Introduction
Gliomas are primary malignant brain tumors that
exhibit aggressive properties and are associated with a poor
prognosis in adults (1,2). Glioma cells are able to invade
healthy brain tissue and possess enhanced resistance to
radiotherapy- and chemotherapy-induced apoptosis (3). Temozolomide (TMZ) comprises an orally
bioavailable alkylating agent, which targets nuclear DNA and
generates nuclear DNA adducts that block the cell cycle leading to
apoptotic cell death (4).
Currently, adjuvant chemotherapy with TMZ is the standard treatment
for patients exhibiting primary glioblastoma multiforme (GBM)
(5,6). The chemotherapeutic regimen is
commonly administered as part of the treatment for GBM; however,
the majority of patients eventually develop resistance to
chemotherapy (7,8). Therefore, understanding the
underlying mechanisms that determine tumor response to TMZ is
considered to be crucial.
The Warburg effect is defined as the increased
utilization of glucose, which is produced by glycolysis (9). Normal tissue relies primarily on
mitochondrial oxidative phosphorylation to generate the energy
required for cellular processes; however, the majority of cancer
cells switch to aerobic glycolysis. This difference suggests that
targeting metabolic dependence may be a selective approach for the
treatment of clinical patients. Therefore, glycolytic inhibition
has been applied as an anticancer strategy in the context of
selected components of the glycolytic pathway, including glucose
transporters (Gluts), hexokinase, pyruvate kinase M2 and lactate
dehydrogenase-A (LDHA) (10). It
has previously been reported that LDHA expression and activity are
higher in Taxol-resistant breast cancer cells, versus those in
parental cells, and down-regulation of LDHA resensitized
Taxol-resistant cells (11)
indicating that dysregulated cellular metabolism may be a
therapeutic target for overcoming drug resistance in cancer
therapy.
The aim of the present study was to investigate the
glucose metabolism of glioma cells in response to treatment with
TMZ. The results demonstrated that glioma cells exhibited decreased
glucose uptake and lactate production.
Materials and methods
Cell culture and antibodies
The U251 and LN229 human glioma cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The cells were grown adherently in Dulbecco's modified
Eagle's medium (DMEM, Sigma-Aldrich China, Inc., Hong Kong, China)
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich China)
and 1% penicillin-streptomycin (Gibco Life Technologies, Grand
Island, NY, USA) and maintained in a humidified incubator
containing 5% CO2 at 37°C. The cells were maintained in
these culture conditions for all experiments. Monoclonal antibodies
used in the present study were as follows: Anti-β-actin (cat. no.
4967; Cell Signaling Technology, Inc., Danvers, MA, USA);
anti-Glut1 (cat. no. sc-7903; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and anti-phosphoinositide-dependent kinase-1
(PDK1; cat. no. 3820; Cell Signaling Technology, Inc.). A vector
containing Myc-DDK-tagged wild-type Glut1 (cat. no. RC222696), was
purchased from OriGene Technologies, Inc., Rockville, MD, USA).
Temozolomide (TMZ), 5-fluorouracil (5-FU) and paclitaxel (Taxol)
were purchased from Sigma-Aldrich China, Inc.
Generation of Taxol- and TMZ-resistant
cell lines
The Taxol- and TMZ-resistant cell lines were
generated according to previously described methods (11,12).
Briefly, U251 Taxol- or TMZ-resistant cells were developed from
parental U251 cells by treating the cells with gradually increasing
concentrations (0.1, 0.5, 1 and 2 µM) of Taxol or TMZ in
regular cell culture medium. Taxol- or TMZ-resistant single clones
were selected and pooled for the subsequent experiments. All
resistant cells were re-selected by treatments every four
weeks.
Cell viability assay
The Taxol- and TMZ-resistant cells were treated with
the indicated concentrations of Taxol, 5-FU or TMZ (0.3, 1 and
0.1%, respectively) for 48 h. The cells were seeded in a 48-well
plate, at a density of 1×104 cells/well in 0.2 ml DMEM
supplemented with 10% FBS. Following an overnight incubation under
the same culture conditions, each well was refreshed with DMEM for
a further 24 h. The cells were then treated with DMEM containing
various concentrations of Taxol, TMZ, 5-FU, Taxol + TMZ, or Taxol +
5-FU. The therapeutic agent-containing DMEM was refreshed after two
days, and the cells were incubated under the same conditions.
Finally, cell viability was assessed using an MTT assay
(Sigma-Aldrich, St. Louis, MO). Five hours prior to the end of the
incubation time, 20 µl MTT solution [5 mg/ml dissolved in
phosphate-buffered saline (PBS)] was added to each well containing
cells, followed by incubation at 37°C for 5 h. The media were
removed by aspiration with a needle and syringe, 200 µl
dimethylsulfoxide was added to each well and formazan crystals were
dissolved by continuous pipetting. Following incubation of the
plate at 37°C for 5 min, the absorbance was measured at 590 nm
using a plate reader (Multiskan™ GO; Thermo Fisher Scientific,
Waltham, MA, USA). The relative viability was obtained from the
absorbance at 590 nm of therapeutic agent-treated cells divided by
the absorbance of untreated cells at 590 nm. The cell viability
experiment was repeated three times.
