
purchased from PeproTech (Rocky Hill, NJ, USA). Cell Counting Kit-8
(CCK-8; cat. no. CK04) and Annexin V-fluorescein isothiocyanate
(FITC) Apoptosis Detection kit (cat. no. KGA107) were purchased
from Dojindo (Kumamoto, Japan) and KeyGen Biotech (Nanjing, China),
respectively. The Total Protein Extraction kit (cat. no. KGP250)
was purchased from KeyGen Biotech. Rabbit polyclonal antibody
against p44/42 mitogen-activated protein kinase (MAPK)
extracellular signal-regulated kinase (ERK)1/2 (cat no. 9102) and
rabbit monoclonal antibody against phospho (p)-p44/42 MAPK (ERK1/2)
(Thr202/Tyr204; cat no. 4370) were from Cell Signaling Technology,
Inc. (Beverly, MA, USA). Mouse monoclonal antibody against GAPDH
(cat no. MB001) was purchased from Bioworld Technology (St. Louis
Park, MN, USA). Horseradish peroxidase (HRP)-conjugated goat
anti-rabbit immunoglobulin (Ig)G (H&L) (cat no. BS13278) and
HRP-conjugated goat anti-mouse IgG (H&L) (cat no. BS12478) were
obtained from Bioworld Technology (St. Louis Park, MN, USA). The
Bicinchoninic acid (BCA) Protein Assay kit (cat. no. P0012) and
Enhanced Chemiluminescence (ECL) Detection kit (cat. no. P1007-1)
were obtained from Beyotime Institute of Biotechnology (Nantong,
China) and Jinan Ubio Biological Technology (Jinan, China),
respectively.
Cell culture
HASMCs were purchased from Sciencell Research
Laboratories (cat. no. 3400; Carlsbad, CA, USA). The cells were
grown in DMEM medium containing 10% FBS, 100 IU/ml penicillin and
100 µg/ml streptomycin (Invitrogen-Gibco), and incubated at
37°C in a humidified 5% CO2 atmosphere (18). Cells at passage 4–8 were used in
all experiments.
Proliferation
HASMCs cultured in DMEM supplemented with
penicillin, streptomycin and 10% FBS were seeded in 96-well plates
at a density of 5,000 or 3,500 cells per well for treatments for 24
and 48 h, respectively. The cells were then serum-deprived in DMEM
containing 0.5% FBS for 24 h when they were ~60–70% confluent.
Cells were treated with chrysin (10, 20 or 40 µM) alone or
pre-treated with chrysin for 30 min prior to stimulation with
PDGF-BB (10 ng/ml). After 24 or 48 h, the Cell Counting kit-8 assay
was used to assess the number of viable cells. After the medium was
aspirated, CCK-8 solution (10 µl) diluted in serum-free DMEM
(100 µl) was added to each well and the cells were further
incubated at 37°C in the presence of 5% CO2 for 2 h. The
absorbance of the wells was measured at 450 nm using the Bio-Rad
iMark™ Microplate Reader (Bio-Rad Laboratories, Hercules, CA,
USA).
Apoptosis assay
An Annexin V-FITC Apoptosis Detection kit was
used to determine the percentage of apoptotic cells. Briefly, cells
were seeded in six-well plates, cultured as described above, and
growth-arrested in DMEM containing 0.5% FBS for 24 h when reaching
~70% confluence. The cells were then stimulated with PDGF-BB (10
ng/ml) with or without 30-min chrysin (20 µM) pre-treatment,
or chrysin (20 µM) alone for 24 h. The treated cells were
trypsinzed and washed with phosphate-buffered saline (PBS) twice,
and subsequently incubated with Annexin V-FITC and propidium iodide
(PI). Quantification of apoptosis and necrosis was performed using
flow cytometry (BD FACSCanto™ II; BD Biosciences, Franklin Lakes,
NJ, USA).
