Introduction
At present, hyperlipidemia is an important global
health concern due to its association with obesity, insulin
resistance and nonalcoholic fatty liver disease (NAFLD), which are
important features of metabolic syndrome (1,2).
Obesity has become a global health problem, with recent statistics
indicating that rates of obesity have risen among adolescents from
5% in 1970 to >18% in 2008, and the number of overweight
adolescents now exceeds 34% in the USA (3,4).
Chronic inflammation in association with insulin
resistance is a key characteristic of obesity. Previous studies
have found associations between chronic inflammation,
hyperlipidemia and insulin resistance (5–7). A
recent study found that toll-like receptors (TLRs), a family of
pattern recognition receptors that contribute to congenital
immunity, have an important role in the outcome and development of
metabolic syndrome (8). Previous
studies by Lee et al (9,10)
have found that saturated fatty acids activate TLR4, and that
polyunsaturated fatty acids (PUFA) inhibit saturated fatty acid-
and LPS-induced activation of TLR4 (9,10).
In addition, the saturated fatty acid lauric acid potentiates,
while the n-3 PUFA docosahexaenoic acid inhibits lipopeptide
(TLR2 agonist)-induced TLR2 activation (11). Fatty acids are able to induce or
inhibit the activation of TLR2 and TLR4; however, it remains to be
elucidated whether TLR2 or TLR4 are associated with hyperlipidemia.
Therefore, the present study detected TLR2 and TLR4 gene expression
in peripheral blood mononuclear cells (PBMCs) among different
individuals and in the skeletal muscle of rats fed a high-fat diet
in order to examine the association with hyperlipidemia.
Patients and methods
Patients
Patients with high triglyceride (HTG) levels, or
high cholesterol (HTC) levels aged between 25 and 55-years-old were
recruited from the Medical Examination Center of Hebei General
Hospital (Shijiazhuang, China). They were divided into the HTG
group (n=43), the HTC group (n=84) or the mixed hyperlipidemia
group (MHL group, n=55). In addition, 68 healthy volunteers were
recruited and assigned to the control group (Con group).
Participants were excluded if they had a history of smoking,
alcohol abuse or use of oral drugs, or evidence of infection within
the past month (ie., C-reactive protein concentrations >5
mg/dl). Participants were also excluded if they had a history of
any the following diseases: Diabetes mellitus, hypertension, blood
disorders, heart disease, liver and renal disease or dystrophia;
had undergone long-term high intensity exercise; or were pregnant
or lactating (12,13). HTG and HTC were defined as TG ≥1.7
mmol/l and TC ≥5.7 mmol/l, respectively. All patients were matched
for age, body mass index and waist circumference (Table II). The present study was approved
by the ethics committee of the Hebei General Hospital (Hebei,
China). Written informed consent was obtained from each
participant.
 | Table IIComparison of clinical parameters
between groups (mean ± standard deviation). |
Table II
Comparison of clinical parameters
between groups (mean ± standard deviation).
| Parameter | Con group | HTC group | HTG group | MHL group |
|---|
| Gender
(male/female) | 40/28 | 51/33 | 27/16 | 35/20 |
| Age (years) | 34.68±4.27 | 35.83±5.05 | 35.37±4.57 | 35.27±4.53 |
| BMI
(kg/m2) | 23.88±1.29 | 23.40±1.68 | 24.37±1.90 | 24.58±2.06 |
| Waist (cm) | 82.97±7.52 | 82.44±8.36 | 82.44±6.54 | 85.29±9.42 |
| TC (mmol/l) | 4.46±0.63 | 6.12±0.20a | 4.82±0.41a | 6.20±0.30a |
| TG (mmol/l) | 0.87±0.30 | 1.18±0.22a | 2.66±0.45a | 2.64±0.49a |
Animals and animal care
A total of 36 male Sprague-Dawley rats (120–150 g;
two week-old) were obtained from the China Experimental Animal
Resources Research Institute for Food and Drug Control [license no.