Western blot analysis
The Taxol- and TMZ-resistant cells, as well as
Taxol-, 5-FU- or TMZ-treated Taxol- and TMZ-resistant cells were
harvested and lysed in a buffer containing 50 mM Tris-HCl (pH 7.5)
150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 1 mM phenylmethylsulfonyl
fluoride and Protease Inhibitor Cocktail (Sigma-Aldrich) for 20 min
on ice. Lysates were cleared by centrifugation at 16873 × g for 10
min at 4°C. Supernatants were collected and protein concentrations
were determined using the Bradford assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The proteins (50 µg) were then
separated by 10% SDS-PAGE and transferred to a nitrocellulose
membrane (Bio-Rad Laboratories, Inc.). After blocking in PBS
containing 5% non-fat dry milk for 1 h, the membranes were
incubated overnight at 4–8°C with the primary antibodies diluted in
PBS (1:5,000) with 5% non-fat dry milk. The membranes were
extensively washed with PBS, and incubated at room temperature for
1 h with horseradish peroxidase-conjugated secondary anti-mouse or
anti-rabbit secondary antibodies (1:2,000; cat. no. SA00001-2;
Proteintech Group, Inc., Chicago, IL, USA). Following additional
washes with PBS, the antigen-antibody complexes were visualized
using an enhanced chemiluminescence kit (Pierce Biotechnology,
Inc., Rockford, IL, USA).
Glucose uptake assay
U251 cells and TMZ-treated U251 cells were seeded in
12-well plates at a density of 1–3×105 cells/well.
Culture media was collected at 48 h and stored at −20°C until
assayed. Glucose uptake was measured using an Amplex Red
Glucose/Glucose Oxidase Assay kit (Molecular Probes Life
Technologies, Carlsbad, CA, USA). Absorbance was measured at 563 nm
using a SpectraMax M5 plate reader (Molecular Devices, Life
Technologies, Sunnyvale, CA, USA), and the results were normalized
to the quantity of total protein compared with that of the control
cells.
Lactate production assay
Lactate production in the cell culture supernatant
was detected using a Lactate Assay kit (BioVision, Inc., Milpitas,
CA, USA). Results were normalized to the quantity of total protein
compared with that of the U251 cells without TMZ treatment.
Plasmid DNA transfection
A vector containing wild-type Glut1 was purchased
from OriGene Technologies, Inc. (cat. no. RC222696). Transfection
was performed using the Oligofectamine Transfection reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions. Taxol-resistant U251 cells at a
density of 106 cells/well in 0.2 ml DMEM were
transfected with vector (1:1,000) and Glut1 (1:1,000). The cells
were collected 48 h post-transfection and prepared for further
analysis by western blotting and an MTT assay.
Statistical analysis
An unpaired Student's t-test was used to analyze the
data. SPSS version 21.0 (International Business Machines, Armonk,
NY, USA) was used for all statistical analyses. All values are
expressed as the mean ± standard error. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
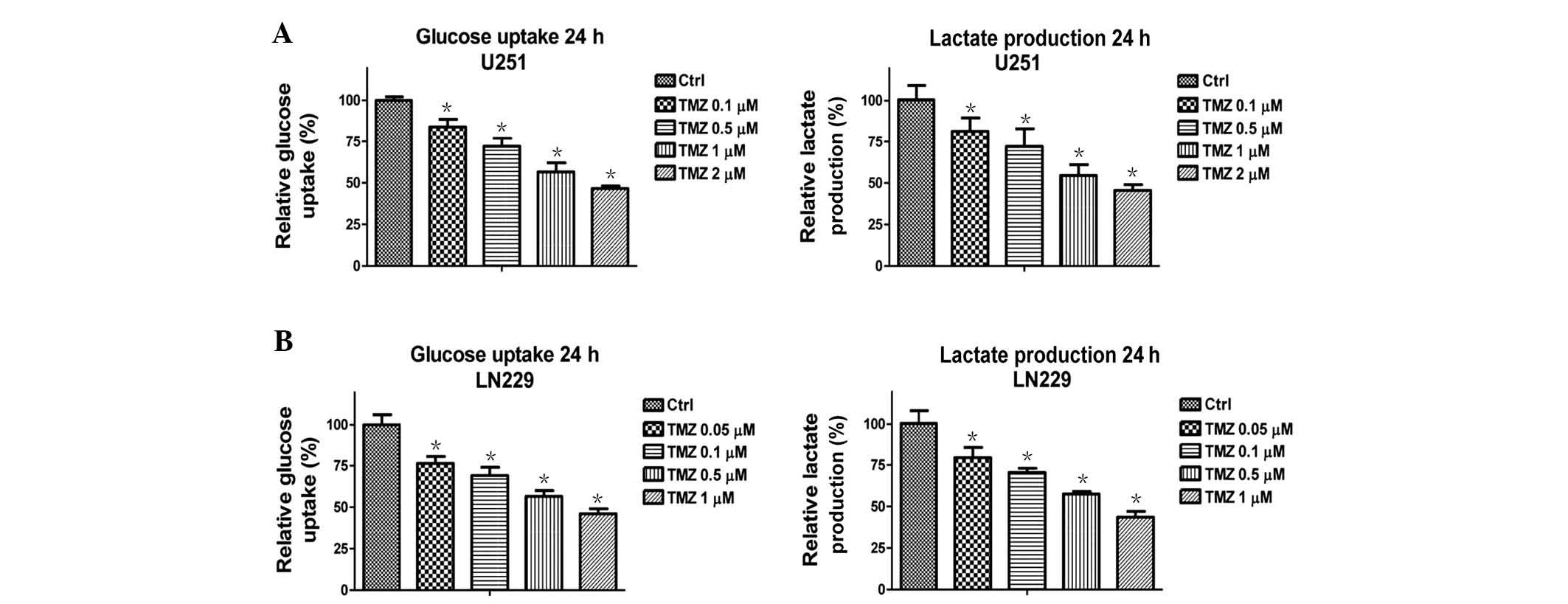
Treatment with TMZ decreases the glucose
metabolism of glioma cells
A previous study demonstrated that TMZ-resistant
glioma cells exhibited elevated mitochondrial function (13), thus suggesting that there is a link
between cellular metabolism and TMZ-induced cell apoptosis.