Western blot analysis
HASMCs were seeded in six-well plates at a density
of 2×105 cells/well and cultured as described above and
subsequently starved in DMEM containing 0.5% FBS overnight. The
growth-arrested cells were stimulated with PDGF-BB (10 ng/ml) for
30 min with or without 30-min chrysin (20 µM) pre-treatment,
or chrysin (20 µM) alone. After the treatments, cells were
immediately washed with ice-cold PBS twice and lysed in lysis
buffer containing 5 µl phosphatase inhibitors, 1 µl
protease inhibitors and 5 µl 100 mM
phenylmethanesulfonylfluoride in 1 ml buffer (Total Protein
Extraction kit). The lysates were centrifuged at 14,000 ×g for 15
min at 4°C to obtain the total cell extracts. The BCA Protein Assay
kit was used to determine the protein concentration. Equal amounts
of protein extracts (20 µg) were subjected to 10%
SDS-PAGEand transferred onto polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). Membranes were blocked in a
blocking buffer (5% non-fat milk, 20 mM Tris-HCl, 150 mM NaCl and
0.05% Tween-20; Biosharp, Hefei, China) at room temperature for 2 h
and then incubated with the primary antibodies, anti-GAPDH (1:5,000
dilution), anti-ERK1/2 (1:1,000 dilution) and anti-p-ERK1/2
(1:1,000 dilution) at 4°C overnight. Subsequently, membranes were
washed with 20 mM Tris-HCl, 150 mM NaCl and 0.05% Tween-20 (TBST)
three times, for 15 min each wash. Following this, the membranes
were incubated for 1.5 h with the HRP-conjugated goat anti-rabbit
IgG antibodies for ERK1/2 and p-ERK1/2, and the HRP-conjugated goat
