Introduction
Spinal cord injury (SCI) is predominantly caused by
accidents associated with falls, vehicle collisions and sport.
Every year there are ~12,000 novel cases of SCI reported in the USA
(1) and 60,000 novel cases in
China, which represents the greatest incidence world-wide (2). SCI may lead to paraplegia or
quadriplegia and patients may be permanently physically disabled
(3,4). Patients with SCI are often confined
to a wheelchair (5). Recent
imaging studies have been developed for predicting the outcomes for
patients with SCI (4). Further
investigations into the mechanisms for regeneration and functional
restoration of patients with SCI are required. Recent advances in
neuroscience research have provided novel insight into the
rehabilitation of patients with SCI. A number of rehabilitative,
cellular and molecular therapies have been tested, using animal
models and clinical trials (3,6).
SCI is a form of central nervous system (CNS)
trauma. Regenerative mechanisms of the CNS are typically suppressed
in response to a number of extrinsic and intrinsic factorsincluding
Nogo, glial scars and chondroitin sulfate proteoglycan activity
(7). Phospholipase A2
(PLA2) mediates multiple injury mechanisms following SCI
and may represent a novel and efficient strategy for inhibiting a
number of injury pathways that occur following SCI (8). Inflammation is one of the
consequences of CNS trauma (9).
Histone H3K27me3 demethylation of PLA2 may regulate
acute inflammatory responses and improve the blood-spinal cord
barrier following SCI (10).
Immune cells, including macrophages and B- and T cells, may protect
and repair the injured CNS, and the latter two are capable of
secreting the bio-active form of brain-derived neurotrophic factor
(11). Previous studies have
demonstrated that the CNS is associated with other diseases,
including hypertension, cardiovascular diseases (12,13)
and cancers (14,15). However, the underlying mechanisms
of SCI development and regeneration have remained to be fully
elucidated.
Recent bioinformatic analyses have explored the
genetic processes and molecular mechanisms underlying SCI. Siebert
et al (16) analyzed the
cellular response of thoracic propriospinal neurons and the
regenerative ability following low thoracic complete SCI using the
gene expression profile of GSE20907. Lai et al (17) identified a number of SCI-associated
pathways, including cell cycle, immune response and olfactory
transduction. Jin et al (18) found that cell cycle and immune
system-associated pathways, as well as oxidative phosphorylation
and CNS disease signaling pathways are important in the development
of SCI. However, Lai et al (17) demonstrated that the identification
of SCI-associated genes is inconsistent due to the different
criteria used for analyzing differentially expressed genes (DEGs).
Furthermore, changes in gene expression over time have not been
investigated.
Therefore, using the expression profile GSE20907,
the present study analyzed time-dependent changes of SCI-associated
DEGs with a cutoff criterion of P<0.01 and Fold-changes of gene
expression (log2 FC) ≥1. In addition, the sub-pathways
in which the DEGs were enriched were identified. Protein-protein
interaction (PPI) network construction and transcription factor
(TF) annotation were performed in order to explore the 'hub' nodes
(highly connected nodes with a large degree) and TFs at various
time-points following SCI. The results of the present study
provided novel insight into the mechanisms underlying SCI.
Materials and methods
Microarray data
The expression profile GSE20907 based on the
Affymetrix Rat Gene 1.0 ST Array (GPL6247; Affymetrix, Inc., Santa
Clara, CA, USA) platform was obtained from the Gene Expression
Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/; accessed June 16,
2014). Data included 12 thoracic non-injured spinal cord control
samples (Ctrl) and 12 thoracic transected spinal cord samples at 3
days (d3, n=4), 1 week (wk1, n=4), 2 weeks (wk2, n=2) and 1 month
(m1, n=2) post-lesion.
Data processing
Expression profile chip data were processed using
the affy package (19) in
R/Bioconductor, version 2.14.1 (20) (http://www.bioconductor.org/). Data were subjected to
background correction, normalization, probe summary and
log2 logarithmic transformation using the robust
multi-array average (RMA Express; version 1.0; http://rmaexpress.bmbolstad.com) method (21). When several probes were found to
project to one gene, the average was used to represent the
expression levels of this gene. There were 27,342 probes in the raw
data and 15,594 genes remained following data processing.
Identification of DEGs between SCI and
controls at four time-points
GSE20907 data included one Ctrl group and four
experimental groups at different time-points (d3, wk1, wk2 and m1).