Therefore, the present study examined whether treatment of glioma
cells with TMZ regulates cellular metabolism. Notably, glucose
metabolism was significantly downregulated following treatment with
TMZ at various concentrations (Fig.
1). U251 (Fig. 1A) and LN229
(Fig. 1B) cells exhibited
decreased glucose uptake and lactate production following treatment
with TMZ at low-toxic concentrations for 48 h. These results
suggest that TMZ may be administered as an anticancer therapeutic
agent through the inhibition of glucose metabolism in glioma
cells.
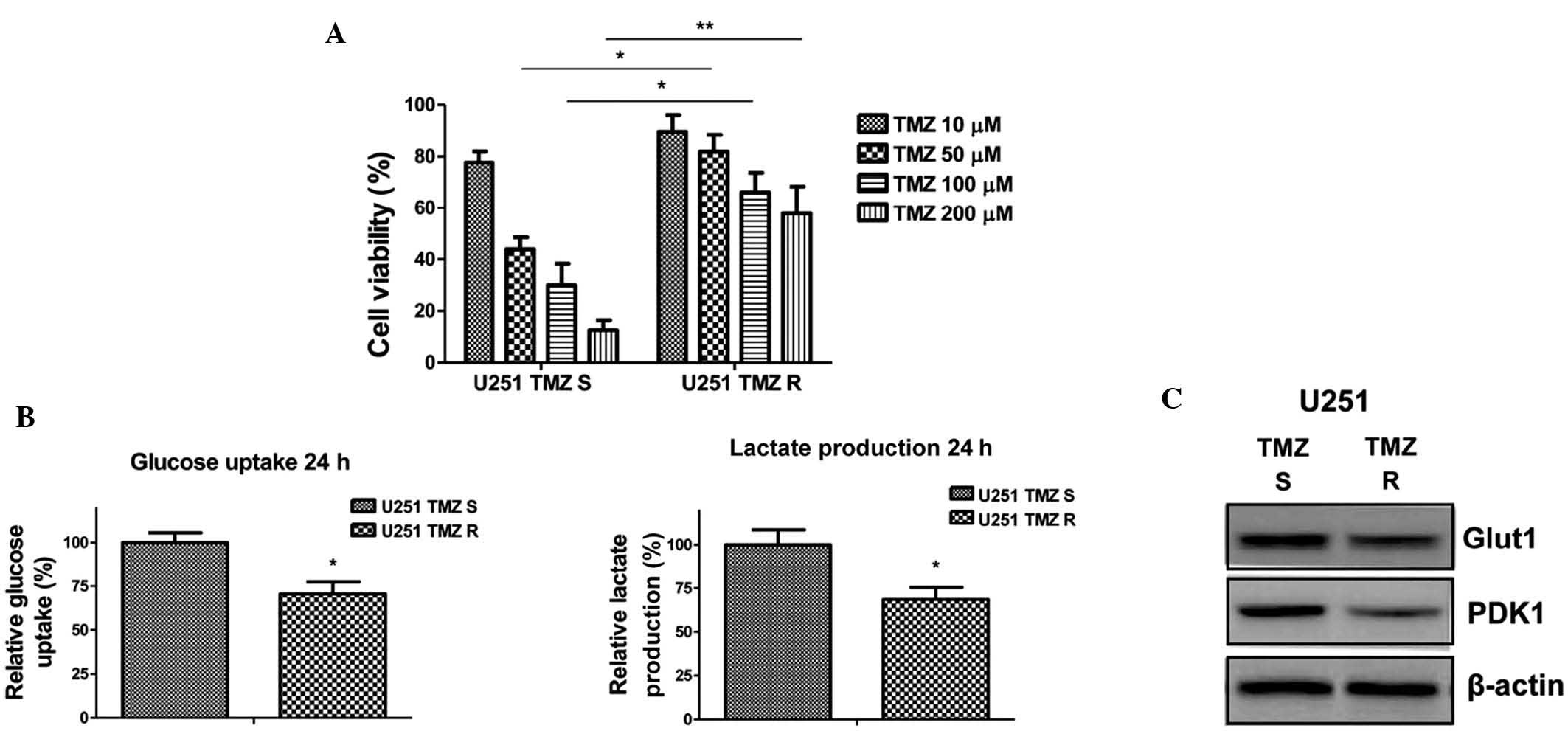
TMZ-resistant glioma cells exhibit
decreased glucose metabolism
To investigate the biological significance of
TMZ-induced downregulation of glucose metabolism, TMZ-resistant
cells were generated using U251 glioma cells, according to the
methods of a previous study (12).