anti-mouse IgG antibody for GAPDH. The membranes were then washed
with TBST 3 times, for 15 min each wash. Immunoreactive bands were
detected using ECL reagents. The intensity of bands was quantified
using the Bio-Rad Gel Doc/Chemi Doc Imaging System and Image Lab
software, version 4.0 (Bio-Rad Laboratories). GAPDH was used as an
internal control for protein loading.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Differences between groups were analyzed using the
two-tailed Student's t-test with SPSS software, version 19 (IBM
SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
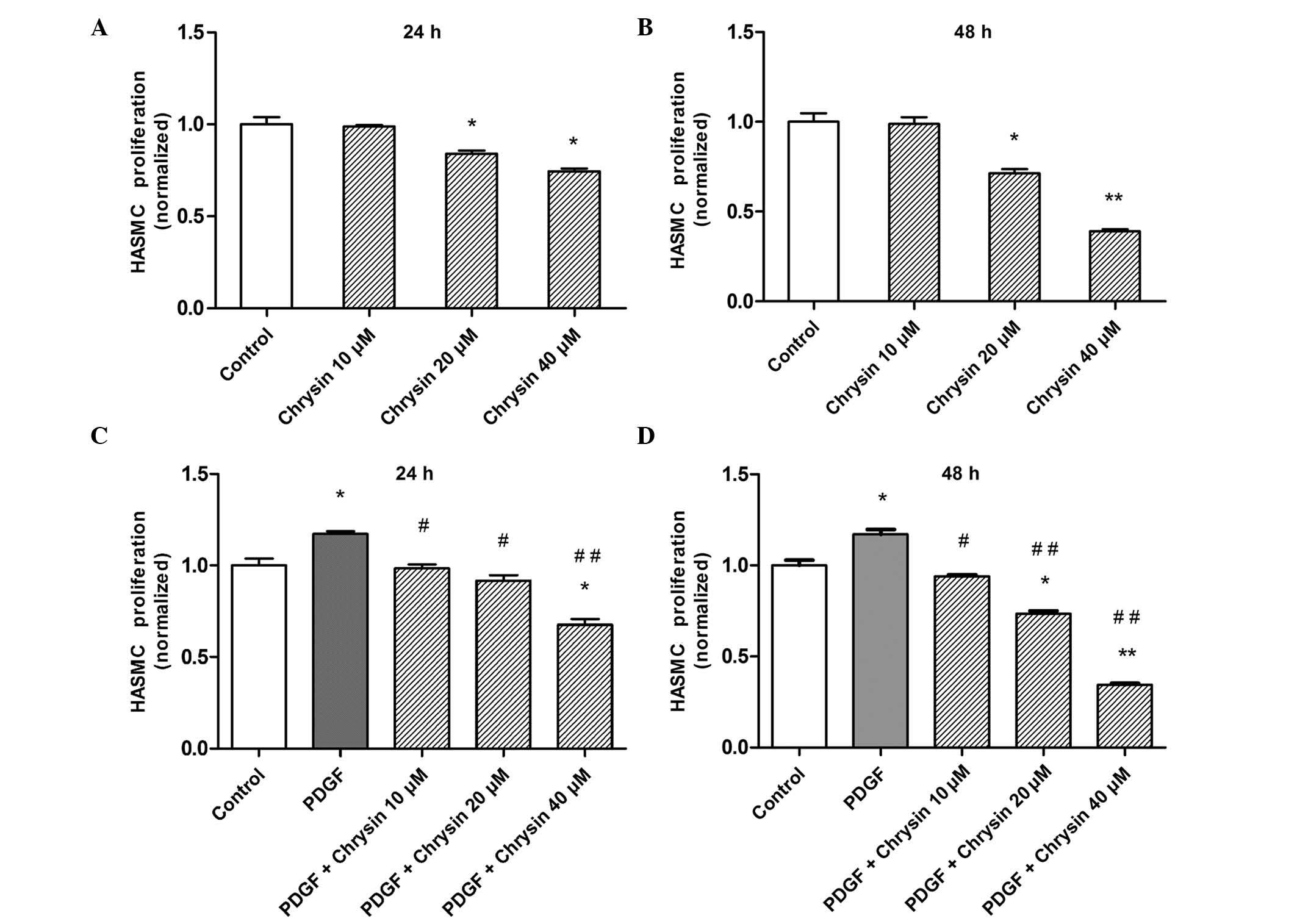
Chrysin inhibits PDGF-induced HASMC
proliferation
To clarify the mechanism of the beneficial effects
of chrysin in patients with asthma, the present study investigated
the role of chrysin on the basal as well as PDGF-induced
proliferation in HASMCs. Chrysin inhibited the proliferation of
HASMCs in a dose- and time-depended manner. Chrysin (20 or 40
µM for 24 or 48 h) significantly suppressed the
proliferation of HASMCs (Fig. 1A and
B). Subsequently, the present study examined whether chrysin
was able to block PDGF-BB-induced HASMC proliferation. The results
showed that PDGF-BB enhanced the proliferation, while chrysin
significantly abrogated the PDGF-BB induced proliferation of
HASMCs, resulting in a decreased number of viable cells compared
with that in the control group at high chrysin concentrations
(Fig. 1C and D).
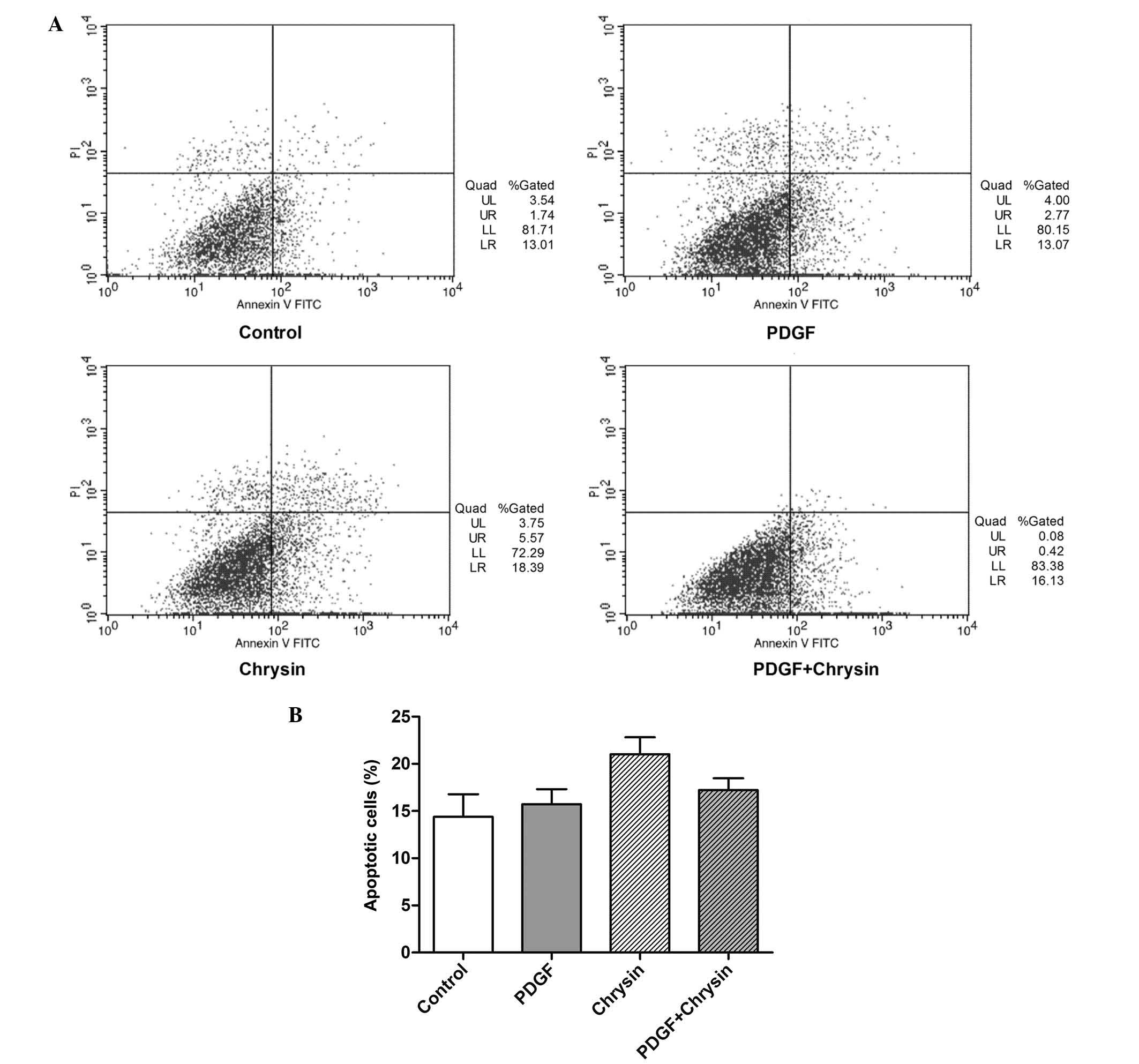