Data were divided into four paired groups: d3-Ctrl, wk1-Ctrl,
wk2-Ctrl and m1-Ctrl. The Limma package (22) in R/Bioconductor was used to analyze
the DEGs in each experimental group. |log2FC| and
P-values from Student's t-test were used to select the DEGs. A
P-value <0.01 and |log2FC|≥1 were set as the cutoff
criteria.
Cluster analysis of DEGs
In order to analyze the changes of DEG expression at
the four time-points, the gplots package (23) in R/Bioconductor was used to
construct a cluster heatmap of DEGs. Mean expression values of DEGs
for the different time-point samples and controls were used to form
the expression matrix.
Kyoto encyclopedia of genes and genomes
(KEGG) pathway enrichment analysis of DEGs
The database for annotation, visualization and
integrated discovery (DAVID; version 6.7) provides a comprehensive
set of functional annotation tools (24). In order to identify DEG functions,
overregulated KEGG (version 59) categories in pathways were
identified using DAVID (25,26).
DAVID was used to identify DEGs associated pathways by calculating
the hyper-geometric test P-values (27). P<0.01 was set as the cut-off
criterion.
Construction of the PPI network
The search tool for the retrieval of interacting
genes (STRING; version 9.0) database (27) was used to annotate functional
interactions between the DEGs encoding proteins. Cytoscape, version
2.6.3 (28) was then used to
construct the PPI networks for the DEGs at different stages
post-SCI.
TF annotation
Based on the rat TFs database TRANSFAC version 6.0
(http://www.gene-regulation.com)
(29), TFs were annotated among
DEGs. Using the TF annotation and PPI network information, the
differences and similarities between TFs at the four time-points as
well as the degrees of TFs in the PPI network were analyzed.
Results
DEGs between the SCI and Ctrl samples at
four time-points
According to the gene expression profiles, 1,942,
396, 188 and 193 DEGs were identified at d3, wk1, wk2 and m1,
respectively (Table I). The number
of DEGs decreased in a time-dependent manner. Upregulated and
downregulated DEGs are summarized in Table I. There was a greater number of
