Introduction
Steroid-induced avascular necrosis of the femoral
head, (SANFH) is a pathological process of femoral bone
degeneration, induced by steroids, which can cause the death of the
dynamic components of bone (1).
Statistical investigations have demonstrated that the hormone is
critical in non-traumatic osteonecrosis (2,3). At
present, the etiology and pathogenesis of steroid-induced
osteonecrosis remains to be fully elucidated. Emerging data has
indicated that various mechanisms are involved, including stasis
bone intraosseous hypertension due to venous stasis, osteoporosis,
fat embolism, fatty degeneration and necrosis of bone cells,
microvascular coagulation with intravenous vasoactive factors,
arterial vascular damage due to the accumulation of immune
complexes, apoptosis, bone marrow stromal stem cell adipogenic
differentiation and gene polymorphism (4,5).
Vascular endothelial growth factor (VEGF) is a glycoprotein, which
is characterized by its ability to promote angiogenesis and
osteogenic differentiation (6–8).
Osteoprotegerin (OPG) is a novel member of the tumor necrosis
factor (TNF) receptor family. It can inhibit osteoclast
differentiation and increase bone density function (9). Alternatively, receptor activator of
nuclear factor (NF)-κB (RANK) ligand (RANKL) can induce
pre-osteoclast differentiation into functional osteoclasts and
dose-dependently activate osteoclast maturation (10). It has been reported that RANKL is
the only cytokine, which can induce osteoclast differentiation and
development (11). RANK, in
combination with RANKL, can induce the osteoclast precursors to
differentiate, survive, integrate and inhibit the apoptosis of
osteoclast cells (12). The
RANKL/RANK/OPG pathway is critical in the regulation of osteoclast
differentiation and bone resorption. Wenyangbushen formula is a
prescription from the Orthopedics Department of the Second People's
Hospital Affiliated to Fujian Traditional Chinese Medicine
University (Fujian, China), and there is compelling evidence that
Wenyangbushen decoction is efficacious in treating SANFH.
Therefore, the present study was designed to investigate whether
treatment with Wenyangbushen formula ameliorates SANFH via VEGF and
the RANKL/RANK/OPG pathways.
Materials and methods
Preparation of Wenyangbushen formula
In the present study Wenyangbushen formula was
produced using the following components: Morinda (15 g), epimedium
(9 g), drynaria (9 g), deerhorn gelatin (6 g), Salvia
miltiorrhiza (9 g), Radix curcumae (9 g), Panax
pseudoginseng (3 g), Astragalus (15 g),
Achyranthes (9 g) and licorice (3 g). All the materials were
obtained from National Medicines Corporation, Ltd. (Beijing,
China). The preparation was processed into a decoction containing
the crude drug (2.5 g/ml) by the Second People's Hospital of Fujian
Province (Fujian, China).
Experimental animals and design
All the procedures and protocols complied with the
Animal Management Rule of the Ministry of Public Health, China
(doc. no. 55, 2001), and the experimental protocol was approved by
the Animal Care and Use Committee of Fujian University of
Traditional Chinese Medicine (Fuzhou, China). A total of 136 New
Zealand rabbits were obtained from the Department of Laboratory
Animal Science, Beijing University (Beijing, China). All animals
were maintained under standardized lighting conditions (12 h
light-dark cycle) and temperature (22±2°C). Normal diet and mineral
water were administered ad libitum. The rabbits were
randomly divided into the following five groups (n=24 rabbits in
each group): Normal group, SANFH model group, and three traditional
Chinese medicine (TCM) Wenyangbushen decoction groups, at low (6.44
g/kg·d), moderate (9.66 g/kg·d) and high (12.88 g/kg·d) doses of
the decoction. Following treatment with Wenyangbushen decoction for
8 weeks, the rabbits were anesthetized via intramuscular injection
of xylazine and ketamine at doses of 2.2 and 21 mg/kg body weight,
respectively. The femoral head tissues were then collected. At the
end of experiment, the rabbits were sacrificed by sodium
pentobarbital overdose (100 mg/kg; Dolethal, Vétoquinol
Especialidades Veterinarias, S.A., Madrid, Spain).