scxk (Beijing) 2009–0017; certificate no. 0270141; Beijing, China]
and received an animal experimental licence from the same
institution. All rats were housed in 12 h light/dark cycle
conditions for 2 weeks. Initially, they were randomly divided into
two groups: Negative control group (NC group; n=12) and high-fat
diet group (HF group; n=24). Six rats were selected randomly from
each group after 6 weeks to harvest blood and skeletal tissue
samples for experimentation. They were anesthetized with 3%
pentobarbital sodium (30 mg/kg; Beijing Pu Bosi Biotechnology Co.,
Ltd., Beijing, China) and sacrificed following blood sample
collection. Subsequently, the remaining rats in the HF group were
randomly divided into three groups: High-fat diet control group
(HFD, N=6), high-fat diet plus Jin Li Da group (JLD, n=6;
Shijiazhuang Yiling pharmaceutical Co., Ltd., Shijiazhuang, China)
and high-fat diet plus pioglitazone group (Ptz, n=6; Takeda
Pharmaceuticals Co., Ltd., Tianjin, China). All rats were housed in
a 12 h light-dark cycle and provided ad libitum access to a
rodent standard diet (65.5% carbohydrate, 10.3% fat and 24.2%
protein) or a HFD (20.1% carbohydrate, 59.8% fat and 20.1%
protein). The drug treatments were intragastrically administered
for 8 weeks (JLD 1.5 g/kg per day and Ptz 4.5 mg/kg per day).
Isolation of PBMCs
Mononuclear cells were isolated from heparinized
blood obtained from all patients who had fasted for 8 h, by Ficoll
Hypaque centrifugation followed by magnetic separation using the
depletion technique (Miltenyi Biotec, Auburn, CA, USA) as described
previously (14–16). Using this technique, >92% of
cells were identified as monocytes by CD14 staining. Whole blood
sample was collected following medical examination PBMCs were
isolated within 3 h
TC and TG detection
All blood samples were assayed using a Hitachi
7300–110 apparatus (Hitachi, Ltd., Tokyo, Japan). The data were
obtained from the Medical Examination Center of Hebei General
Hospital.
TLR2 and TLR4 mRNA expression
RNA was extracted from monocytes or skeletal muscles
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer's instructions. The RNA quality
was assessed using a NanoDrop 1000 spectrophotometer (Thermo Fisher
Scientific, Waltham, MA, USA). cDNA was synthesized using the
iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed on an ABI PRISM 7300 PCR system
(Applied Biosystems Life Technologies, Foster City, CA, USA) using
SYBR Green I GoTaq® qPCR Master mix (Promega
Corporation, Madison, WI, USA) with GAPDH as a control and a mixed
cDNA sample control incorporated into each PCR run (17,18).
All target gene primers were designed with DNAMan 6.0.40 (Lynnon
Biosoft, San Ramon, CA, USA) and the primers are shown in Table I. PCR was performed as follows:
Denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec, 58°C for 20 sec and 72°C for 27 sec. Subsequently, the PCR
products were assessed using a melting curve analysis to confirm
the specificity of the amplification. The mRNA expression of target
genes was expressed as a ratio to glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) (12).
 | Table IReverse transcription-polymerase chain
reaction primer sequences. |
Table I
Reverse transcription-polymerase chain
reaction primer sequences.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| TLR2-h |
GACTTCTCCCATTTCCGTCT |
GGTGTTCATTATCTTCCGCA |
| TLR4-h |
ATCATTGGTGTGTCGGTCC |
GCTCATTCCTTACCCAGTCC |
| GAPDH-h |
TGAACGGGAAGCTCACTG |
GCTTCACCACCTTCTTGATG |
| TLR2-r |
TCGGGACTCACAGCAAACA |
TTCACACAGGCTCGCAAGT |
| TLR4-r |
TGGTCAGTGTGCTTGTGGTA |
GTTTCTCACCCAGTCCTCATT |
| GAPDH-r |
TGAACGGGAAGCTCACTG |
GCTTCACCACCTTCTTGATG |
Western blot analysis
Frozen-dried muscle tissues (50 mg) were homogenized
in 100 µg/ml phenylmethylsulfonyl fluoride (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China), including
three cycles of homogenization for 8 sec and then standing for 10
min. The samples were subjected to centrifugation at 10,000 x g for
10 min at 4°C. The protein concentration of the supernatant was
determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher
Scientific). Muscle protein fractions (30 µg) were separated
by 10% SDS-polyacrylamide gel (Sigma-Aldrich, St. Louis, MO, USA)
electrophoresis and transferred onto polyvinylidene difluoride
(PVDF) membranes (EMD Millipore, Billerica, MA, USA). Following
protein transfer, the membranes were blocked with 5% non-fat dry
milk in Tris-buffered saline containing 0.05% Tween-20 (TBST)
overnight at 4°C. Following blocking, the membranes were incubated
overnight at 4°C with anti-TLR2 (polyclonal rabbit anti-mouse; cat.