upregulated DEGs than that of downregulated DEGs at the four
time-points.
 | Table IDEG counts (n) at four time-points
following spinal cord injury in rats. |
Table I
DEG counts (n) at four time-points
following spinal cord injury in rats.
| DEGs | Upregulated
genes | Downregulated
genes |
|---|
| d3-Ctrl | 1,942 | 1,038 | 904 |
| wk1-Ctrl | 396 | 204 | 192 |
| wk2-Ctrl | 188 | 146 | 42 |
| m1-Ctrl | 193 | 154 | 39 |
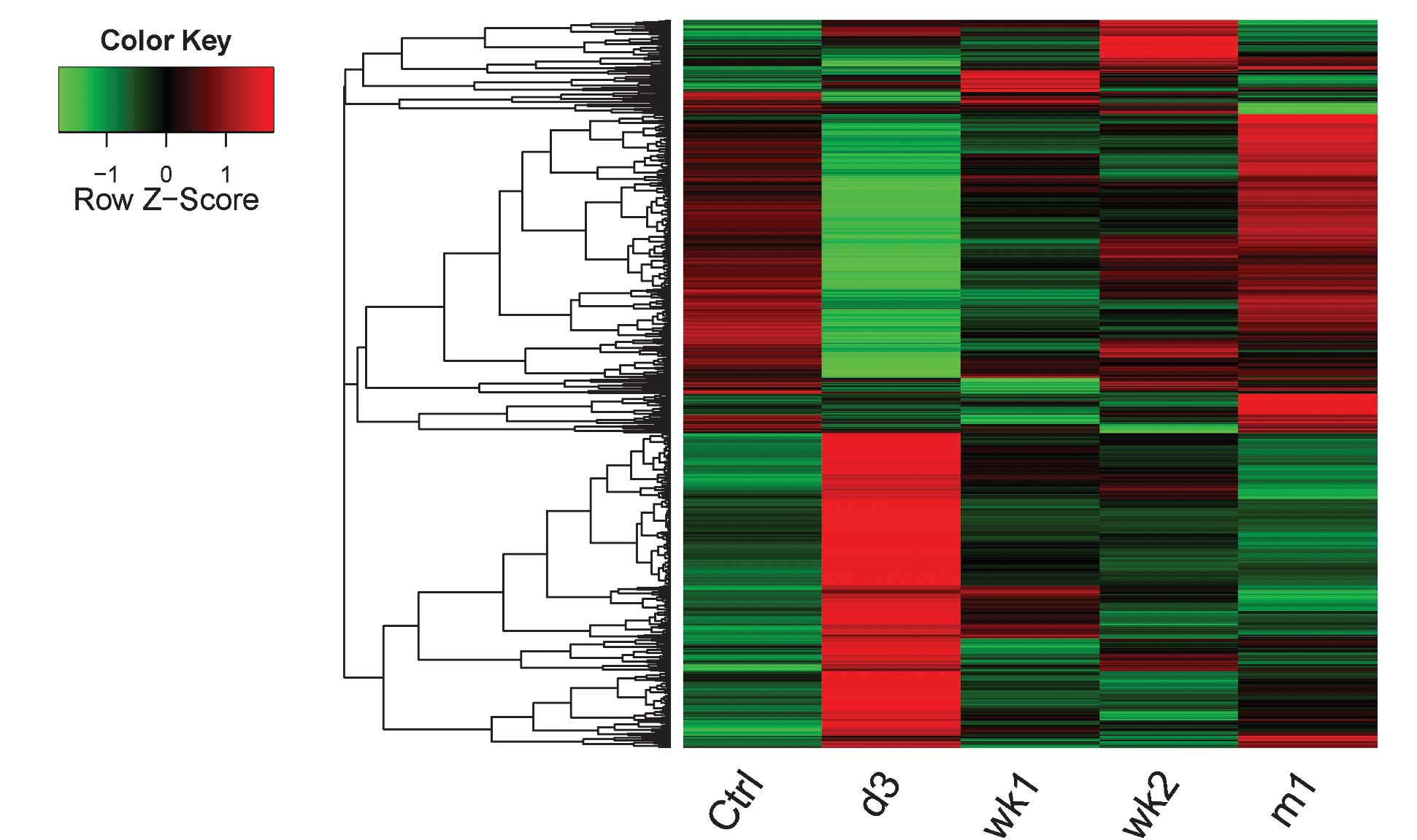
Cluster analysis of the DEGs
In order to explore the changes of the DEG
expression levels at the four time-points following SCI, a cluster
analysis was performed. A cluster heat map of the four experimental
groups compared with the Ctrl group is shown in Fig. 1. DEG expression levels of d3
samples were markedly different from those of the Ctrl samples. DEG
expression levels of m1 samples were similar to those in the Ctrl
group.
KEGG pathway enrichment analysis
The KEGG pathways of the significantly upregulated
and downregulated genes are summarized in Table II. Results demonstrated that the
significantly enriched KEGG pathways of the downregulated genes
were relatively similar between d3, wk1 and wk2, which were
predominantly associated with pathways of neurological diseases,
including Parkinson's disease, oxidative phosphorylation,
Huntington's disease and Alzheimer's disease. At d3 and wk1, the
upregulated genes were enriched in immune response-associated
pathways, including Fc γ R-mediated phagocytosis, lysosome,
leukocyte transendothelial migration, B-cell receptor signaling
pathway, complement and coagulation cascades, systemic lupus
erythematosus and natural killer cell-mediated cytotoxicity. At wk2
and m1, upregulated genes were enriched in pathways associated with
cancer and pyrimidine metabolism, respectively. Overall, DEGs were
predominantly associated with pathways of immune and nervous
system-associated diseases.
 | Table IIKEGG pathways of significantly up-
and downregulated genes at four time-points following spinal cord
injury in rats. |
Table II
KEGG pathways of significantly up-
and downregulated genes at four time-points following spinal cord
injury in rats.