Briefly, the cells were treated with gradually increasing
concentrations of TMZ in cell culture medium and the TMZ-resistant
cells were selected (14). After
successive treatments for three months, numerous resistant cell
clones were developed and pooled for the subsequent experiments. To
verify TMZ resistance, the parental cells and resistant pool cells
were treated with TMZ at various concentrations for 48 h. As was
expected, a cell viability assay demonstrated that TMZ-resistant
U251 cells tolerated markedly higher concentrations of TMZ, when
compared with TMZ-sensitive cells, which exhibited significant
inhibition of viability at 50, 100 and 200 µM (Fig. 2A). In addition, the glucose uptake
and lactate production of the TMZ-resistant U251 cells were
decreased (Fig. 2B). In addition,
the expression levels of key enzymes associated with glycolysis
were detected. Glut1 facilitates the transport of glucose across
the plasma membranes of mammalian cells, whereas PDK1 inactivates
pyruvate dehydrogenase (PDH), which catalyzes the oxidative
decarboxylation of pyruvate through the phosphorylation of PDH
(15). The present study
demonstrated that Glut1 and PDK1 were downregulated in
TMZ-resistant cells (Fig. 2C),
supporting the results presented in Fig. 2B demonstrating that TMZ-resistant
glioma cells exhibit decreased glucose metabolism.
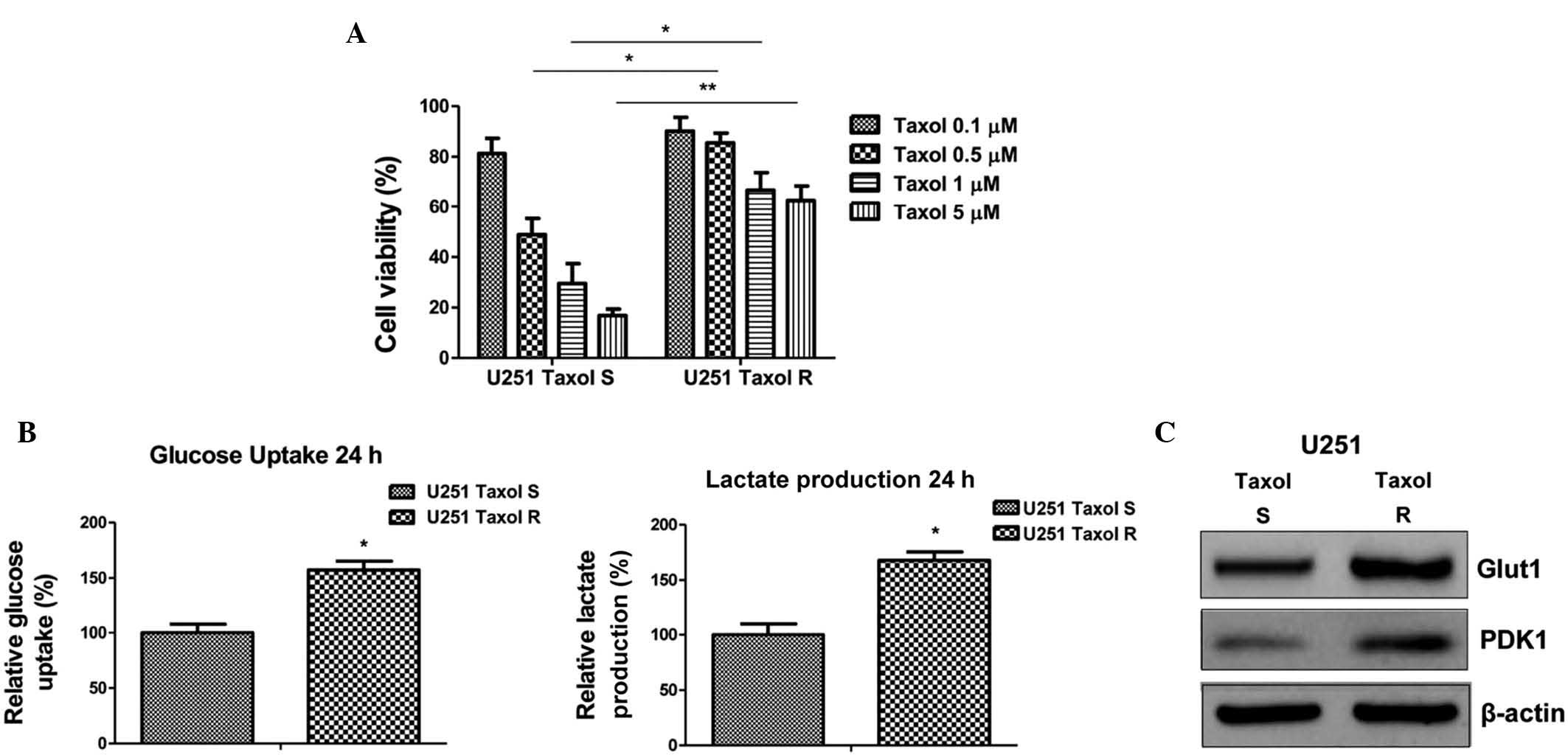
Taxol-resistant glioma cells display
upregulated glucose metabolism
The combination of Taxol with numerous anticancer
therapeutic agents has been assessed and clinically applied for the
treatment of patients with cancer. To investigate the association
between dysregulated glucose metabolism and chemosensitivity, a
Taxol-resistant cell line was generated from U251 cells.