Model establishment
The SANFH model was replicated using horse serum
combined with methylprednisolone (Sigma-Aldrich, St. Louis, MO,
USA), according to a study by Hofstaetter et al (13). Briefly, the rabbits were
administered with 10 ml/kg horse serum via the ear vein and, after
3 weeks, 6 ml/kg horse serum was administered. After 2 weeks,
methylprednisolone was intraperitoneally injected at a dose of 45
mg/kg, once a day for three consecutive days. In addition,
penicillin at 100,000 units/rabbit was administered, once a day for
7 days, to prevent infection. The control animals were injected
with an equal volume of saline. After 4 weeks, the animal model was
established.
Histological analysis of the femoral head
tissues
Following the establishment of the animal model, two
rabbits in the normal control group and four rabbits in the model
group were randomly selected. Their femoral heads were removed,
fixed with 4% paraformaldehyde (Sigma-Aldrich) for 48 h (pH 7.4)
and decalcified with 10% EDTA-Tris buffer (Sigma-Aldrich). Paraffin
sections at 4 µm were stained with hematoxylin and eosin
(HE; Sigma-Aldrich). The pathological changes of the femoral head
were observed under an optical microscope (Olympus Corporation,
Tokyo, Japan). The successfully established animal model was
characterized by a reduction in bony trabeculae, disappearance of
bone cells in the lacunae and an increase in empty bone lacuna.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for detection of the mRNA
expression levels of VEGF, OPG, RANK and RANKL
The mRNA levels of VEGF, OPG, RANK and RANKL in the
left femoral head tissues were determined using a RT-qPCR system
(Beckman Coulter, Fullerton, CA, USA). Total RNA was isolated using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Total RNA (2 µg)
was reverse-transcribed using a Superscript First Strand Synthesis
System (Invitrogen; Thermo Fisher Scientific, Inc.) to generate
complementary DNA (cDNA). cDNA was immediately reverse-transcribed
from the isolated RNA. The sequences of the primers used are
indicated in Table I. Primers were
obtained from Invitrogen (Thermo Fisher Scientific, Inc.). The cDNA
samples were amplified in a DNA thermal cycler under the following
conditions: The mixture was annealed at 57°C for 30 sec, extended
at 72°C for 45 sec, and denatured at 94°C for 30 sec, with a total
of 30 cycles. This was followed by a final extension step at 72°C
for 10 min to ensure complete product extension. PCR was performed
in a volume of 20 µl containing 2 µl buffer (10X),
0.5 µl deoxynucleotide triphosphates (10 mM), 0.5 µl
Taq enzyme, 0.5 µl Primer F (10 mM), 0.5 µl Primer R
(10 mM), 14 µl ddH2O and 2 µl DNA
template. The qPCR products were resolved on a 1.5% agarose gel
(Sigma-Aldrich) and analyzed on a white/ultraviolet
transilluminator digital science and analysis system (14). The expression levels of the target
genes were determined in triplicate.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| VEGF |
CGTTTCCTTCCTCATCTCCTC |
GCTTGTTCCTCCTTCTTGCTC |
| OPG |
ACAATGAACAAGTGGCTGTGCTG |
CGGTTTCTGGGTCATAATGCAAG |
| RANK | TTCAGGTTTGCTGTTCCTACA
A |
CGCCGTTTTATCCTCTCTACAC |
| RANKL |
GCAGCATCGCTCTGTTCCTGTA |
GCATGAGTCAGGTAGTGCTTCTGTG |
| β-actin |
AGACCACCTTCAACTCGATCAT |
ACTCGTCATACTCCTGCTTGCT |
Western blotting to determine the protein
expression levels of VEGF, OPG, RANK and RANKL
The right femoral head tissues were collected and
lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich),
containing 50 mM Tris-Cl (pH 8.0), 150 mM NaCl, 1% Nonidet P-40,
0.5% sodium deoxycholate, 0.1% SDS, 100 µg/ml
phenylmethyl-sulfonyl fluoride and 2 µg/ml aprotinin. The
suspension was incubated on ice and then centrifuged (14,000 g, 10
min, 4°C). The soluble fraction was stored at −80°C. Protein
concentrations were measured using a Bradford assay with bovine
serum albumin as a standard. Proteins (40 µg) were run on a
12% SDS-PAGE gel (for VEGF and RANKL) or 8% SDS-PAGE gel (for RANK
and OPG), and then transferred onto polyvinylidene fluoride
membranes. Following incubation with 10% non-fat milk for 1 h, the
membranes were probed with primary antibodies overnight at 4°C and
then incubated with goresradish peroxidase-labeled anti-rabbit
secondary antibodies (1:2,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The protein levels were normalized using β-actin
as a loading control. The relative optical density of the protein
bands was measured using a Zeineh Laser Densitometer (Biomed
Instruments, Inc., Fullerton, CA, USA) following subtracting the
film background. Incubation with primary antibodies was then
performed at following dilutions: Primary antibodies were used as
follows: Mouse monoclonal anti-VEGF (1:500, cat. no. ab1316; Abcam,
Cambridge, UK); rabbit polyclonal anti-OPG (1:400; cat. no.