| Contrast group | KEGG pathway | Gene count (n) | P-value |
|---|
| d3-Ctrl | | | |
| Upregulated
genes | rno04666: Fc gamma
R-mediated phagocytosis | 24 |
5.25×10−8 |
| rno04142:
Lysosome | 28 |
6.27×10−8 |
| rno04670: Leukocyte
transendothelial migration | 27 |
1.72×10−7 |
| rno04810:
Regulation of actin cytoskeleton | 37 |
1.10×10−6 |
| rno04662: B cell
receptor signaling pathway | 20 |
1.31×10−6 |
| Downregulated
genes | rno05012:
Parkinson's disease | 55 |
1.13×10−34 |
| rno00190: Oxidative
phosphorylation | 54 |
1.79×10−34 |
| rno05016:
Huntington's disease | 54 |
2.27×10−26 |
| rno05010:
Alzheimer's disease | 54 |
1.56×10−25 |
| wk1-Ctrl | | | |
| Upregulated
genes | rno04610:
Complement and coagulation cascades | 9 |
4.69×10−06 |
| rno05322: Systemic
lupus erythematosus | 8 |
2.32×10−04 |
| rno04650: Natural
killer cell mediated cytotoxicity | 7 | 0.0026 |
| rno04666: Fc gamma
R-mediated phagocytosis | 6 | 0.0074 |
| Downregulated
genes | rno05012:
Parkinson's disease | 14 |
9.93×10−10 |
| rno00190: Oxidative
phosphorylation | 13 |
7.99×10−9 |
| rno05016:
Huntington's disease | 13 |
3.11×10−7 |
| rno05010:
Alzheimer's disease | 13 |
4.61×10−7 |
| rno00100: Steroid
biosynthesis | 6 |
5.20×10−7 |
| rno00900: Terpenoid
backbone biosynthesis | 5 |
9.02×10−6 |
| wk2-Ctrl | | | |
| Upregulated
genes | rno05200: Pathways
in cancer | 8 | 0.0035 |
| Downregulated
genes | rno05012:
Parkinson's disease | 6 |
2.41×10−5 |
| rno05016:
Huntington's disease | 6 |
9.79×10−5 |
| rno04260: Cardiac
muscle contraction | 4 | 0.0010 |
| rno00190: Oxidative
phosphorylation | 4 | 0.0053 |
| m1-Ctrl | | | |
| Upregulated
genes | rno00240:
Pyrimidine metabolism | 6 |
7.87×10−4 |
PPI network
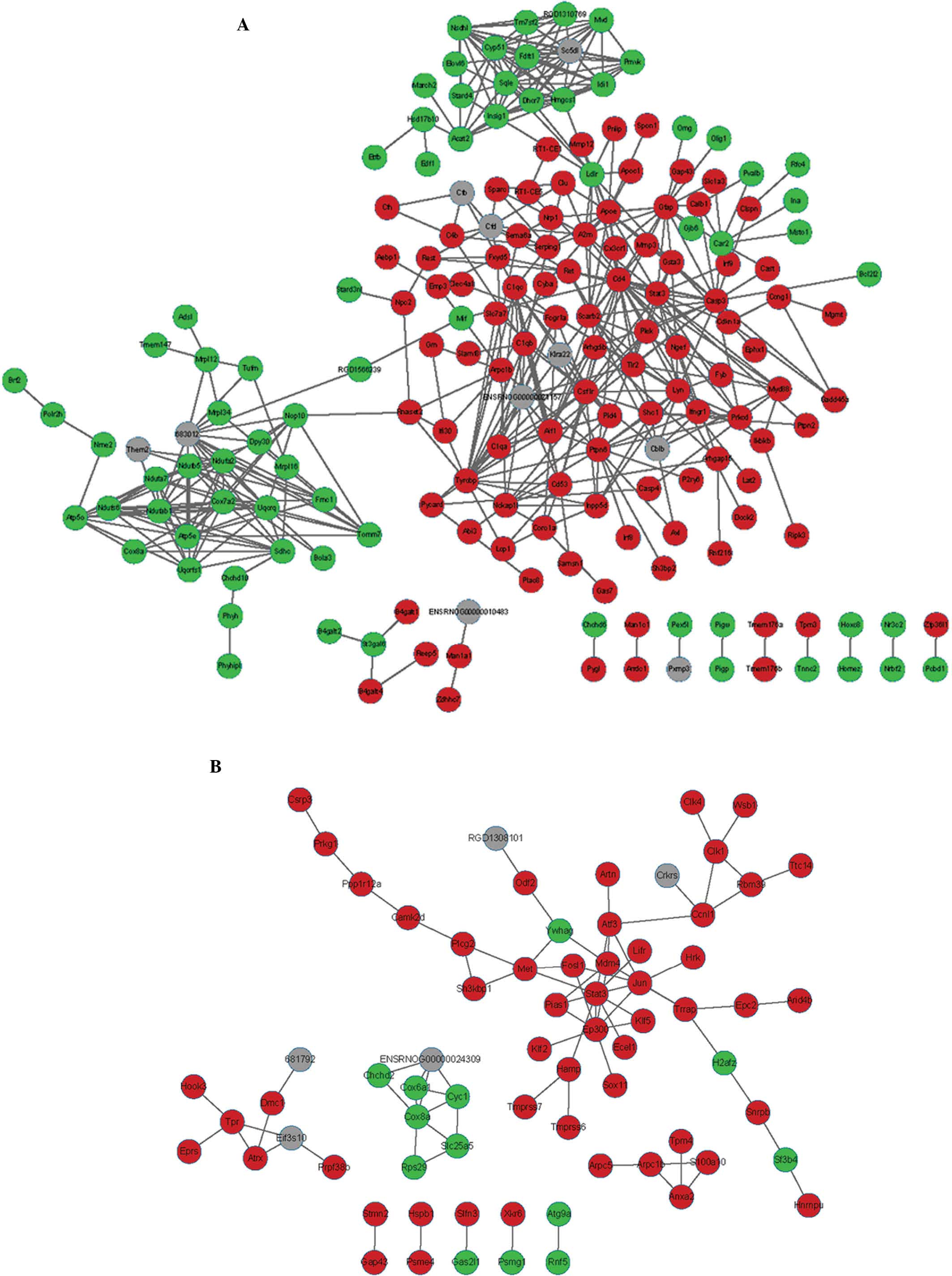
The PPI network of d3 consisted of 1,524 protein
nodes and 10,390 pairs of mutual protein associations (data not
shown). The PPI network of the DEGs based on three time-points wk1,
wk2 and m1 are demonstrated in Figs.
