Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumor types, with a rate of incidence that is
increasing worldwide (1).
According to the 'Word Cancer Report 2014' of the Word Health
Organization, new cases reported of HCC and the mortality rate in
China are the highest worldwide, with an incidence rate of
~25.7/100,000 individuals (2).
Previous studies reported that the predominant risk factor of HCC
in China is chronic infection by hepatitis B virus, although other
major risk factors include infection with the hepatitis C virus, an
excessive alcohol consumption, tobacco smoking and aflatoxins
(3–5). At present, observable symptoms of HCC
at an early stage are lacking, and therefore clinically symptomatic
HCC is often only identified when the disease is already well
advanced in patients, which makes treatment difficult, and the
prognosis is poor (1). In common
with a number of other types of tumor, distant metastasis is the
major cause of mortality for patients with HCC. Therefore, the
ability to control the dissemination of cancer cells at an early
stage is the focus of numerous studies.
N-glycosyltransferase-V (GnT-V), as a key member of
the glycosyltransferase family, is closely associated with the
proliferation, migration and invasion of cancer cells (6–7).
Previous studies reported that GnT-V is commonly overexpressed in
various advanced tumor types, including prostate cancer, oral
squamous cell carcinoma, colorectal and breast cancer, gastric
cancer cells and ovarian mucinous cancer (8–13).
Notably, previous studies identified that the downregulation of
GnT-V markedly suppressed the proliferation and migration of tumor
cells, and induced cell apoptosis (11,14,15).
Preliminary experiments in our laboratory demonstrated that GnT-V
is highly expressed in SMMC7721 cells (unpublished data), although
the effect of reducing the expression of GnT-V on the
proliferation, migration and invasion of SMMC7721/R cells remains
to be fully elucidated.
In the present study, the role of GnT-V-knockdown on
the growth of human HCC was investigated. The expression of GnT-V
was suppressed in SMMC7721/R cells using short hairpin (sh)RNA
analysis, and the effects of GnT-V-knockdown on the proliferation,
adhesion, invasion and apoptosis of the SMMC7721/R cells in
vitro were examined. Furthermore, the potential mechanisms
underlying the observed effects were investigated using western
blotting and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR).
Materials and methods
Reagents and cell lines
The human SMMC7721 HCC cell line was obtained from
the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The human HCC drug-resistant cell line
(SMMC7721/R) was developed from the SMMC7721 cell line using
continuous exposure to adriamycin, as previously described
(16). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
0.25% trypsin solution, sodium dodecyl sulfate (SDS),
phosphate-buffered saline (PBS) and Giemsa stain were obtained from
Sigma-Aldrich (St. Louis, MO, USA). The annexin V/fluorescein
isothiocyanate (FITC) kit and Matrigel™ were purchased from BD
Biosciences (San Jose, CA, USA), and Transwell® culture
chambers were purchased from Corning Costar, Inc. (Corning, NY,
USA). Propidium iodide (PI) was purchased from Beyotime Institute
of Biotechnology (Jiangsu, China). Rabbit GAPDH polyclonal antibody
(cat no. sc-25778; 1:200 dilution), rabbit B-cell lymphoma 2
(Bcl-2) polyclonal antibody (cat no. sc-492; 1:200 dilution),
rabbit Bcl-2-associated X protein (Bax) polyclonal antibody (cat
no. sc-493; 1:200 dilution), rabbit matrix metalloproteinase
(MMP)-2 polyclonal antibody (cat no. sc-10736; 1:200 dilution),
rabbit MMP-9 polyclonal antibody (cat no. sc-10737; 1:200 dilution)
and goat-anti-rabbit horseradish-peroxidase-conjugated secondary
antibody (cat no. sc-2004; 1:10,000 dilution) were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit polyclonal
antibody to active caspase-3 (cat no. ab2302; 1:200 dilution) and
rabbit polyclonal antibody to active caspase-9 (cat no. ab2324;
1:200 dilution) were obtained from Abcam (Cambridge, MA, USA). The
enhanced chemiluminescence (ECL) reagent was provided by Beyotime
Institute of Biotechnology (Haimen, China).