Taxol-resistant U251 cells demonstrated a significant tolerance to
Taxol at the doses of 0.5 µM, 1 µM and 5 µM
(Fig. 3A). In addition, glucose
uptake and lactate production (Fig.
3B) were detected in Taxol-sensitive and -resistant glioma
cells. Taxol-resistant cells exhibited upregulated glucose
metabolism, as compared with the Taxol-sensitive cells, suggesting
that activated glucose metabolism may be an underlying mechanism of
resistance, and may be a therapeutic target for overcoming Taxol
resistance in patients with glioma. Concordantly, the expression
levels of Glut1 and PDK1 were observed to be upregulated in the
Taxol-resistant cells (Fig.
3C).
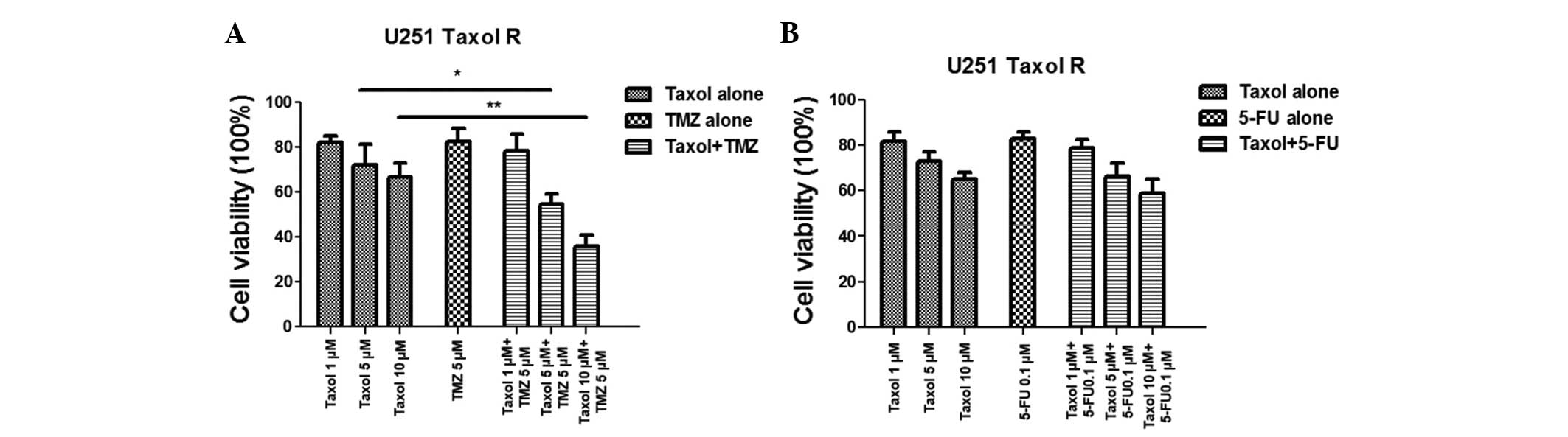
A combination of Taxol and TMZ exerts a
synergistic effect on Taxol-resistant cells via inhibition of
glucose metabolism
It has previously been reported that Taxol-resistant
breast cancer cells exhibit upregulated glucose metabolism, and
treatment with Taxol and glucose metabolism inhibitors demonstrated
a greater inhibitory effect on breast cancer (11). The present study observed that
treatment with TMZ inhibited glucose metabolism, however
Taxol-resistant glioma cells exhibited activated glucose
metabolism; therefore, it was hypothesized that a combination of
TMZ and Taxol may act synergistically to overcome Taxol resistance
via the inhibition of glucose metabolism. Experiments were designed
to examine whether treating Taxol-resistant glioma cells with TMZ
would result in synergistic therapeutic effects. As shown in
Fig. 4A, no significant inhibitory
effects were observed in the Taxol-resistant cells treated with
Taxol or TMZ alone; however, administering a combination of Taxol
and TMZ resulted in significant inhibition of cell viability. It
has previously been reported that treatment with 5-FU may induce
glucose metabolism in colon cancer cells and has similar regulatory
effects on glucose metabolism as Taxol (15). Therefore, in the present study,
resistant cells were treated with a combination of Taxol and 5-FU;
however, no improvement in the chemotherapeutic effects was noted
(Fig. 4B), indicating that the
synergistic inhibitory effects exerted by the combination of Taxol
and TMZ may be due to the inhibition of glucose metabolism.
To support this conclusion, the glucose uptake and
lactate production of the cells were determined following the
combined treatment (Fig. 5A). The
glucose uptake and lactate production were significantly decreased
following treatment with a combination of Taxol and TMZ. To further
verify the hypothesis, an expression vector containing Glut1 was
transiently transfected into the Taxol-resistant U251 cells
(Fig. 5B). Restoration of Glut1
expression recovered the glucose metabolism in U251 Taxol-resistant
cells (data not shown), and rendered cells resistant to the
combined treatment (Fig. 5B).
These data indicate that the inhibition of glucose metabolism may
specifically account for the synergistic effects of Taxol and TMZ
combined treatment in Taxol-resistant glioma cells.