2 and 3. The PPI network of
wk1 consisted of 184 protein nodes and 475 protein pairs. THe PPI
network of wk2 consisted of 71 protein nodes and 82 protein pairs.
The PPI network of m1 consisted of 49 protein nodes and 43 protein
pairs.
The top 10 hub node genes exhibiting enhanced
expression at the four time-points are summarized in Table III. Hub node genes in the PPI
network were differentially expressed at the four time-points.
STAT3 was the hub gene at wk1 and wk2, JUN was the hub gene at wk2
and m1 and CD4 was the hub gene at d3, wk1 and m1, while RAC2 was
the hub gene at d3.
 | Table IIITop 10 upregulated hub genes at four
time-points following spinal cord injury in rats. |
Table III
Top 10 upregulated hub genes at four
time-points following spinal cord injury in rats.
d3
| wk1
| wk2
| m1
|
|---|
| Gene | Degree | Gene | Degree | Gene | Degree | Gene | Degree |
|---|
| Rac2 | 130 | Cd4 | 22 | Stat3 | 9 | Jun | 10 |
| Mapk3 | 128 | Casp3 | 21 | Ep300 | 8 | Nos3 | 7 |
| Il6 | 128 | Tyrobp | 19 | Cox8a | 6 | Ins1 | 4 |
| Cdc2 | 113 | Apoe | 18 | Jun | 6 | Cd4 | 4 |
| Vegfa | 105 | Stat3 | 17 | Met | 5 | Dusp1 | 4 |
| Pcna | 101 | Csf1r | 16 | Atf3 | 5 | Egr1 | 3 |
| Cd4 | 99 | Lyn | 16 | Mdm4 | 5 | Dvl3 | 3 |
| Calm1 | 96 | Gfap | 16 | Ccnl1 | 4 | Nfatc2 | 3 |
| Fn1 | 95 | Sqle | 15 | Cyc1 | 4 | Pofut2 | 2 |
| Fos | 91 | Cox7a2 | 15 | Tpr | 4 | Tfip11 | 2 |
TFs
TFs among the DEGs at the four time-points were
identified (Table IV). The
greatest number of TFs was identified at d3, including ATF3, JUN
and EGR1. The lowest number of TFs was identified at m1, including
EGR1 and JUN. Combined with the PPI network, ATF3, EGR1 and JUN
were the most important TFs associated with the development of
SCI.
 | Table IVTranscription factors among DEGs at
four time-points following spinal cord injury in rats. |
Table IV
Transcription factors among DEGs at
four time-points following spinal cord injury in rats.