Cell culture and transient
transfection
The SMMC7721/R cells were cultured in Gibco
BRL® RPMI-1640 medium, supplemented with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin and streptomycin (Beyotime Institute of
Biotechnology) at 37°C in a 5% CO2 humidified
atmosphere.
The expression of GnT-V was knocked down using
short-hairpin (sh)RNAs in a pGenesil-4 shRNA lentiviral vector
provided by Wuhan Cell Marker and Machine Technology Co., Ltd.
(Wuhan, China). The recombinant lentiviruses were transfected into
SMMC7721/R cells using Invitrogen® Lipofectamine 2000
reagent (Thermo Fisher Scientific, Inc.). The stably transfected
cells were selected in RPMI-1640 medium, containing G418, and were
termed SMMC7721/R-GnT-V and SMMC7721/R-GnT-V/NC, (signifying
treatment with control vector, or as a control group,
respectively).
RT-qPCR
The mRNA expression levels of GnT-V, the breast
cancer resistance protein (BCRP) and P-glycoprotein [also termed
the ATP-binding cassette, subfamily B protein (ANCB1)] were
quantified using SYBR Green-based RT-qPCR analysis (Bio-Rad
Laboratories, Shanghai, China). The primer sequences are listed in
Table I (17,18).
The total RNA from cells was isolated using RNAiso Plus extraction
reagent (Takara Biotechnology Co., Ltd., Dalian, China).
Subsequently, cDNA was synthesized from 1 µg total RNA using
PrimeScript™ RT reagent kits (Takara Biotechnology Co., Ltd.). The
cDNAs were amplified using SYBR Green mixture on a CFX96 Touch
Real-Time PCR Detection system (Bio-Rad Laboratories). The cycle
threshold values were normalized against the amplification of
GAPDH, and the relative mRNA expression levels of GnT-V, BCRP and
ABCB1 were assessed using 2−∆∆Cq relative quantitative
analysis of each sample. All samples were analyzed in
triplicate.
 | Table IPrimers for reverse
transcription-quantitative polymerase chain reaction used in the
present study. |
Table I
Primers for reverse
transcription-quantitative polymerase chain reaction used in the
present study.
| Gene | Sequence (5′-3′) | Amplicon size
(bp) | Reference |
|---|
| GAPDH | Forward:
GACCCCTTCATTGACCTCAAC | 219 | (17) |
| Reverse:
CTTCTCCATGGTGGTGAAGA | | |
| GnT-V | Forward:
GAAAATGGAATCTGAACCCTCA | 160 | (11) |
| Reverse:
ACTTTGCCATACACAAGGGACT | | |
| BCRP | Forward:
CACCACCTCCTTCTGTCATCAA | 127 | (17) |
| Reverse:
GGCACCTATAACCAGTCCCAGTA | | |
| ABCB1 | Forward:
CCCATCATTGCAATAGCAGG | 158 | (18) |
| Reverse:
TGTTCAAACTTCTGCTCCTGA | | |
Western blot analysis
The cells were lysed using western blotting and an
immunoprecipitation cell lysis buffer kit (Sangon Biotech Co.,
Ltd., Shanghai, China) and the total proteins were quantified using
a bicinchoninic acid protein assay reagent kit (Sangon Biotech Co.,
Ltd.). The total protein (20 µg) was loaded onto
SDS-polyacrylamide electrophoresis gels (Bio-Rad Laboratories), and
subsequently transferred onto a nitrocellulose filter membrane
(Bio-Rad Laboratories). Anti-Bcl2, Bax, caspase-3, caspase-9,
MMP-2, MMP-9 and GAPDH antibodies were used to assess the
corresponding protein expression at 4°C for 12 h. The
horseradish-peroxidase-conjugated anti-rabbit and anti-rat
immunoglobulins were used as secondary antibodies, and the
immunoreactive bands were visualized using ECL-detecting
reagents.
MTT assay
A total of 5×103 cells/well were seeded
into 96-well plates, and following an incubation for 0, 12, 24 or
48 h, the proliferation of the cells was determined using an MTT
assay, according to the manufacturer's protocol. Subsequently, the
optical density values were measured at 490 nm using a 96-well
plate reader (Thermo Scientific® Multiscan MK3; Thermo
Fisher Scientific, Inc.).