no. bs-1019R; 1:300, BIOSS, Beijing, China), anti-TLR4 (polyclonal
rabbit anti-mouse; cat. no. BA1717; 1:200, Wuhan Boster Biological
Technology, Ltd., Wuhan, China) and anti-β-actin antibodies
(polyclonal rabbit anti-mouse; cat. no. 85-14-6496-82; 1:5,000,
eBioscience, Inc., San Diego, CA, USA). They were then incubated
with the appropriate horseradish peroxidase-conjugated secondary
antibodies (polyclonal goat anti-rabbit; cat. no. SA00002-2;
1:10,000, ProteinTech Group, Inc., Chicago, IL, USA) for 1 h at
room temperature. All antibodies were diluted with TBST. The
membranes were washed three times for 10 min in TBST. The
immunoreactive proteins were visualized by enhanced
chemiluminescence (Pierce Biotechnology, Rockford, IL, USA). X-ray
film (Ruike Medical Devices Co., Ltd., Xiamen, China) was exposed
to the PVDF membranes for 5 min. The reaction product of each blot
was analyzed by densitometry using Bandscan 5.0 software
(http://soft.bio1000.com/show-149.html).
Statistical analysis
All data analysis was performed with SAS 8.0
software for Windows XP (Hebei Medial University, Shijiazhuang,
China). The key outcome variables were compared between study
groups and the Con group using unpaired two-sample/group t-tests
for continuous variables, and χ2 or Fisher's exact test,
were used for categorical variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
TLR2 and TLR4 are highly expressed in
patients with hyperlipidemia
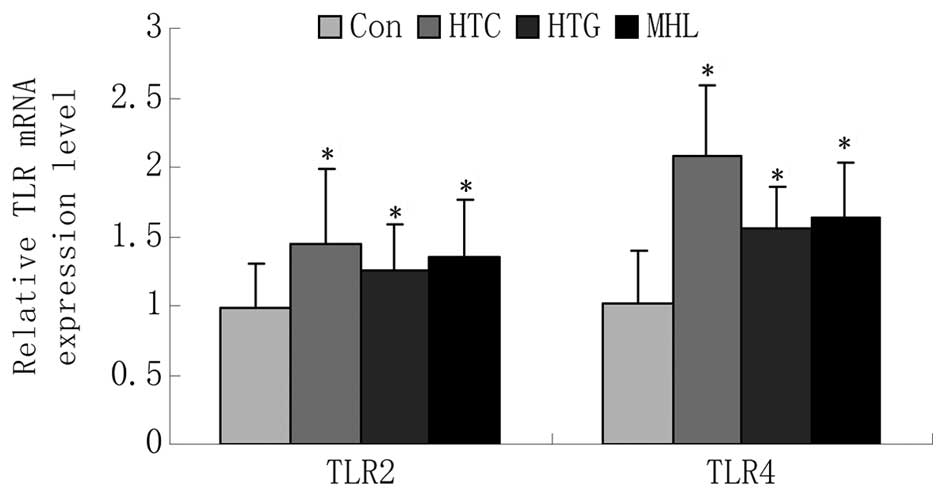
The gene expression of the TLRs are shown in
Table III and Fig. 1. The gene expression levels of TLR2
and TLR4 were significantly higher in the HTG and HTC groups
compared with the Con group (P<0.05). The gene expression levels
of TLR2 and TLR4 in the MHL group were also increased compared with
that in the Con group (TLR2: 1.34±0.42 vs. 0.98±0.32, P<0.05;
TLR4: 1.63±0.41 vs. 1.01±0.39, P<0.05).
 | Table IIIComparison of relative TLR gene
expression levels (mean ± standard deviation). |
Table III
Comparison of relative TLR gene
expression levels (mean ± standard deviation).
| Gene | Con group | HTC group | HTG group | MHL group |
|---|
| TLR2 | 0.98±0.32 | 1.45±0.54a | 1.26±0.33a | 1.34±0.42a |
| TLR4 | 1.01±0.39 | 2.08±0.51a | 1.56±0.30a | 1.63±0.41a |
TLR2 and TLR4 expression in the skeletal
muscle of the rat model
To further demonstrate the association between TLR2
and TLR4 with hyperlipidemia, a high-fat diet rat model was used.