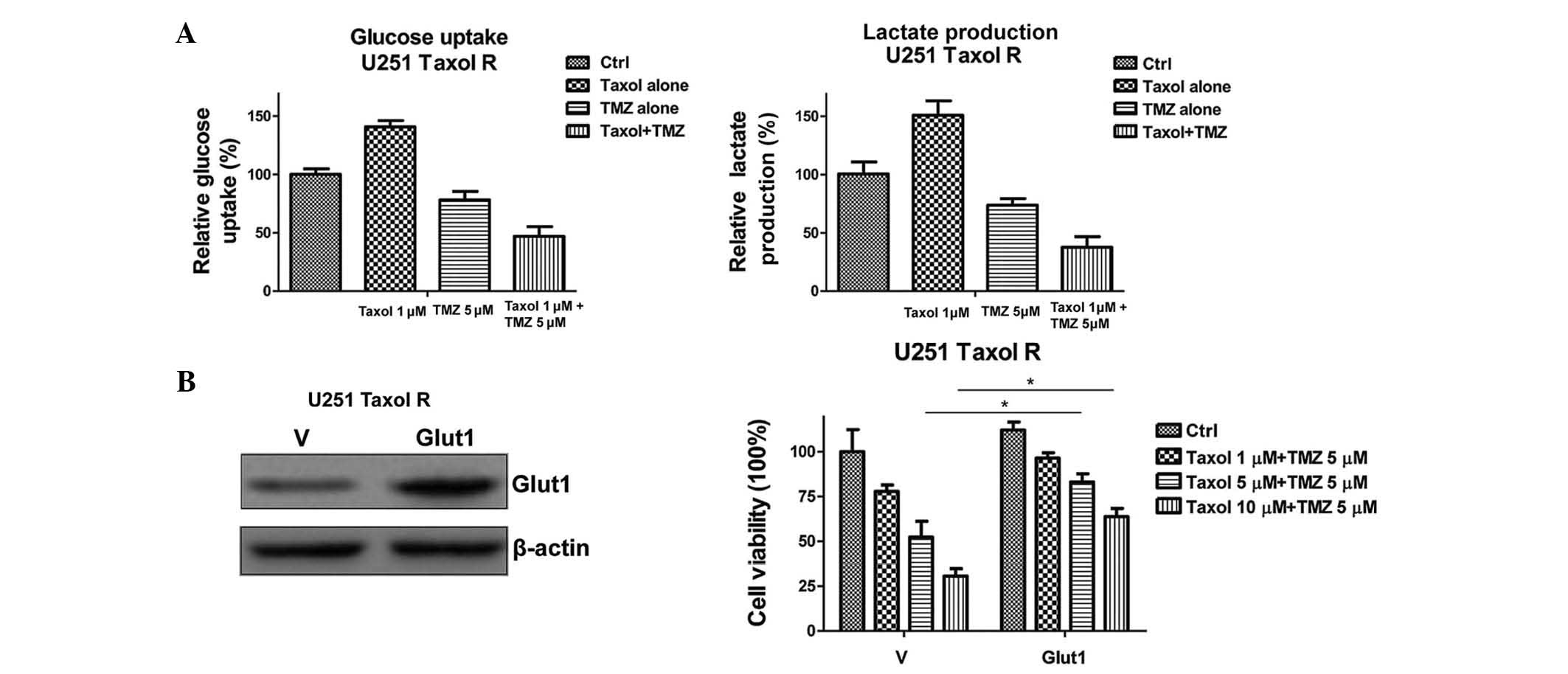 | Figure 5Overexpression of Glut1 renders
Taxol-resistant cells unsusceptible to Taxol and TMZ combined
treatment. (A) Treatment with a combination of Taxol and TMZ
inhibited glucose uptake (left) and lactate production (right).
Taxol-resistant U251 glioma cells were treated with Taxol alone at
1 µM; TMZ alone at 5 µM, or Taxol + TMZ for 48 h,
following which glucose uptake (left) and lactate production
(right) were measured. (B) Western blotting demonstrated that the
expression levels of Glut1 in U251 Taxol-resistant cells
transiently transfected with Glut1 were higher, as compared with
cells transfected with a vector control (left). U251
Taxol-resistant cells were transiently transfected with vector
control or wild-type Glut1 for 48 h, and were plated into 48-well
plates for the indicated treatments for 48 h, followed by
measurements of cell viability. β-actin served as a loading
control. Data are presented as the mean ± standard error of three
independent experiments. *P<0.05. TMZ, temozolomide;
Ctrl, control; R, resistant; Glut1, glucose transporter 1; V,
vector. |
Discussion
Paclitaxel (Taxol) is one of numerous tumoricidal
agents that target microtubules, and is widely administered as a
chemotherapeutic agent in the treatment of various types of human
cancer (16). The primary cellular
targets of Taxol are cancer cell microtubules, which are vital for
mitotic activity, cellular motility and proliferative capacity
(17). However, despite an
impressive initial clinical response, the majority of patients
eventually develop resistance to Taxol. Therefore, the ability to
reduce chemoresistance is particularly important for patients with
cancer. One known mechanism associated with cancer cell resistance
to Taxol, and other microtubule-stabilizing agents, is the high
expression of the membrane, P-glycoprotein, which functions as a
drug-efflux pump (18). Other
cellular mechanisms include cell cycle deregulation (19,20)
and alterations to tubulin structure (21,22).