| Time-points | Transcription
factors
|
|---|
| Upregulated
gene(s) | Downregulated
gene(s) |
|---|
| d3 | Atf3, Crem, Jun,
Maf, Nfe2l2, Pax6, Rest, Tceb3, Tfec, Ybx1 | Atf4, Bmyc, Dbp,
Egr1, Mef2d, Nr1d1, Nr3c2, Nr4a2, Olig1, Pou6f1, Rxrg, Thrb, |
| wk1 | Rest | Nr3c2, Olig1 |
| wk2 | Atf3, Csrp3, Fosl1,
Jun | |
| m1 | Egr1, Jun | |
Discussion
At present, no completely restorative treatments for
SCI are available (3,30). The present study was performed in
order to explore potential biomarkers and molecular mechanisms
underlying SCI using bioinformatic methods. Thousands of DEGs were
identified by comparing the gene expression profiles of samples
from d3, wk1, wk2 and m1 post-SCI with those of healthy Ctrl
samples. DEGs were shown to be enriched in pathways associated with
immune response, nervous system diseases and cancer. According to
the PPI network for d3, wk1, wk2 and m1, a number of hub nodes were
identified, including CD4, STAT3 and JUN. TFs were identified in
the DEGs, including ATF3, EGR1, OLIG1 and JUN. These genes may be
involved in the mechanisms underlying regeneration or self-repair
following SCI.
Siebert et al (16) have demonstrated that there is a
strong regenerative response during the early stages of SCI. The
present study found that OLIG1 was differentially expressed at d3
and wk1 but not at wk2 or m1. OLIG1 is expressed during the
maturation and regeneration of human oligodendrocytes (31). Arnett et al (32) demonstrated that OLIG1 is associated
with CNS repair in mice. Therefore, OLIG1 may participate in early
regenerative responses to SCI. ATF3 is a member of the mammalian
activation transcription factor protein family and was found to be
differentially expressed at d3, wk1 and wk2. ATF3 was suggested to
be a useful marker for regenerative response following nerve root
injury (33) and a novel indicator
of nerve injury (34). ATF3 is
able to bind with other members of the ARF/CREB family, including
ATF2, c-JUN and JUNB, and form dimers, which exert transcriptional
activation and inhibitory effects (35). In the present study, ATF3 and JUN
were present in the PPI network. ATF3 and c-Jun induces the
anti-apoptotic factor Hsp27 (36),
which activates protein kinase B, thereby inhibiting apoptosis and
inducing nerve elongation. The results of the present study
suggested that ATF3- and c-JUN-induced Hsp27 expression may be a
novel neuron survival response to nerve injury.
A number of immune response-associated DEGs and
pathways were identified in the present study. CD4 is a membrane
glycoprotein, which is associated with the T-cell receptor
signaling pathway. CD4 may be involved in neuronal damage
associated with infectious and immune-mediated diseases of the CNS
(37,38). STAT3 is activated via
phosphorylation in response to various cytokines and growth factors
such as interleukins (ILs). STAT3 is associated with a number of
chemokine signaling pathways, including the IL-9 signaling pathway,
immune response IL-23 signaling pathway and certain pathways
associated with cancer. SCI or amyotrophic lateral sclerosis
damages spinal motor neurons and forms a glial scar, which prevents
neural regeneration. STAT3 is involved in astrogliogenesis and scar
formation, and therefore, modulation of STAT3 signaling may be
useful for controlling the excessive gliogenic environment and
neural repair in patients with SCI (39). In the present study, STAT3 was
significantly upregulated at wk1 and wk2, but not at d3, which
suggested that there was a regenerative effect associated with
STAT3 expression. In addition, RAC2, which exhibited the highest
degree of expression at d3, regulates a number of cellular
responses and is associated with neutrophil immunodeficiency
syndrome (40). Immune responses
maintain neurogenesis in adult germinal centers of the damaged CNS,
even under non-pathological conditions (41). Treatments to decrease inflammatory
responses are likely to be beneficial to CNS recovery following
SCI.
The DEGs identified in the present study at four
time-points following SCI are involved in a number of
disease-associated pathways, including those associated with
certain cancers. These results are in accordance with those of
other studies. For example, Myers et al (13) demonstrated that patients with SCI
exhibited higher morbidity of the cardiovascular system with
greater incidences of diabetes, compared with healthy patients. van
den Berg et al (42) found
that cancer and bacterial infection may enhance SCI. Furthermore,
SCI may induce cardiovascular disease and alter immune responses.