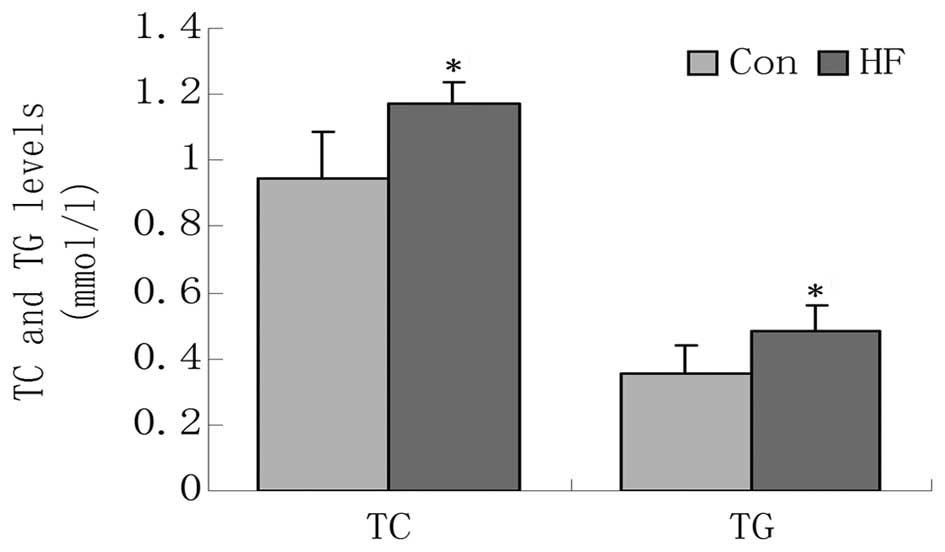
After 6 weeks on the high-fat diet, the TG and TC levels of the HF
group were significantly higher compared with the NC group
(Fig. 2). TLR2 and TLR4 gene
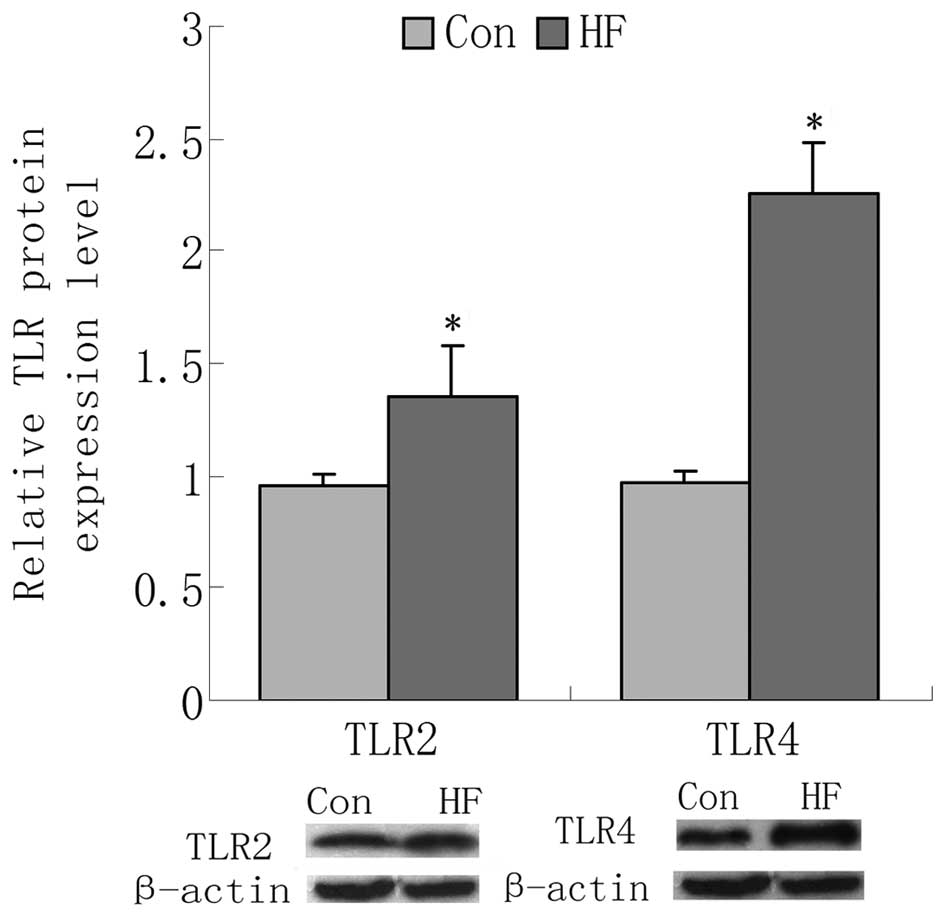
expression in the skeletal muscle was detected using RT-qPCR. It
was identified that the mRNA levels of two TLRs were upregulated in
the HF group compared with the NC group, as were the protein levels
(Figs. 3 and 4). To confirm the reliability of the
results, lipid levels in the HF group were decreased through
intragastric administration of JLD and Ptz. At the end of the
8-week drug intervention, TC, TG and TLR gene and protein levels in
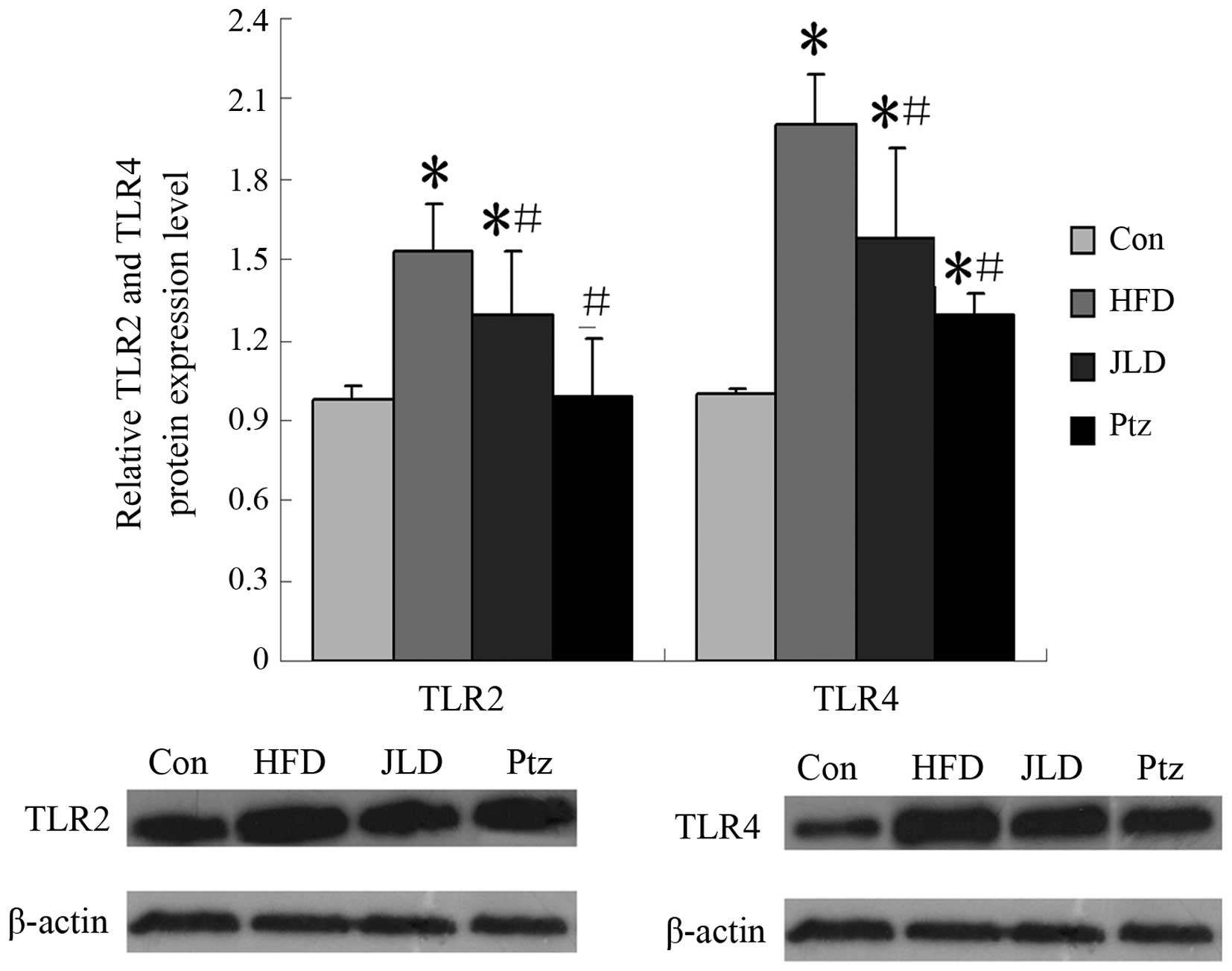
the skeletal muscle were analyzed again. From Fig. 5, it can be observed that levels of
TC in the JLD and Ptz groups were decreased compared with the HFD
group and NC group. TG levels were altered in the same manner. TLR
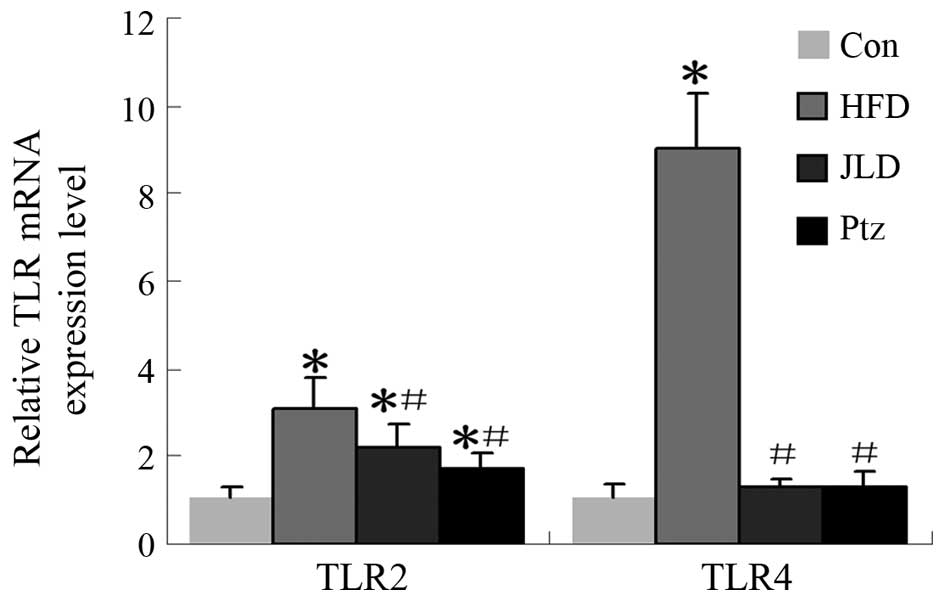
gene and protein levels in skeletal muscle are shown in Figs. 6 and 7. TLR2 gene expression was significantly
decreased in the JLD and Ptz groups compared with the HFD group
(P<0.05), remaining higher than that of the NC group
(P<0.05). The TLR2 protein level in the JLD group was
significantly decreased and remained higher than the NC group
(P<0.05). For the Ptz group, no significant differences were
identified compared with the NC group (P>0.05). TLR4 gene levels
were significantly decreased in the JLD and Ptz groups compared
with those in the HFD group and no significant differences were
identified compared with the NC group. The protein levels in
skeletal muscle also declined in the drug intervention groups, but