A recent study reported that Taxol resistance in breast cancer
cells was mediated by the hippo signaling pathway (23). In addition, a correlation between
Aurora kinase A and Taxol resistance has been detected in a
xenograft model of breast cancer (24). However, the detailed mechanisms for
the development of Taxol resistance in glioma cells remain to be
elucidated.
Cancer cells use aerobic glycolysis, with reduced
mitochondrial oxidative phosphorylation, to metabolize glucose,
whereas healthy cells rely on oxidative phosphorylation. Therefore,
the metabolic dependencies of cancer cells may be exploited for
cancer treatment via inhibition of metabolic enzymes, in order to
improve the efficacy of cancer therapy and overcome therapeutic
resistance. It has been reported that numerous anticancer agents
may be combined with glycolysis inhibitors, such as 5-FU (25), cisplatin (26), daunorubicin (27), Taxol (28), trastuzumab (29) and doxorubicin (30), in order to exert a synergistic
inhibitory effect on cancer cells. In addition, the inhibition of
glucose uptake has been shown to sensitize cancer cells to
daunorubicin, and resensitize drug-resistant cells (27). A previous study demonstrated that
targeting dysregulated glucose metabolism in breast cancer cells
led to reduced trastuzumab resistance (29). Furthermore, in vivo
experiments demonstrated that 2-deoxy-D-glucose increased the
efficacy of adriamycin and paclitaxel in human osteosarcoma and
non-small cell lung cancers (31).
However, the detailed mechanisms for the efficacy of combining
chemotherapeutic agents with glycolysis inhibitors remains
unclear.
The present study reported that a combination
treatment with TMZ and Taxol sensitized Taxol-resistant glioma
cells via the inhibition of glucose metabolism. Significant
inhibition of glucose metabolism was observed in the TMZ-resistant
cells, whereas increased glucose metabolism was observed in the
Taxol-resistant cells. This phenotype triggered the investigation
of the synergistic effects of a combination of these two
chemotherapeutic agents. It has been reported that inhibition of
glucose metabolism by oxamate resensitizes Taxol-resistant breast
cancer cells to Taxol (11). Since
TMZ was able to inhibit glucose metabolism, the present results
demonstrate that treatment with TMZ enhances the cytotoxic effects
on Taxol-resistant glioma cells by inhibiting glucose metabolism. A
previous study reported that 5-FU treatment in cancer cells
activated glucose uptake and lactate production (15). Conversely, in the present study, as
compared with the combination of TMZ and Taxol, 5-FU treatment did
not exert an improved inhibitory effect on glioma cells when
combined with Taxol, thus suggesting that the inhibition of glucose
metabolism may be the mechanism for the synergistic effect of TMZ
and Taxol on Taxol-resistant cells. In addition, recovering the
glycolysis signaling pathway by exogenous overexpression of Glut1
facilitated glioma cells with obtaining resistance to Taxol,
indicating that dysregu-lated glucose metabolism may be the
underlying mechanism of the synergistic effects of TMZ and Taxol.
However, the detailed mechanisms are currently being investigated.
The aim of future studies will be to screen additional
chemotherapeutic agents and assess increasingly efficient
chemotherapeutic strategies, and in vivo experiments will be
performed to verify the results of animal models.
In conclusion, the present study provides a novel
perspective on the synergistic effects of combined chemotherapeutic
agents, and contributes to the development of clinical strategies
for the treatment of patients with glioma.
References
|
1
|
Huse JT, Holland E and DeAngelis LM:
Glioblastoma: Molecular analysis and clinical implications. Annu
Rev Med. 64:59–70. 2013. View Article : Google Scholar
|
|
2
|
Tanase CP, Enciu AM, Mihai S, Neagu AI,
Calenic B and Cruceru ML: Anti-cancer therapies in high grade
gliomas. Curr Proteomics. 10:246–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas RP, Recht L and Nagpal S: Advances
in the management of glioblastoma: The role of temozolomide and
MGMT testing. Clin Pharmacol. 5:1–9. 2013.PubMed/NCBI
|
|
5
|
Fiorentino A, Chiumento C, Caivano R,
Cozzolino M, Pedicini P and Fusco V: Adjuvant radiochemotherapy in
the elderly affected by glioblastoma: Single-institution experience
and literature review. Radiol Med. 118:870–881. 2013.In Italian.
View Article : Google Scholar
|
|
6
|
Sengupta S, Marrinan J, Frishman C and
Sampath P: Impact of temozolomide on immune response during
malignant glioma chemotherapy. Clin Dev Immunol. 2012:8310902012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chamberlain MC: Temozolomide: Therapeutic
limitations in the treatment of adult high-grade gliomas. Expert
Rev Neurother. 10:1537–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang G, Wei ZP, Pei DS, Xin Y, Liu YQ and
Zheng JN: A novel approach to overcome temozolomide resistance in
glioma and melanoma: Inactivation of MGMT by gene therapy. Biochem
Biophys Res Commun. 406:311–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z,
Fodstad O, Riker AI, Kamarajugadda S, Lu J, Owen LB, et al: Warburg
effect in chemosensitivity: Targeting lactate dehydrogenase-A
re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer.
9:332010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan Q, Yang XJ, Wang HM, Dong XT, Wang W,
Li Y and Li JM: Chemoresistance to temozolomide in human glioma
cell line U251 is associated with increased activity of
O6-methylguanine-DNA methyltransferase and can be overcome by
metronomic temo-zolomide regimen. Cell Biochem Biophys. 62:185–191.