In addition, immune-associated pathways were predominantly observed
during the early stages of SCI (d3, wk1). By contrast, pathways
associated with cancer were predominantly observed in the later
stage of SCI (wk2). The results of the present study suggested that
genes associated with myocardial contraction and immune response
may be involved in the mechanisms underlying early-stage SCI.
In conclusion, a number of SCI
regeneration-associated genes have been identified using a
computational bioinformatics analysis of gene expression, including
OLIG1, ATF3 and JUN. The involvement of inflammation in SCI was
investigated and associated genes were highlighted, including CD4,
STAT3 and RAC2. Furthermore, the results of the present study
suggested that SCI may be associated with a number of diseases,
including cardiovascular disease and cancers. The present study
provided novel insight into the molecular mechanisms of SCI
regeneration, which may aid in the development of strategies to
enhance recovery following SCI. Further investigations using a
larger sample size should be performed to confirm the results of
the present study. Since the present study was based on microarray
data alone, further studies should incorporate different data
types.
References
|
1
|
National Spinal Cord Injury Statistical
Center: Spinal cord injury facts and figures at a glance. J Spinal
Cord Med. 36:1–2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu J: China spinal cord injury network:
changes from within. Lancet Neurol. 8:606–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thuret S, Moon LD and Gage FH: Therapeutic
interventions after spinal cord injury. Nat Rev Neurosci.
7:628–643. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freund P, Weiskopf N, Ashburner J, et al:
MRI investigation of the sensorimotor cortex and the corticospinal
tract after acute spinal cord injury: a prospective longitudinal
study. Lancet Neurol. 12:873–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McDonald JW and Sadowsky C: Spinal-cord
injury. Lancet. 359:417–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mariano ED, Batista CM, Barbosa BJ, et al:
Current perspectives in stem cell therapy for spinal cord repair in
humans: a review of work from the past 10 years. Arq
Neuropsiquiatr. 72:451–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young W: Spinal cord regeneration. Cell
Transplant. 23:573–611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu NK and Xu XM: Phospholipase A2 and its
molecular mechanism after spinal cord injury. Mol Neurobiol.
41:197–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donnelly DJ and Popovich PG: Inflammation
and its role in neuroprotection, axonal regeneration and functional
recovery after spinal cord injury. Exp Neurol. 209:378–388. 2008.
View Article : Google Scholar
|
|
10
|
Lee K, Na W, Lee JY, et al: Molecular
mechanism of Jmjd3-mediated interleukin-6 gene regulation in
endothelial cells underlying spinal cord injury. J Neurochem.
122:272–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kerschensteiner M, Gallmeier E, Behrens L,
et al: Activated human T cells, B cells and monocytes produce
brain-derived neurotrophic factor in vitro and in inflammatory
brain lesions: a neuroprotective role of inflammation? J Exp Med.
189:865–870. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yekutiel M, Brooks M, Ohry A, Yarom J and
Carel R: The prevalence of hypertension, ischaemic heart disease
and diabetes in traumatic spinal cord injured patients and
amputees. Paraplegia. 27:58–62. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myers J, Lee M and Kiratli J:
Cardiovascular disease in spinal cord injury: an overview of
prevalence, risk, evaluation and management. Am J Phys Med Rehabil.
86:142–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Groah SL, Weitzenkamp DA, Lammertse DP,
Whiteneck GG, Lezotte DC and Hamman RF: Excess risk of bladder
cancer in spinal cord injury: evidence for an association between
indwelling catheter use and bladder cancer. Arch Phys Med Rehabil.
83:346–351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalisvaart JF, Katsumi HK, Ronningen LD
and Hovey R: Bladder cancer in spinal cord injury patients. Spinal
Cord. 48:257–261. 2010. View Article : Google Scholar
|
|
16
|
Siebert JR, Middelton FA and Stelzner DJ:
Intrinsic response of thoracic propriospinal neurons to axotomy.