BA1475-1; Boster Biological Engineering Co. Ltd., Wuhan, China),
rabbit polyclonal anti-RANK (1:300; cat. no. BA1323; Boster
Biological Engineering Co., Ltd.) and rabbit polyclonal anti-RANKL
(1:650; cat. no. ab22113; Abcam). The intensity of the bands was
quantified using densitometry. The resulting blots are
representative of at least three experiments.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean, unless otherwise indicated. Group comparisons
were performed using Student's t-test (two sample test) or one-way
analysis of variance. A Mann-Whitney U test was used when the
variance was not normally distributed. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
Histological analysis using HE
staining
In the normal control group, the cartilage thickness
of the femoral head layer was normal. The cartilage surface was
smooth and lined with dense and regular bony trabeculae. The bone
structure was integral and the cells in each layer were evenly
distributed. Cartilage cells were arranged regularly. Bone cells
with normal morphology were clearly visible, located in the central
position of the bone lacuna, with occasional, scattered, empty
lacunae. The bone marrow cavity was rich in bone marrow cells, and
a small number of fat cells with normal morphology were noted
(Fig. 1A). In the model group, the
cartilage of the femoral head layer was thinner, compared with that
of the normal group (Fig. 1B).
Areas of the cartilage surface appeared exfoliated with cracks. The
bony trabeculae were loose, irregular, slender and sparse, and even
broken. All types of cells were scattered and sparsely distributed.
The numbers of chondrocytes were reduced substantially and often
clustered together. The number of bone cells was decreased,
compared with the normal model, and empty lacunae were observed.
The numbers of adipose cells were increased and were hypertrophied,
degenerate and becoming necrotic. By contrast, treatment with
Wenyangbushen decoction at the indicated concentrations (Fig. 1C–E) led to improvements in these
pathological changes.
In addition, the number of empty lacunae in the
normal group and in the model group were counted under a light
microscope. The ratios of empty lacunae in the normal group and
model group were 9.5±1.152 and 21±1.078%, respectively. The
difference between the two groups was statistically significant
(P<0.01). Wenyangbushen decoction treatment decreased the number
of empty lacunae.
Characteristics of bone cell
ultrastructure visualized under an electron microscope
In the normal control group (Fig. 2A), the bone cells were
predominantly elongated-oval shaped, located in the bone lacunae
and were plump in appearance. There were several prominentia on the
cell surface. The nuclear heterochromatin was uniform. The
mitochondria and rough endoplasmic reticulum were well-developed.
The number of glycogen granules were rich in the cytoplasm. A small
number of lysosomes were observed. The peripheral collagen fibers
were densely distributed and their composition was regular and
uniform.
In the model group (Fig. 2B), the bone cells exhibited
karyopyknosis, chromatin aggregation and nuclear fragmentation. The
bone cells were squeezed aside by large lipid droplets, and
exhibited various degrees of necrosis, apoptosis and empty bone
lacuna formation. The collagen fibers were thick and
disorganized.
In the low dose TCM-treatment group (Fig. 2C), the bone cells were increasedin
number, compared with the model group. The arrangement of rough
endoplasmic reticulum, mitochondria and collagen fibers were also
improved, compared with the model group. In the moderate- and
high-dose TCM treatment groups (Fig.
2D and E), the bone cells were further increased in number and
the organelles were well-organized and abundant in mitochondria and
rough endoplasmic reticulum. The distribution of collagen fibers
were dense and well arranged.