Chrysin does not significantly affect the
apoptotic rate of HASMCs
In order to investigate whether the
anti-proliferative effects of chrysin were attributable to the
induction of apoptosis, the present study next examined the effects
of chrysin on HASMC apoptosis. An Annexin V-FITC apoptosis assay
was used to determine the percentage of apoptotic cells. The
results showed that PDGF-BB had no effects on the apoptotic rate
compared with that in the control group. Treatment with chrysin
increased the apoptotic rate of the HASMCs, but not significantly.
However, chrysin had no effect on the apoptotic rate of cells
treated with PDGF-BB (Fig. 2).
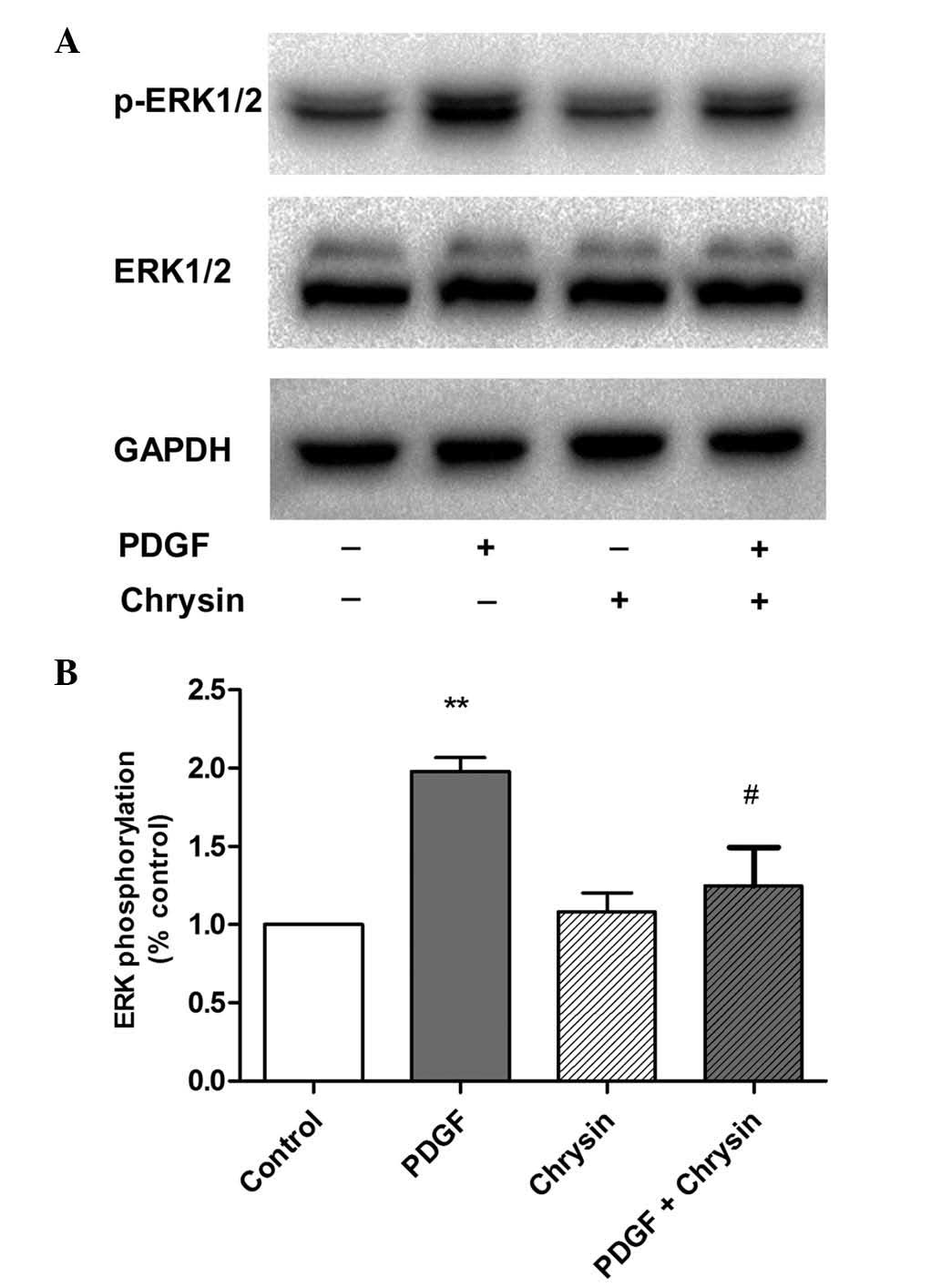
Chrysin abrogates PDGF-induced ERK1/2
phosphorylation
It has been established that ERK1/2 is activated
during PDGF-stimulated cell proliferation (19). Therefore, the present study
explored whether chrysin inhibited the proliferation of HASMCs by
reducing the phosphorylation of ERK1/2. As shown in Fig. 3, PDGF-BB significantly increased
the phosphorylation of ERK1/2 compared with that in the control
group, which was significantly abrogated by chrysin. However,
chrysin treatment alone did not affect the phoshorylation levels of
ERK1/2. In addition, neither PDGF-BB nor chrysin had any effect on
total ERK1/2 expression.
Discussion
The present study investigated the effects of
chrysin on HASMCs. The results showed that chrysin inhibited basal
and PDGF-induced proliferation and slightly, but not significantly,