2012. View Article : Google Scholar
|
|
13
|
Oliva CR, Moellering DR, Gillespie GY and
Griguer CE: Acquisition of chemoresistance in gliomas is associated
with increased mitochondrial coupling and decreased ROS production.
PLoS One. 6:e246652011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujiwara S, Kawano Y, Yuki H, Okuno Y,
Nosaka K, Mitsuya H and Hata H: PDK1 inhibition is a novel
therapeutic target in multiple myeloma. Br J Cancer. 108:170–178.
2013.PubMed/NCBI
|
|
15
|
Shin YK, Yoo BC, Hong YS, Chang HJ, Jung
KH, Jeong SY and Park JG: Upregulation of glycolytic enzymes in
proteins secreted from human colon cancer cells with 5-fluorouracil
resistance. Electrophoresis. 30:2182–2192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yusuf RZ, Duan Z, Lamendola DE, Penson RT
and Seiden MV: Paclitaxel resistance: Molecular mechanisms and
pharmacologic manipulation. Curr Cancer Drug Targets. 3:1–19. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of Taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Donnenberg VS and Donnenberg AD: Multiple
drug resistance in cancer revisited: The cancer stem cell
hypothesis. J Clin Pharmacol. 45:872–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kavallaris M, Kuo DY, Burkhart CA, Regl
DL, Norris MD, Haber M and Horwitz SB: Taxol-resistant epithelial
ovarian tumors are associated with altered expression of specific
beta-tubulin isotypes. J Clin Invest. 100:1282–1293. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan M, Jing T, Lan KH, Neal CL, Li P, Lee
S, Fang D, Nagata Y, Liu J, Arlinghaus R, et al: Phosphorylation on
tyrosine-15 of p34(Cdc2) by ErbB2 inhibits p34(Cdc2) activation and
is involved in resistance to taxol-induced apoptosis. Mol Cell.
9:993–1004. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martello LA, Verdier-Pinard P, Shen HJ, He
L, Torres K, Orr GA and Horwitz SB: Elevated levels of microtubule
destabilizing factors in a Taxol-resistant/dependent A549 cell line
with an alpha-tubulin mutation. Cancer Res. 63:1207–1213.
2003.PubMed/NCBI
|
|
22
|
Panda D, Miller HP, Banerjee A, Ludueña RF
and Wilson L: Microtubule dynamics in vitro are regulated by the
tubulin isotype composition. Proc Natl Acad Sci USA.
91:11358–11362. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lai D, Ho KC, Hao Y and Yang X: Taxol
resistance in breast cancer cells is mediated by the hippo pathway
component TAZ and its downstream transcriptional targets Cyr61 and
CTGF. Cancer Res. 71:2728–2738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Tang K, Zhang H, Zhang Y, Zhou W and
Chen X: Function of Aurora kinase A in Taxol-resistant breast
cancer and its correlation with P-gp. Mol Med Rep. 4:739–746.
2011.PubMed/NCBI
|
|
25
|
Tong J, Xie G, He J, Li J, Pan F and Liang
H: Synergistic antitumor effect of dichloroacetate in combination
with 5-fluorouracil in colorectal cancer. J Biomed Biotechnol.
2011:7405642011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo W, Zhang Y, Chen T, Wang Y, Xue J,
Zhang Y, Xiao W, Mo X and Lu Y: Efficacy of RNAi targeting of
pyruvate kinase M2 combined with cisplatin in a lung cancer model.
J Cancer Res Clin Oncol. 137:65–72. 2011. View Article : Google Scholar
|
|
27
|
Cao X, Fang L, Gibbs S, Huang Y, Dai Z,
Wen P, Zheng X, Sadee W and Sun D: Glucose uptake inhibitor
sensitizes cancer cells to daunorubicin and overcomes drug
resistance in hypoxia. Cancer Chemother Pharmacol. 59:495–505.
2007. View Article : Google Scholar
|
|
28
|
Liu Y, Cao Y, Zhang W, Bergmeier S, Qian
Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, et al: A small-molecule
inhibitor of glucose transporter 1 downregulates glycolysis,
induces cell-cycle arrest, and inhibits cancer cell growth in vitro
and in vivo. Mol Cancer Ther. 11:1672–1682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Liu H, Liu Z, Ding Y, Ledoux SP,
Wilson GL, Voellmy R, Lin Y, Lin W, Nahta R, et al: Overcoming
trastuzumab resistance in breast cancer by targeting dysregulated
glucose metabolism. Cancer Res. 71:4585–4597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakano A, Tsuji D, Miki H, Cui Q, El Sayed
SM, Ikegame A, Oda A, Amou H, Nakamura S, Harada T, et al:
Glycolysis inhibition inactivates ABC transporters to restore drug
sensitivity in malignant cells. PLoS One. 6:e272222011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hadzic T, Aykin-Burns N, Zhu Y, Coleman
MC, Leick K, Jacobson GM and Spitz DR: Paclitaxel combined with
inhibitors of glucose and hydroperoxide metabolism enhances breast
cancer cell killing via H2O2-mediated
oxidative stress. Free Radic Biol Med. 48:1024–1033. 2010.
View Article : Google Scholar : PubMed/NCBI
|