remained higher than the NC group (P<0.05).
Discussion
The World Health Organization reports that obesity
is one of today's most obvious public-health problems due to its
high prevalence and its association with a wide range of chronic
diseases, such as insulin resistance, type 2 diabetes,
atherosclerosis, hypertension, immune-mediated disorders, certain
types of cancer and NAFLD (19,20).
Chronic low level inflammation and hyperlipidemia, have been
established to be key factors of obesity. TLRs mediate
infection-induced inflammation by recognizing invading pathogens
and activating downstream signaling pathways that lead to the
expression of diverse arrays of pro-inflammatory marker gene
products (21,22). Recent evidence suggests that fatty
acids are able to modulate TLR4 activation and that mice deficient
in TLR2 were protected from high-fat diet-induced adiposity
(23,24).
In the present study, 250 patients were selected
from the Medical Examination Center at Hebei General Hospital,
including 84 patients with high levels of TC and normal levels of
TG, 43 patients with high levels of TG and normal levels of TC, 55
patients with high TC and TG levels and 68 healthy controls with
normal biochemical indicators. The present data revealed that TLR2
and TLR4 gene expression levels were significantly higher in the
hyperlipidemia groups compared with the Con group. This suggests an
association between the increase in TLR levels and and an increase
in lipid levels.
In order to confirm the reliability of the results,
a high-fat diet induced hyperlipidemia rat model was used, then the
rats were protected from the high-fat diet-induced TC and TG
increase through intragastric administration of JLD and Ptz. In our