enhanced apoptosis. This effect was associated with decreased
phosphorylation of ERK1/2.
Asthma is a complex chronic airway inflammatory
disease, which is accompanied with oxidative stress. Increased
production of reactive oxygen species has been identified to cause
airway inflammation, bronchial hyperreactivity, increased vascular
permeability, tissue injury and airway remodeling (1,20,21).
Previous studies have demonstrated that chrysin has an anti-oxidant
function (12,13). Chrysin may therefore be a suitable
medication for treating asthma. A previous study reported that
chrysin inhibits mast cell-derived allergic inflammatory reactions
in vivo and in vitro by blocking histamine release
and pro-inflammatory cytokine expression (16). Du et al (15) and Lee et al (22) have demonstrated that chrysin exerts
anti-asthmatic effects in animal models. However, the target cells
and mechanisms involved in chrysinmodulated amelioration of asthma
have remained elusive. Studies have confirmed that chrysin exerts
anti-proliferative effects on numerous cancer cell lines, including
A549 and PC-3 (23,24), and also significantly suppresses
the proliferation of human umbilical vein endothelial cells in a
concentration-dependent manner (25). Since ASM have a crucial role in
airway remodeling of asthma, primarily due to their increased mass,
the present study investigated whether chrysin had an effect on the
proliferation of HASMCs. The results revealed that chrysin
inhibited HASMCs proliferation in a dose- and time-depended manner.