Apoptosis assay using flow cytometric
analysis
The extent of cell apoptosis was analyzed using an
Annexin V-FITC kit (BD Biosciences) according to the manufacturer's
instructions. In brief, cells were harvested following an overnight
incubation in serum-free medium and 5×104 cells were
washed with PBS and stained PI and Annexin V for flow-cytometric
analysis on a FACSCalibur flow cytometer (BD Biosciences). The
percentage of cells undergoing early-stage apoptosis was determined
by quantifying the Annexin V-positive and the PI-negative cell
population, whereas the percentage of cells undergoing late-stage
apoptosis was determined by quantifying the Annexin V-positive and
the PI-positive cell population. Finally, data were analyzed using
FlowJo 7.6 software (FlowJo, LLC, Ashland, OR, Canada).
Cell adhesion assay
Matrigel™-coated 12-well plates were used to assess
cell adhesion. Prior to use, the cells were grown until they
reached 90% confluence on 12-well plates, and they were
subsequently cultured in serum-free RPMI-1640 medium for 24 h. The
cells were harvested following an overnight incubation in
serum-free medium and subsequently suspended in RPMI-1640 medium
containing 10% fetal bovine serum (FBS), prior to adding the cells
to 12-well plates that were pre-coated with Matrigel™
(2×104 cells/well). Following an incubation for 1 h at
37°C in 5% CO2, the unattached cells were removed by
washing the cells twice with warmed PBS, and the number of adhering
cells was determined using the Giemsa staining method for 10 min.
The stained cells were observed under an optical microscope (IX53;
Olympus, Tokyo, Japan).
In vitro invasion assay
The invasive ability of the cells was measured using
Transwell culture chambers with Matrigel™. Briefly, the cells were
grown until they reached 90% confluence on 24-well plates, and they
were subsequently cultured in the serum-free RPMI-1640 medium for
24 h prior to use. A series of 24-well plates and Transwell well
culture chambers pro-coated with Matrigel™ were washed in PBS for 5
min, and dried immediately. Aliquots of 0.75 ml RPMI-1640 medium
supplemented with 10% FBS were added to the upper chamber and 0.5
ml cells (at a density of 1×105/ml) in RPMI-1640 medium,
containing 1% FBS, were placed in the upper chamber. Following
incubation for 48 h at 37°C in 5% CO2, the number of
cells which had invaded through the Matrigel™-coated polyvinylidene
fluoride filter was determined by counting the cells stained with
0.5% crystal violet solution. The stained cells were observed under
an optical microscope (IX53; Olympus).
Statistical analysis
The data are expressed as the mean ± standard
deviation. The data analysis was performed using the SPSS 19.0
statistical software package (IBM SPSS, Armonk, NY, USA), and
one-way analysis of variance was used to compare the means between
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of GnT-V in SMMC7721/R cells
following transfection
Following transfection, the expression of GnT-V in
the SMMC7721/R cells was determined using RT-qPCR and western
blotting. As shown in Fig. 1A, the
gene expression of GnT-V was markedly downregulated in the
SMMC-7721/R-GnT-C cells compared with the control and mock groups.
This trend was also observed in the protein expression level of
GnT-V following transfection (Fig.
1B). These results indicated that GnT-V-knockdown in the
SMMC-7721/R cells was successful.
GnT-V-knockdown inhibits the
proliferation of the SMMC7721/R cells
The proliferation of the SMMC-7721/R cells was
assessed using an MTT assay following transfection. As shown in
Fig. 2, compared with the two
control cell groups, the proliferation of the SMMC-7721/R-GnT-C
cells decreased significantly at 12, 24 and 48 h following
transfection (P<0.01 for all the time points). However, no
significant differences were identified between the untreated
SMMC-7721/R and the SMMC-7721/R-GnT-V/NC groups (P>0.05). These
results therefore revealed that GnT-V-knockdown significantly
inhibited the proliferation of the SMMC-7721/R cells.