previous study, it was found that JLD had a significant protective
effect against high-fat diet-induced lipid increase (25,26).
In addition, Ptz has been established to decrease hyperlipidemia
and has been widely used in the clinical treatment of diabetes and
combined hyperlipidemia (2,27).
In the present study, it was identified that a
6-week high-fat diet is able to induce significant TC and TG
increases, and JLD and Ptz were shown to significantly protect
against these effects (Figs. 2 and
5). Although rats in the JLD and
Ptz groups were fed a high-fat diet, their TC and TG levels were
lower compared with the HFD group, and even lower than the Con
group. However, it was also observed that TLR2 and TLR4 gene and
protein expression levels in skeletal muscle were increased
following a 6-weeks high-fat diet regimen, and their levels were
decreased following a decrease in lipid levels.
Previous studies have established contribution of
the TLR family to innate immunity and recent research has found
that a high-fat meal induces low-grade endotoxemia, which is
associated with obesity (8,28,29).
A further study reported that gut microbiota is a key modulator of
insulin resistance (30), which
together suggests a complex correlation between a high-fat diet,
obesity, gut microbiota and insulin resistance. In the present
study, the data revealed that TLR2 and TLR4 were increased in the
hyperlipidemia groups and their gene and protein levels in skeletal
muscle were consistent with the lipid level. This suggests that
TLR2 and TLR4 were associated with the outcome of development of
hyperlipidemia, however the mechanism for this requires further
investigation.