PDGF-BB is a mitogen, which was extensively proved to potently
induce the proliferation of ASM cells in vitro as well as
in vivo (26–28). Furthermore, it has been reported
that chrysin can inhibit PDGF-induced proliferation in rat vascular
smooth muscle cell (19). In line
with this finding, the results of the present study also indicated
that chrysin suppressed the proliferation of HASMCs induced by
PDGF-BB. This capacity may be of potential clinical value in the
treatment of airway remodeling.
The increased ASM mass in patients with asthma is
associated with reduced apoptosis (4). Apoptosis has been observed to be
responsible for the growth inhibition by chrysin in A549 and HepG2
cells (23,29). The present study explored whether
chrysin inhibited the growth of ASM through inducing apoptosis. The
results showed that chrysin induced apoptosis of HASMCs, although
without statistical significance. This finding implied that the
anti-proliferative effects of chrysin were not primarily the
promotion of apoptosis.
ERK is required for HASMC proliferation and the
phosphorylation of ERK1/2 is enhanced during PDGF-induced ASM-cell
proliferation (30-32). Furthermore, chrysin has been
confirmed to restore PDGF-induced ERK1/2 phos-phorylation in rat
vascular smooth muscle cell (19).
To further elucidate the molecular mechanisms involved in the
anti-proliferative effects of chrysin, the present study assessed
whether chrysin inhibited the proliferation of HASMCs through the
ERK pathway. The results revealed that chrysin reduced PDGF-induced
ERK1/2 phosphorylation in HASMCs, indicating that chrysin may
inhibit PDGF-induced proliferation through the ERK signaling
pathway. However, chrysin alone had no effect on the basal
phosphorylation of ERK1/2 and total ERK1/2. This finding indicated
that other pathways contribute to the anti-proliferative effects of
chrysin. Phosphoinositol-3-kinase (P13K), p38-MAPK, c-Jun
N-terminal kinase, phospholipase C, protein kinase C, tyrosine
kinases and CCAAT/enhancer binding protein-a are also involved in
ASM-cell proliferation (7,33). Chrysin has been proved to induce
growth inhibition and reduce the phosphorylation of Akt in A549
cells (23). Decreased
proliferation induced by chrysin was observed in human and murine
melanoma cells, which is associated with the activation of p38-MAPK
(34). Therefore, the
abovementioned signaling molecules may be additional targets of
chrysin in ASM cells.
The PDGF - PDGF receptor (PDGFR) axis is involved in
ASM-cell proliferation (35,36).
It has remained elusive whether chrysin affects the interaction
between PDGF and PDGFR or the downstream signaling of this axis to
inhibit proliferation. A complex signaling network consisting of
numerous signaling molecules participates in the proliferative
process (33). Hence, it is
possible that chrysin has a role in other pathways. In addition,
asthma remodeling is associated with ASM migration (4). Previous studies suggested that
chrysin inhibited the migration of cancer cells, endothelial cells
and even vascular smooth muscle cells (19,37,38),
indicating that chrysin may also affect ASM-cell migration. Since
numerous other cell types, including inflammatory cells,
participate in the pathology of asthma, ASM may not be the sole
target cell type of chrysin in ameliorating asthma. Therefore,
additional studies are required to gain deeper insight into the
mechanisms of action of chrysin and provide data supporting its
clinical application.
In conclusion, the present study demonstrated that
chrysin inhibited PDGF-induced proliferation of HASMCs through
reducing the phosphorylation of ERK1/2, suggesting that chrysin mat
be a promising medication for controlling airway remodeling and
clinical manifestations of asthma.
Acknowledgments
This study was supported by the Natural Science
Foundation of Jiangsu Province (no. BK20131436).
References
|
1
|
Al-Muhsen S, Johnson JR and Hamid Q:
Remodeling in asthma. J Allergy Clin Immunol. 128:451–462. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergeron C and Boulet LP: Structural
changes in airway diseases: Characteristics, mechanisms,
consequences and pharmacologic modulation. Chest. 129:1068–1087.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manuyakorn W, Howarth PH and Holgate ST:
Airway remodelling in asthma and novel therapy. Asian Pac J Allergy
Immunol. 31:3–10. 2013.PubMed/NCBI
|
|
4
|
Ozier A, Allard B, Bara I, Girodet PO,
Trian T, Marthan R and Berger P: The pivotal role of airway smooth
muscle in asthma pathophysiology. J Allergy (Cairo).