Downregulation of GnT-V enhances cell
apoptosis
The extent of cell apoptosis was analyzed using flow
cytometry to investigate whether the antiproliferative effect of
GnT-V downregulation was associated with apoptosis in the
SMMC-7721/R cells. As shown in Fig.
3, the apoptosis rate of the GnT-V knockdown group was
significantly higher compared with that in the untreated
SMMC-7721/R cells and the SMMC-7721/R-GnT-V/NC group (43.5, 4.2 and
6.3%, respectively), which indicated that decreasing the expression
of GnT-V may markedly increase the levels of apoptosis in the
SMMC-7721/R cells.
Downregulation of GnT-V inhibits cell
adhesion and invasion in vitro
To determine the role of GnT-V on the in
vitro adhesion and invasion of the SMMC-7721/R cells, cell
adhesion and invasion assays were performed. As shown in Fig. 4A, compared with the untreated cells
and the mock group, cell adhesion was significantly inhibited by
GnT-V-knockdown in the SMMC-7721/R cell line. Notably, the results
of the cell invasion assay were similar to those of the cell
adhesion assay (Fig. 4B). The
results of these experiments revealed that down-regulating the
expression of GnT-V clearly suppressed the adhesion and the
invasion of the SMMC-7721/R cells in vitro.
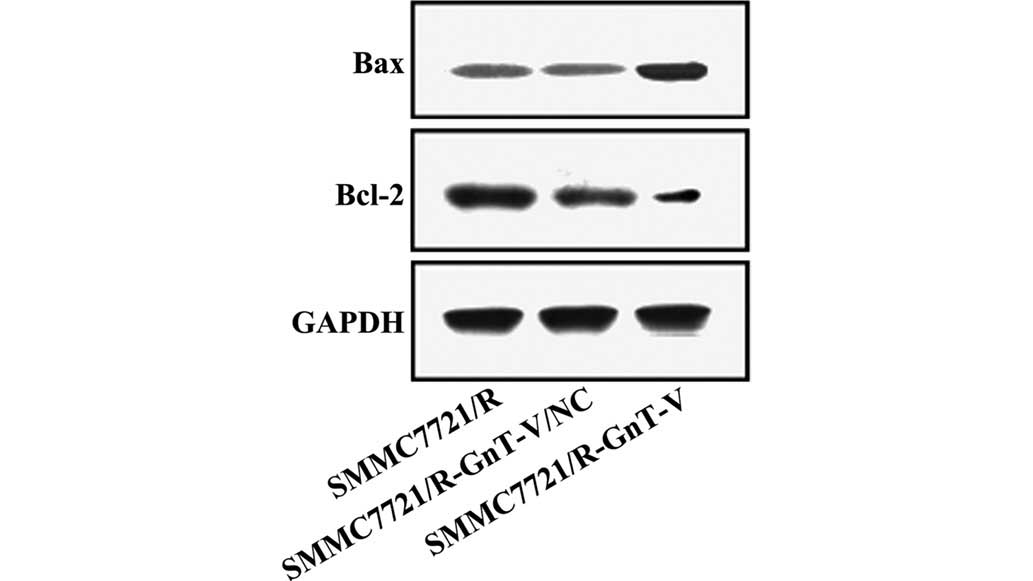
Protein expression levels of caspase-3,
caspase-9, Bcl-2, Bax, MMP-2 and MMP-9
Thus far, the experiments performed in the present
study revealed that GnT-V-knockdown may inhibit the proliferation,
adhesion and invasion of the SMMC-7721/R cells in vitro. To
further investigate the possible mechanisms underlying these
changes, the protein expression levels of caspase-3, caspase-9,
Bcl-2, Bax, MMP-2 and MMP-9 were assessed by western blotting. As
shown in Figs. 5Figure 6–7, no significant differences were
observed between the untreated SMMC-7721/R cells and the
SMMC-7721/R-GnT-V/NC group with respect to the expression levels of
any of the proteins. Notably, compared with the untreated
SMMC-7721/R cells and the SMMC-7721/R-GnT-V/NC group, the protein
expression levels of caspase-3, caspase-9, Bcl-2, MMP-2 and MMP-9
were clearly decreased in the GnT-V knockdown group, whereas the
protein expression of Bax was markedly upregulated.