BMC Neurosci. 11:692010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai J, He X, Wang F, et al: Gene
expression signature analysis and protein-protein interaction
network construction of spinal cord injury. Eur Rev Med Pharmacol
Sci. 17:2941–2948. 2013.PubMed/NCBI
|
|
18
|
Jin L, Wu Z, Xu W, et al: Identifying gene
expression profile of spinal cord injury in rat by bioinformatics
strategy. Mol Biol Rep. 41:3169–3177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: Affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gentleman RC, Carey VJ, Bates DM, et al:
Bioconductor: open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smyth GK: Limma: Linear Models for
Microarray Data. Bioinformatics and Computational Biology Solutions
Using R and Bioconductor. Gentleman R, Carey V, Huber W, Irizarry R
and Dudoit S: Springer; New York: pp. 397–420. 2005, View Article : Google Scholar
|
|
23
|
Warnes GR, Bolker B, Bonebakker L, et al:
gplots: Various R programming tools for plotting data. R package
version 2.7.4. 2009
|
|
24
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanehisa M and Goto S: KEGG: kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
26
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2008. View Article : Google Scholar
|
|
27
|
Szklarczyk D, Franceschini A, Kuhn M, et
al: The STRING database in 2011: functional interaction networks of
proteins, globally integrated and scored. Nucleic Acids Res.
39(Database Issue): D561–D568. 2011. View Article : Google Scholar :
|
|
28
|
Shannon P, Markiel A, Ozier O, et al:
Cytoscape: a software environment for integrated models of
biomolecular interaction networks. Genome Res. 13:2498–2504. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matys V, Fricke E, Geffers R, et al:
TRANSFAC: transcriptional regulation, from patterns to profiles.
Nucleic Acids Res. 31:374–378. 2003. View Article : Google Scholar :
|
|
30
|
Courtine G, van den Brand R and Musienko
P: Spinal cord injury: Time to move. Lancet. 377:1896–1898. 2011.
View Article : Google Scholar
|
|
31
|
Othman A, Frim DM, Polak P, Vujicic S,
Arnason BG and Boullerne AI: Olig1 is expressed in human
oligodendrocytes during maturation and regeneration. Glia.
59:914–926. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arnett HA, Fancy SP, Alberta JA, et al:
bHLH transcription factor Olig1 is required to repair demyelinated
lesions in the CNS. Science. 306:2111–2115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindå H, Sköld MK and Ochsmann T:
Activating transcription factor 3, a useful marker for regenerative
response after nerve root injury. Front Neurol. 2:302011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flatters S: ATF3: novel signpost for nerve
injury. Neuroreport. 11:A72000. View Article : Google Scholar
|
|
35
|
Koh IU, Lim JH, Joe MK, et al: AdipoR2 is
transcriptionally regulated by ER stress-inducible ATF3 in HepG2
human hepatocyte cells. Febs J. 277:2304–2317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakagomi S, Suzuki Y, Namikawa K,
Kiryu-Seo S and Kiyama H: Expression of the activating
transcription factor 3 prevents c-Jun N-terminal kinase-induced
neuronal death by promoting heat shock protein 27 expression and
Akt activation. J Neurosci. 23:5187–5196. 2003.PubMed/NCBI
|
|
37
|
Kohm AP, Carpentier PA, Anger HA and
Miller SD: Cutting edge: CD4+ CD25+ regulatory T cells suppress
antigen-specific autoreactive immune responses and central nervous
system inflammation during active experimental autoimmune
encephalomyelitis. J Immunol. 169:4712–4716. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liblau RS, Gonzalez-Dunia D, Wiendl H and
Zipp F: Neurons as targets for T cells in the nervous system.
Trends Neurosci. 36:315–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Natarajan R, Singal V, Benes R, et al:
STAT3 modulation to enhance motor neuron differentiation in human
neural stem cells. PLoS One. 9:e1004052014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ambruso DR, Knall C, Abell AN, et al:
Human neutrophil immunodeficiency syndrome is associated with an
inhibitory Rac2 mutation. Proc Natl Acad Sci USA. 97:4654–4659.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ziv Y, Avidan H, Pluchino S, Martino G and
Schwartz M: Synergy between immune cells and adult neural
stem/progenitor cells promotes functional recovery from spinal cord
injury. Proc Natl Acad Sci. 103:13174–13179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van den Berg ME, Castellote JM, de
Pedro-Cuesta J and Mahillo-Fernandez I: Survival after spinal cord
injury: A systematic review. J Neurotrauma. 27:1517–1528. 2010.
View Article : Google Scholar : PubMed/NCBI
|