2011:7427102011.
|
|
5
|
Bara I, Ozier A, Tunon de Lara JM, Marthan
R and Berger P: Pathophysiology of bronchial smooth muscle
remodelling in asthma. Eur Respir J. 36:1174–1184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Damera G, Tliba O and Panettieri RA Jr:
Airway smooth muscle as an immunomodulatory cell. Pulm Pharmacol
Ther. 22:353–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart AG: Airway wall remodelling and
hyperresponsiveness: Modelling remodelling in vitro and in vivo.
Pulm Pharmacol Ther. 14:255–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Girodet PO, Ozier A, Bara I, Tunon de Lara
JM, Marthan R and Berger P: Airway remodeling in asthma: New
mechanisms and potential for pharmacological intervention.
Pharmacol Ther. 130:325–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moon YJ, Wang X and Morris ME: Dietary
flavonoids: Effects on xenobiotic and carcinogen metabolism.
Toxicol In Vitro. 20:187–210. 2006. View Article : Google Scholar
|
|
10
|
Williams CA, Harborne JB, Newman M,
Greenham J and Eagles J: Chrysin and other leaf exudate flavonoids
in the genus pelargonium. Phytochemistry. 46:1349–1353. 1997.
View Article : Google Scholar
|
|
11
|
Rapta P, Misik V, Stasko A and Vrabel I:
Redox intermediates of flavonoids and caffeic acid esters from
propolis: An EPR spectroscopy and cyclic voltammetry study. Free
Radic Biol Med. 18:901–908. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho H, Yun CW, Park WK, Kong JY, Kim KS,
Park Y, Lee S and Kim BK: Modulation of the activity of
pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives.
Pharmacol Res. 49:37–43. 2004. View Article : Google Scholar
|
|
13
|
Lapidot T, Walker MD and Kanner J:
Antioxidant and prooxidant effects of phenolics on pancreatic
beta-cells in vitro. J Agric Food Chem. 50:7220–7225. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phan T, Yu XM, Kunnimalaiyaan M and Chen
H: Antiproliferative effect of chrysin on anaplastic thyroid
cancer. J Surg Res. 170:84–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du Q, Gu X, Cai J, Huang M and Su M:
Chrysin attenuates allergic airway inflammation by modulating the
transcription factors T-bet and GATA-3 in mice. Mol Med Rep.
6:100–104. 2012.PubMed/NCBI
|
|
16
|
Bae Y, Lee S and Kim SH: Chrysin
suppresses mast cell-mediated allergic inflammation: Involvement of
calcium, caspase-1 and nuclear factor-κB. Toxicol Appl Pharmacol.
254:56–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lommatzsch M: Airway hyperresponsiveness:
New insights into the pathogenesis. Semin Respir Crit Care Med.
33:579–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zha WJ, Qian Y, Shen Y, Du Q, Chen FF, Wu
ZZ, Li X and Huang M: Galangin abrogates ovalbumin-induced airway
inflammation via negative regulation of NF-κB. Evidence-Based
Complement Alternat Med. 2013:7676892013. View Article : Google Scholar
|
|
19
|
Lo HM, Wu MW, Pan SL, Peng CY, Wu PH and
Wu WB: Chrysin restores PDGF-induced inhibition on protein tyrosine
phosphatase and reduces PDGF signaling in cultured VSMCs. J Nutr
Biochem. 23:667–678. 2012. View Article : Google Scholar
|
|
20
|
Kirkham P and Rahman I: Oxidative stress
in asthma and COPD: Antioxidants as a therapeutic strategy.
Pharmacol Ther. 111:476–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sugiura H and Ichinose M: Oxidative and
nitrative stress in bronchial asthma. Antioxid Redox Signal.
10:785–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JY, Kim JM and Kim CJ: Flavones
derived from nature attenuate the immediate and late-phase
asthmatic responses to aerosolized-ovalbumin exposure in conscious
guinea pigs. Inflamm Res. 63:53–60. 2014. View Article : Google Scholar
|
|
23
|
Shao JJ, Zhang AP, Qin W, Zheng L, Zhu YF
and Chen X: AMP-activated protein kinase (AMPK) activation is
involved in chrysin-induced growth inhibition and apoptosis in
cultured A549 lung cancer cells. Biochem Biophys Res Commun.