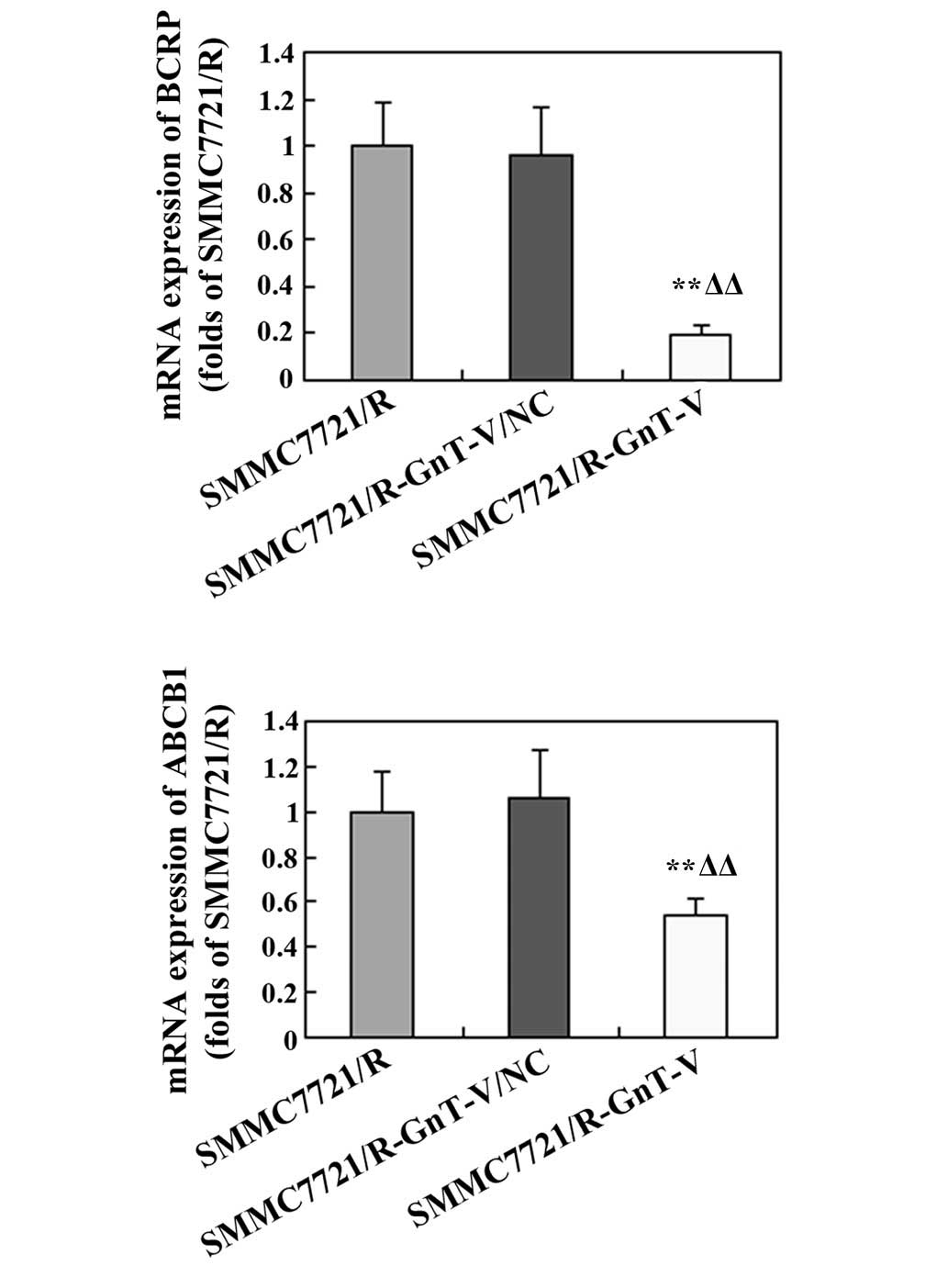
mRNA expression levels of BCRP and
ANCB1
To further examine whether GnT-V-knockdown affected
the drug-sensitivity of the SMMC-7721/R cells, the mRNA expression
levels of BCRP and ABCB1 were assessed by RT-qPCR. As shown in
Fig. 8, the expression levels of
these genes were markedly reduced in the GnT-V-knockdown cells
compared with the untreated SMMC-7721/R cells and the
SMMC-7721/R-GnT-V/NC group.