Wenyangbushen decoction upregulates the
mRNA expression levels of VEGF and OPG, and downregulates the mRNA
expression levels of RANK and RANKL in the SANFH model
Compared with the normal control rabbits, the mRNA
levels of VEGF and OPG were decreased (Fig. 3A and B), while those of RANK and
RANKL were increased (Fig. 3C and
D), in the SANFH model group. Wenyangbushen treatment prevented
these changes, which exhibited upregulation in the mRNA expression
levels of VEGF and OPG, and downregulation in the mRNA expression
levels of RANK and RANKL, compared with the model group. These
changes occurred in a concentration-dependent manner. In the
presence of a high dose of the Wenyangbushen decoction, the mRNA
expression levels of VEGF and OPG were increased by 45.5 and 42.1%,
respectively, and the expression levels of RANK and RANKL were
attenuated by 29.4 and 26.7% respectively, compared with those in
the model group (Fig. 3).
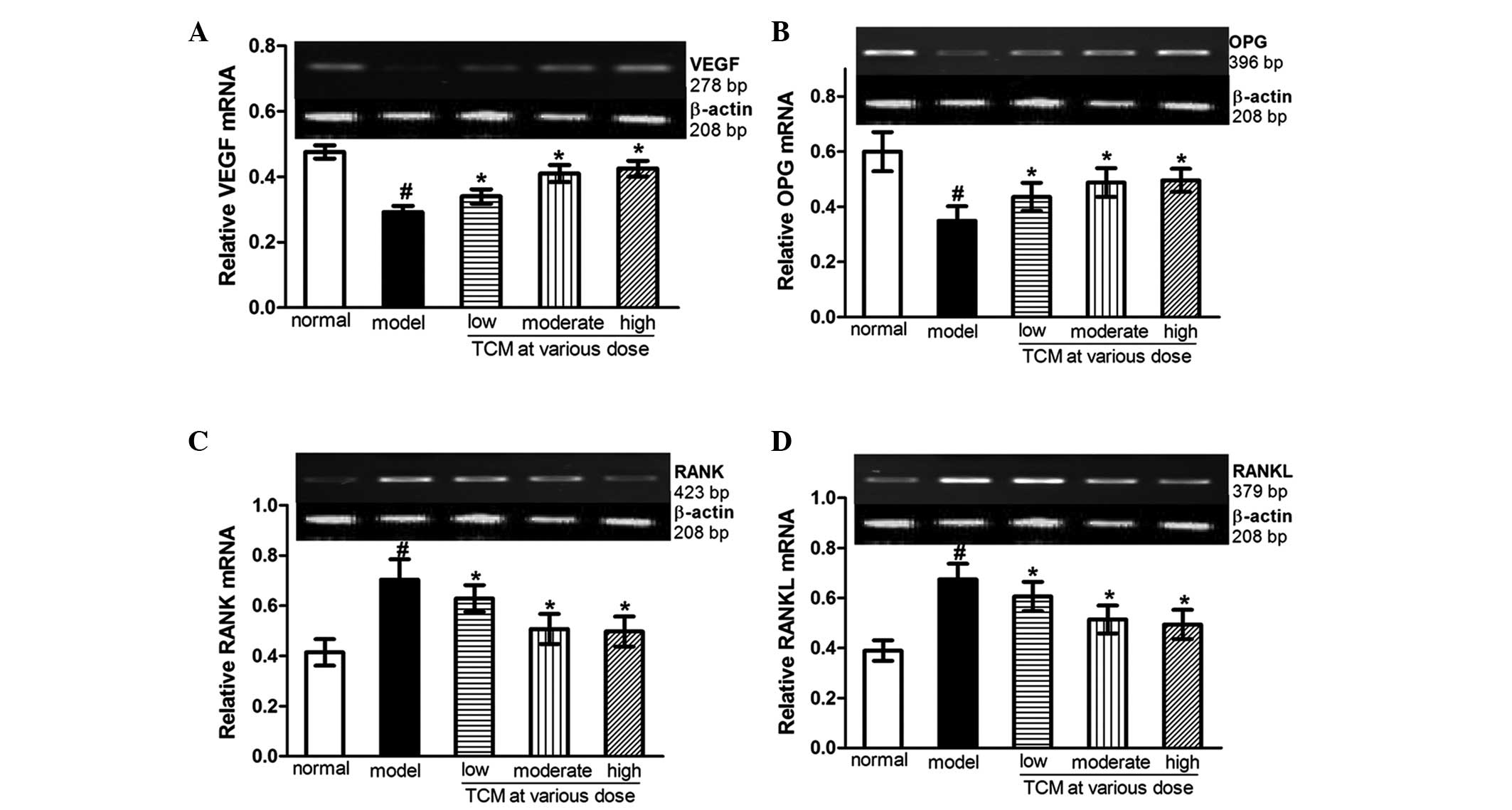 | Figure 3Effects of Wenyangbushen decoction on
the mRNA expression levels of VEGF, OPG, RANK and RANKL in the
steroid-induced avascular necrosis of the femoral head animal
model. The expression of (A) VEGF, (B) OPG, (C) RANK and (D) RANKL
were determined using reverse transcription-quantitative polymerase
chain reaction. β-actin served as an endogenous control. Results
are presented as the mean ± standard error of the mean of three
independent experiments in triplicate. #P<0.05, vs.