In conclusion, for the first time, to the best of
our knowledge the association between TLR and hyperlipidemia was
demonstrated, and it was found that the levels of TLR2 and TLR4
were correlated with the lipid level. The results suggest that TLRs
are important in hyperlipidemia and may provide a deeper
understanding of the mechanisms underlying hyper-lipidemia for
future studies.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundations of China (grant nos. 30971391 and
81170742) and the Hebei Natural Science Foundation of China (grant
nos. C2010001638 and C2011307008).
Abbreviations:
|
TLRs
|
Toll-like receptors
|
|
NAFLD
|
nonalcoholic fatty liver disease
|
|
PUFA
|
polyunsaturated fatty acids
|
|
TG
|
triglyceride
|
|
TC
|
total cholesterol
|
|
HTC group
|
high TC group
|
|
HTG group
|
high TG group
|
|
MHL group
|
mixed hyperlipidemia group
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
Con group
|
control group
|
|
NC group
|
negative control group
|
|
HF group
|
high-fat diet group
|
|
HFD group
|
high-fat diet control group
|
|
JLD group
|
high-fat diet plus Jin Li Da group
|
|
Ptz group
|
high-fat diet plus pioglitazone
group
|
References
|
1
|
López-Sánchez I, Sanz-Garcia M and Lazo
PA: Plk3 interacts with and specifically phosphorylates VRK1 in
Ser342, a downstream target in a pathway that induces Golgi
fragmentation. Mol Cell Biol. 29:1189–1201. 2009. View Article : Google Scholar :
|
|
2
|
Niranjan G, Mohanavalli V, Srinivasan AR
and Ramesh R: Serum lipid peroxides and magnesium levels following
three months of treatment with pioglitazone in patients with type-2
diabetes mellitus. Diabetes Metab Syndr. 7:35–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogden CL, Carroll MD, Curtin LR, Lamb MM
and Flegal KM: Prevalence of high body mass index in US children
and adolescents, 2007–2008. JAMA. 303:242–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holl MG, Jaser SS, Womack JA, Jefferson VL
and Grey M: Metabolic risk and health behaviors in minority youth
at risk for type 2 diabetes. Diabetes Care. 34:193–197. 2011.
View Article : Google Scholar
|
|
5
|
Sambataro M, Perseghin G, Lattuada G,
Beltramello G, Luzi L and Pacini G: Lipid accumulation in
overweight type 2 diabetic subjects: Relationships with insulin
sensitivity and adipokines. Acta Diabetol. 50:301–307. 2013.
View Article : Google Scholar
|
|
6
|
Bozzetto L, De Natale C, Di Capua L, Della
Corte G, Patti L, Maione S, Riccardi G, Rivellese AA and Annuzzi G:
The association of hs-CRP with fasting and postprandial plasma
lipids in patients with type 2 diabetes is disrupted by dietary
monounsaturated fatty acids. Acta Diabetol. 50:273–276. 2013.
View Article : Google Scholar
|
|
7
|
Oda E: High-sensitivity C-reactive protein
and white blood cell count equally predict development of the
metabolic syndrome in a Japanese health screening population. Acta
Diabetol. 50:633–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tilich M and Arora RR: Modulation of
toll-like receptors by insulin. Am J Ther. 18:e130–e137. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao
L, Sizemore N and Hwang DH: Reciprocal modulation of Toll-like
receptor-4 signaling pathways involving MyD88 and
phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated
fatty acids. J Biol Chem. 278:37041–37051. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JY, Plakidas A, Lee WH, Heikkinen A,
Chanmugam P, Bray G and Hwang DH: Differential modulation of
toll-like receptors by fatty acids: Preferential inhibition by n-3
polyunsaturated fatty acids. J Lipid Res. 44:479–486. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JY, Zhao L, Youn HS, Weatherill AR,
Tapping R, Feng L, Lee WH, Fitzgerald KA and Hwang DH: Saturated
fatty acid activates but polyunsaturated fatty acid inhibits
toll-like receptor 2 dimerized with toll-like receptor 6 or 1. J
Biol Chem. 279:16971–16979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Devaraj S, Dasu MR, Rockwood J, Winter W,
Griffen SC and Jialal I: Increased toll-like receptor (TLR) 2 and
TLR4 expression in monocytes from patients with type 1 diabetes:
Further evidence of a proinflammatory state. J Clin Endocrinol
Metab. 93:578–583. 2008. View Article : Google Scholar
|
|
13
|
Bian H, Yan H, Zeng M, Rao S, Yao X, Zhou
J, Jia W and Gao X: Increased liver fat content and unfavorable
glucose profiles in subjects without diabetes. Diabetes Technol
Ther. 13:149–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Devaraj S, Glaser N, Griffen S,
Wang-Polagruto J, Miguelino E and Jialal I: Increased monocytic
activity and biomarkers of inflammation in patients with type 1
diabetes. Diabetes. 55:774–779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Devaraj S, Cheung AT, Jialal I, Griffen
SC, Nguyen D, Glaser N and Aoki T: Evidence of increased
inflammation and microcirculatory abnormalities in patients with
type 1 diabetes and their role in microvascular complications.