Discussion
In the present study, GnT-V knockdown in
vitro was revealed to markedly decrease the proliferation,
migration and invasion of human HCC drug-resistant SMMC-7721/R
cells. In addition, the inhibitory effects may be involved in
inducing apoptosis of the cells, in inhibiting the degradation of
the extracellular matrix (ECM) and in restoring drug-sensitivity.
Previous studies reported that protein glycosylation exerts a
crucial role in cell growth, differentiation and tumor metastasis,
and β1,6-branched oligosaccharides are key compounds associated
with the process of malignant transformation (6,19,20).
A previous study demonstrated that GnT-V is an important
glycosyltransferase, which promotes this malignant transformation
process by catalyzing the formation of β1,6-branched
oligosaccharides (19).
Furthermore, GnT-V is overexpressed in various malignant tumor
types, thereby promoting the malignant transformation process
(21,22). Therefore, it was hypothesized that
reducing the expression of GnT-V may provide a suitable strategy
for ameliorating the progression of certain tumor types. To
determine the effect of downregulating the expression of GnT-V on
the progression of HCC, a GnT-V-knockdown cell model was
successfully constructed by transferring short hairpin (sh) RNA
into SMMC-7721/R cells.
As demonstrated by preliminary in vitro
experiments in our group, GnT-V-knockdown markedly decreased the
proliferation, migration and invasion of human HCC drug-resistant
SMMC-7721/R cells (unpublished data). Notably, increased levels of
apoptosis were also observed in the GnT-V-knockdown cells.
According to these results, the antiproliferative effects of
GnT-V-knockdown on SMMC-7721/R cells may be closely associated with
the increased apoptosis that is induced by the downregulation of
the expression of GnT-V. Apoptosis, or programmed cell death, is a
complex biological process, which is important for the development
and maintenance of cells. It was reported that caspases exert a
crucial role during cell apoptosis (23). Among them, caspase-3 is an
important effector of cell mortality, whereas caspase-9 functions
as a crucial upstream activator (23). In the present study, the protein
expression levels of caspase-3 and caspase-9 were significantly
upregulated in the GnT-V-knockdown SMMC-7721/R cells, which was
consistent with the results of a previous study performed in H7721
human HCC cells (21).
Furthermore, the proteins Bcl-2 and Bax are also essential for cell
apoptosis, functioning as the predominant controller and mediator
of apoptosis, respectively, and the ratio of Bcl-2 to Bax is a key
factor determining whether the switch to apoptosis is made
(24,25). The results of the present study
demonstrated that the protein expression level of Bcl-2 was
downregulated, whereas that of Bax was upregulated. Furthermore,
GnT-V-knockdown may increase the expression of Bcl-2, whereas the
protein expression of caspase-3, caspase-9 and Bax were decreased.
Therefore, inhibiting the expression of GnT-V promoted
mitochondrial-associated apoptosis, which is mediated by caspase-3,
caspase-9, Bcl-2 and Bax.
Furthermore, previous studies demonstrated that
GnT-V exerts an important role in the metastasis/invasion of
various types of tumor. For example, a close association was
identified between GnT-V activity and tumor invasiveness in the
sera of patients with HCC (19).
Additionally, in vitro GnT-V-knockdown may decrease the
invasive ability of the BGC823 gastric cancer cell line (11). Notably, the present results
demonstrated that suppressing the expression of GnT-V markedly
reduced cell-to-cell adherence and the invasive abilities of the
SMMC-7721/R cells. Cellular metastasis and invasion is a complex
and crucial process in cancer. It is well known that cell-to-cell
adherence and invasion of the ECM is responsible for malignant
neoplasms, and ECM and basement membranes are predominantly
degraded by MMPs (26). MMP-2 and
MMP-9 exert key roles in degrading the ECM components, and are also
important in tumor progression, as demonstrated by their
overexpression in advanced tumor types (27,28).