normal control; *P<0.05, vs. model. Low dose, 6.44
g/kg·d; moderate dose, 9.66 g/kg·d; high dose, 12.88 g/kg·d; TCM,
traditional Chinese medicine (Wenyangbushen decoction); VEGF,
vascular endothelial growth factor; OPG, osteoprotegerin; RANK,
receptor activator of nuclear factor-κβ; RANKL, RANK ligand. |
Effects of Wenyangbushen decoction on the
protein expression levels of VEGF, OPG, RANK and RANKL in the SANFH
model
Consistent with the results observed in the
examination of the mRNA expression levels, the protein expression
levels of VEGF and OPG (Fig. 4A and
B) were also reduced, while those of RANK and RANKL (Fig. 4C and D) were increased in the SANFH
group, compared with the model group. The protein expression levels
of VEGF and OPG were markedly enhanced, while those of RANK and
RANKL were inhibited by Wenyangbushen treatment, compared with the
model group, and this also occurred in a dose-dependant manner.
Compared with the SANFH model counterparts, treatment with a
high-dose of Wenyangbushen increased the expression of VEGF
1.43-fold and OPG 1.48-fold. Compared with the SANFH model group,
the protein expression levels of RANK and RANKL were decreased by
32.9 and 44.3%, respectively.
 | Figure 4Effects of Wenyangbushen decoction on
the protein expression levels of VEGF, OPG, RANK and RANKL in the
steroid-induced avascular necrosis of the femoral head animal
model. The expression levels of (A) VEGF, (B) OPG, (C) RANK and (D)
RANKL were determined using western blotting. β-actin served as a
loading control. Results are presented as the mean ± standard error
of the mean of three independent experiments in triplicate.
#P<0.05, vs. normal control; *P<0.05,
vs. model. Low dose, 6.44 g/kg·d; moderate dose, 9.66 g/kg·d; high
dose, 12.88 g/kg·d; TCM, traditional Chinese medicine
(Wenyangbushen decoction); VEGF, vascular endothelial growth
factor; OPG, 0steoprotegerin; RANK, receptor activator of nuclear
factor-κβ; RANKL, RANK ligand. |
Discussion
The pathogenesis of steroid induced femoral head
necrosis is complex and remains to be fully elucidated. Emerging
data suggests that SANFH is a multifactorial disease, which is
predominantly associated with bone structure destruction, cell
necrosis and vascular stasis. Therefore, the fundamental method of
treatment for SANFH is to promote the formation of bone and
regeneration of vessels (15,16),
and the effective management of these two aspects is a priority.
The fundamental prin ciple of Warming Yang and Tonifying Kidney was
established, according to the traditional Chinese medicine theory
'the kidney governs the bones and engenders marrow' (17). Our previous study indicated that
Wenyangbushen decoction promotes the differentiation of bone marrow
stromal cells, chondrocyte proliferation and ultimately prevents
the development of SANFH (18).