Diabetes. 56:2790–2796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dasu MR, Devaraj S, Park S and Jialal I:
Increased toll-like receptor (TLR) activation and TLR ligands in
recently diagnosed type 2 diabetic subjects. Diabetes Care.
33:861–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gan KX, Wang C, Chen JH, Zhu CJ and Song
GY: Mitofusin-2 ameliorates high-fat diet-induced insulin
resistance in liver of rats. World J Gastroenterol. 19:1572–1581.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roth CL, Elfers CT, Figlewicz DP, Melhorn
SJ, Morton GJ, Hoofnagle A, Yeh MM, Nelson JE and Kowdley KV:
Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty
liver disease and increases hepatic resistin and toll-like receptor
activation. Hepatology. 55:1103–1111. 2012. View Article : Google Scholar
|
|
19
|
Obesity: Preventing and managing the
global epidemic. Report of a WHO consultation. World Health Organ
Tech Rep Ser. 894:i–xii. 1–253. 2000.
|
|
20
|
Choukem SP and Gautier JF: How to measure
hepatic insulin resistance? Diabetes Metab. 34(6 Pt 2): 664–673.
2008. View Article : Google Scholar
|
|
21
|
Uematsu S and Akira S: Toll-like receptors
and innate immunity. J Mol Med (Berl). 84:712–725. 2006. View Article : Google Scholar
|
|
22
|
Kawai T and Akira S: Signaling to
NF-kappaB by toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong SW, Kwon MJ, Choi AM, Kim HP,
Nakahira K and Hwang DH: Fatty acids modulate toll-like receptor 4
activation through regulation of receptor dimerization and
recruitment into lipid rafts in a reactive oxygen species-dependent
manner. J Biol Chem. 284:27384–27392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davis JE, Braucher DR, Walker-Daniels J
and Spurlock ME: Absence of Tlr2 protects against high-fat
diet-induced inflammation and results in greater insulin-stimulated
glucose transport in cultured adipocytes. J Nutr Biochem.
22:136–141. 2011. View Article : Google Scholar
|
|
25
|
Zang SS, Song A, Liu YX, Wang C, Song GY,
Li XL, Zhu YJ, Yu X, Li L, Liu CX, et al: Chinese medicine Jinlida
(JLD) ameliorates high-fat-diet induced insulin resistance in rats
by reducing lipid accumulation in skeletal muscle. Int J Clin Exp
Med. 8:4620–4634. 2015.PubMed/NCBI
|
|
26
|
Liu Y, Song A, Zang S, Wang C, Song G, Li
X, Zhu Y, Yu X, Li L, Wang Y and Duan L: Jinlida reduces insulin
resistance and ameliorates liver oxidative stress in high-fat fed
rats. J Ethnopharmacol. 162:244–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurisu S, Iwasaki T, Ishibashi K, Mitsuba
N, Dohi Y, Nishioka K and Kihara Y: Effects of low-dose
pioglitazone on glucose control, lipid profiles,
renin-angiotensin-aldosterone system and natriuretic peptides in
diabetic patients with coronary artery disease. J Renin Angiotensin
Aldosterone Syst. 14:51–55. 2013. View Article : Google Scholar
|
|
28
|
Sturton G, Persson C and Barnes PJ: Small
airways: An important but neglected target in the treatment of
obstructive airway diseases. Trends Pharmacol Sci. 29:340–345.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kucuk T and Deveci S: Can
'chromohysteroscopy' help target endometrial biopsy in
postmenopausal bleeding? Eur J Gynaecol Oncol. 29:165–167.
2008.
|
|
30
|
Wang S and Fischer PM: Cyclin-dependent
kinase 9: A key transcriptional regulator and potential drug target
in oncology, virology and cardiology. Trends Pharmacol Sci.
29:302–313. 2008. View Article : Google Scholar : PubMed/NCBI
|