423:448–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samarghandian S, Afshari JT and Davoodi S:
Chrysin reduces proliferation and induces apoptosis in the human
prostate cancer cell line Pc-3. Clinics (Sao Paulo). 66:1073–1079.
2011. View Article : Google Scholar
|
|
25
|
Ahn MR, Kunimasa K, Kumazawa S, Nakayama
T, Kaji K, Uto Y, Hori H, Nagasawa H and Ohta T: Correlation
between antiangiogenic activity and antioxidant activity of various
components from propolis. Mol Nutr Food Res. 53:643–651. 2009.
View Article : Google Scholar
|
|
26
|
Barnes PJ: Immunology of asthma and
chronic obstructive pulmonary disease. Nat Rev Immunol. 8:183–192.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirota JA, Ask K, Farkas L, Smith JA,
Ellis R, Rodriguez-Lecompte JC, Kolb M and Inman MD: In vivo role
of platelet-derived growth factor-BB in airway smooth muscle
proliferation in mouse lung. Am J Respir Cell Mol Biol. 45:566–572.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirst SJ, Barnes PJ and Twort CH: PDGF
isoform-induced proliferation and receptor expression in human
cultured airway smooth muscle cells. Am J Physiol. 270:L415–L428.
1996.PubMed/NCBI
|
|
29
|
Deng X, Zhao X, Lan Z, Jiang J, Yin W and
Chen L: Anti-tumor effects of flavonoids from the ethnic medicine
docynia delavayi (Franch.) Schneid And its possible mechanism. J
Med Food. 17:787–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu ZH, Wang YX, Song Y, Lu HZ, Hou LN, Cui
YY and Chen HZ: Up-regulation of KCa3.1 promotes human airway
smooth muscle cell phenotypic modulation. Pharmacol Res. 77:30–38.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Movassagh H, Shan L, Halayko AJ, Roth M,
Tamm M, Chakir J and Gounni AS: Neuronal chemorepellent Semaphorin
3E inhibits human airway smooth muscle cell proliferation and
migration. J Allergy Clin Immunol. 133:560–567. 2014. View Article : Google Scholar
|
|
32
|
Lee JH, Johnson PR, Roth M, Hunt NH and
Black JL: ERK activation and mitogenesis in human airway smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 280:L1019–L1029.
2001.PubMed/NCBI
|
|
33
|
Pelaia G, Renda T, Gallelli L, Vatrella A,
Busceti MT, Agati S, Caputi M, Cazzola M, Maselli R and Marsico SA:
Molecular mechanisms underlying airway smooth muscle contraction
and proliferation: Implications for asthma. Respir Med.
102:1173–1181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pichichero E, Cicconi R, Mattei M and
Canini A: Chrysin-induced apoptosis is mediated through p38 and Bax
activation in B16–F1 and A375 melanoma cells. Int J Oncol.
38:473–483. 2011.
|
|
35
|
Tallquist M and Kazlauskas A: PDGF
signaling in cells and mice. Cytokine Growth Factor Rev.
15:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gosens R, Stelmack GL, Dueck G, McNeill
KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J and
Halayko AJ: Role of caveolin-1 in p42/p44 MAP kinase activation and
proliferation of human airway smooth muscle. Am J Physiol Lung Cell
Mol Physiol. 291:L523–L534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang B, Huang J, Xiang T, Yin X, Luo X,
Huang J, Luo F, Li H, Li H and Ren G: Chrysin inhibits metastatic
potential of human triple-negative breast cancer cells by
modulating matrix metalloproteinase-10, epithelial to mesenchymal
transition and PI3K/Akt signaling pathway. J Appl Toxicol.
34:105–112. 2014. View
Article : Google Scholar
|
|
38
|
Lin CM, Shyu KG, Wang BW, Chang H, Chen YH
and Chiu JH: Chrysin suppresses IL-6-induced angiogenesis via
downregulation of JAK1/STAT3 and VEGF: An in vitro and in ovo
approach. J Agric Food Chem. 58:7082–7087. 2010. View Article : Google Scholar : PubMed/NCBI
|