In the process of bone formation and absorption,
RANKL, RANK and OPG act in a combination to regulate the
differentiation of osteoblasts and osteoclasts. When various
stimulating factors act on osteoblasts or stromal cells to express
RANKL on their surface, they identify specifically the osteoclast
precursors and bind RANK receptor on the cell membrane (19). In the presence of M-CSF, the
RANK-RANKL signal can be transduced into the cells and stimulate
the maturation of osteoclast precursors into osteoclasts (20,21).
In addition, the actin ring in mature osteoclasts develops and is
activated via the RANK-RANKL pathway, which can promote bone
resorption, whereas OPG can competitively inhibit the binding of
RANKL and RANK, and inhibits the biological effects of
RANK-RANKL.
OPG also binds RANKL/RANK to form a trimer, which
can directly inhibit the function of RANKL/RANK. RANKL- or
RANK-deficient mice exhibit abnormal bone resorption and severe
bone sclerosis. OPG-deficient mice also develop osteoporosis
following birth, which can be alleviated by treatment with
recombinant OPG (22). Therefore,
the RANKL/RANK/OPG pathway is a key loop regulator of osteoblast
and osteoclast differentiation, as well as bone resorption
(23). The data of the present
study suggested that the expression levels of RANK and RANKL were
significantly increased, while that of OPG was decreased in the
femoral head tissues of the model group, compared with the normal
control. However, treatment with Wenyangbushen decoction was
observed to reverse these effects by inhibiting the expression of
RANK and RANKL, while upregulating the expression of OPG. This led
to the inhibition of osteoclast differentiation and function, as
well as the promotion of osteoblast activity.
The expression of VEGF in bone tissue is affected by
hormones. Decreased expression of VEGF can lead to a reduction in
angiogenesis in bone tissues and a lack of blood supply to the
femoral head, leading eventually to femoral head necrosis (24). Lee et al reported that the
VEGF gene promotes angiogenesis and stimulates bone growth in SANFH
(25). Similarly, the results of
the present study suggested that the expression of VEGF in the
model group was significantly decreased, compared with the normal
control group. Additionally, its expressionlevel was substantially
increased by Wenyangbushen decoction treatment, which indicated
that VEGF is critical in SANFH.
In conclusion, the results of the present study
demonstrated that Wenyangbushen decoction effectively promoted bone
cell, osteoblast and chondrocyte growth, and prevented cell
apoptosis. The mechanism underlying these effects may be associated
with it inhibiting the expression of RANK and RANKL, and promoting
the expression of OPG and VEGF in SANFH. Accordingly, Wenyangbushen
decoction may be considered a potential candidate drug for SANFH
treatment.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant. no. 81173283) and
Project of Fujian Province Educational Department (grant. no.
JA13156).
References
|
1
|
Beckmann R, Shaheen H, Kweider N, Ghassemi
A, Fragoulis A, Hermanns-Sachweh B, Pufe T, Kadyrov M and Drescher
W: Enoxaparin prevents steroid-related avascular necrosis of the
femoral head. Scientific World Journal. 2014:3478132014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheng H, Sheng CJ, Cheng XY, Zhang G, Lee
KM, Leung KS, Qu S and Qin L: Pathomorphological changes of bone
marrow adipocytes in process of steroid-associated osteonecrosis.
Int J Clin Exp Pathol. 6:1046–1050. 2013.PubMed/NCBI
|
|
3
|
Bekler H, Uygur AM, Gökçe A and
Beyzadeoğlu T: The effect of steroid use on the pathogenesis of
avascular necrosis of the femoral head: An animal model. Acta
Orthop Traumatol Turc. 41:58–63. 2007.In Turkish.
|
|
4
|
Steffen RT, Athanasou NA, Gill HS and
Murray DW: Avascular necrosis associated with fracture of the
femoral neck after hip resurfacing: Histological assessment of
femoral bone from retrieval specimens. J Bone Joint Surg Br.
92:787–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tong P, Wu C, Jin H, Mao Q, Yu N, Holz JD,
Shan L, Liu H and Xiao L: Gene expression profile of
steroid-induced necrosis of femoral head of rats. Calcif Tissue
Int. 89:271–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varoga D, Drescher W, Pufe M, Groth G and
Pufe T: Differential expression of vascular endothelial growth
factor in glucocorticoid-related osteonecrosis of the femoral head.
Clin Orthop Relat Res. 467:3273–3782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh LC and Lee JC: Osteogenic protein-1
increases gene expression of vascular endothelial growth factor in
primary cultures of fetal rat calvaria cells. Mol Cell Endocrino.
153:113–124. 1999. View Article : Google Scholar
|
|
8
|
Wang G, Zhang CQ, Sun Y, Feng Y, Chen SB,
Cheng XG and Zeng BF: Changes in femoral head blood supply and
vascular endothelial growth factor in rabbits with steroid-induced
osteonecrosis. J Int Med Res. 38:1060–1069. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simonet WS, Lacey DL, Cunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osetoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiotani A, Takami M, Itoh K, Shibasaki Y
and Sasaki T: Regulation of osteoclast differentiation and function
by recep to ractivator of NFkB ligand and osteoprotegerin. Anat
Rec. 268:137–146. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boyce BF and Xing L: The RANKL/RANK/OPG
pathway. Curr Osteoporos Rep. 5:98–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hofstaetter JG, Wang J, Yan J and Glimcher
MJ: The effects of alendronate in the treatment of experimental
osteonecrosis of the hip in adult rabbits. Osteoarthritis
Cartilage. 17:362–370. 2009. View Article : Google Scholar
|
|
14
|
Zhong X, Lin J, Zhou J, Xu W and Hong Z:
Anti-proliferative effects of qianliening capsules on prostatic
hyperplasia in vitro and in vivo. Mol Med Rep. 12:1699–1708.
2015.PubMed/NCBI
|
|
15
|
Seamon J, Keller T, Saleh J and Cui Q: The
pathogenesis of nontraumatic osteonecrosis. Arthritis.
2012:6017632012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang L, Boyd K, Kaste SC, Kamdem L, Rahija
RJ and Relling MV: A mouse model for glucocorticoid-induced
osteonecrosis: effect of a steroid holiday. J Orthop Res.
7:169–175. 2009. View Article : Google Scholar
|
|
17
|
Liu R, Kang X, Xu L, Nian H, Yang X, Shi H
and Wang X: Effect of the combined extracts of Herba epimedii and
Fructus ligustri lucidi on sex hormone functional levels in
osteoporosis rats. Evid Based Complement Alternat Med.
2015:1848022015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen WH and Wang HM: Experimental research
progress of warming yang and reinforcing kidney of Chinese medicine
to promote the differentiation of bone marrow stromal cells.
Zhongguo Gu Shang. 24:352–356. 2011.In Chinese. PubMed/NCBI
|
|
19
|
Bai YD, Yang FS, Xuan K, Bai YX and Wu BL:
Inhibition of RANK/RANKL signal transduction pathway: a promising
approach for osteoporosis treatment. Med Hypotheses. 71:256–258.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HK, Morgan-Bagley S and Kostenuik P:
RANKL inhibition: A novel strategy to decrease femoral head
deformity after ischemic osteonecrosis. J Bone Miner Res.
21:1946–1954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wittrant Y, Theoleyre S, Couillaud S,
Dunstan C, Heymann D and Rédini F: Relevance of an in vitro
osteoclastogenesis system to study receptor activator of NF-kB
ligand and osteoprotegerin biological activities. Exp Cell Res.
293:292–301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Geusens P: The role of RANK
ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv
Musculoskelet Dis. 4:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mandelin J, Li TF, Liljeström M, Kroon ME
and Hanemaaijer R: I mba la nce of RANKL/RANK/OPG system in
interface tissue in loosening of total hip replacement. J Bone
Joint Surg Br. 85:1196–1201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Varoga D, Drescher W, Pufe M, Groth G and
Pufe T: Differential expression of vascular endothelial growth
factor in glucocorticoid-related osteonecrosis of the femoral head.
Clin Orthop Relat Res. 467:3273–3282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee YJ, Lee JS, Kang EH, Lee YK, Kim SY,
Song YW and Koo KH: Vascular endothelial growth factor
polymorphisms in patients with steroid-induced femoral head
osteonecrosis. J Orthop Res. 30:21–27. 2012. View Article : Google Scholar
|


