The mRNA expression of MMP-2 and MMP-9 were demonstrated to be
upregulated in HCCs, although they were expressed with different
intensities, and had a different cellular origin (29). The mRNA expression of MMP-9 was
higher in HCCs with capsular infiltration compared with HCCs that
lacked capsular infiltration, whereas the mRNA expression of MMP-2
exhibited no marked difference between tumorous and non-tumorous
tissues (30). In the present
study, the protein expression levels of MMP-2 and MMP-9 were
markedly decreased in the SMMC-7721/R-GnT-V cells. According to
these results, the reduced invasive ability of the
SMMC-7721/R-GnT-V cells is closely associated with the
downregulation of the protein expression of MMP-2 and MMP-9.
In addition, multidrug resistance (MDR), resulting
from the overexpression of MDR proteins, is a major obstacle in
cancer chemotherapy. A number of previous studies revealed that MDR
proteins are predominantly encoded by ATP-binding cassette (ABC)
families, which may mediate drug efflux to the cytomembrane,
leading to lower levels of chemicals in the plasma (31,32).
BCRP and ABCB1, as members of the ABC transporter family of
proteins, are important mediators of MDR in cancer cells (33–35).
The overexpression of BCRP and ABCB1 was frequently identified in
various tumor types, including HCC, ovarian tumor and breast cancer
(36–39). In the present study, the mRNA
expression of BCRP and ANCB1 was notably reduced in the
GnT-V-knockdown SMMC-7721/R cells, indicating that GnT-V-knockdown
may improve the sensitivity of the human HCC drug-resistant cell
line, SMMC7721/R, to chemotherapies.
In conclusion, GnT-V-knockdown inhibited the
proliferation, migration and invasion of human HCC drug-resistant
SMMC7721/R cells in vitro. The underlying mechanisms may be
associated with the induction of mitochondrial-mediated apoptosis,
a suppression of the degradation of ECM components of the basement
membrane, and a strengthening of the drug sensitivity of the
cells.
Acknowledgments
The present study was supported by the Support
Program of the Department of Science and Technology of Sichuan
Province (no. 2009JY0096).
References
|
1
|
Lafaro KJ, Demirjian AN and Pawlik TM:
Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am.
24:1–17. 2015. View Article : Google Scholar
|
|
2
|
Stewart BW and Wild CP: World Cancer
Report 2014. IARC; Nonserial Publication: 2014
|
|
3
|
Nordenstedt H, White DL and El-Serag HB:
The changing pattern of epidemiology in hepatocellular carcinoma.
Dig Liver Dis. 42(Suppl 3): S206–S214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB and Kanwal F: Epidemiology of
hepatocellular carcinoma in the United States: Where are we? Where
do we go? Hepatology. 60:1767–1775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kew MC and Kew MC: Hepatocellular
carcinoma: Epidemiology and risk factors. J Hepa Carc. 1:115–125.
2014.
|
|
6
|
Chakraborty AK and Pawelek JM: GnT-V,
macrophage and cancer metastasis: A common link. Clin Exp
Metastasis. 20:365–373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song K, Ko JH and Kim YS: Role of
N-acetylglucosaminyltra nsferase-V and galectin-3 binding protein
in anoikis stress of cancer cells (788.1). FASEB J. 28:781–788.
2014.
|
|
8
|
Murata K, Miyoshi E, Kameyama M, Ishikawa
O, Kabuto T, Sasaki Y, Hiratsuka M, Ohigashi H, Ishiguro S, Ito S,
et al: Expression of N-acetylglucosaminyltransferase V in
colorectal cancer correlates with metastasis and poor prognosis.
Clin Cancer Res. 6:1772–1777. 2000.PubMed/NCBI
|
|
9
|
Handerson T, Camp R, Harigopal M, Rimm D
and Pawelek J: β1, 6-branched oligosaccharides are increased in
lymph node metastases and predict poor outcome in breast carcinoma.
Clin Cancer Res. 11:2969–2973. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi N, Yamamoto E, Ino K, Miyoshi E,
Nagasaka T, Kajiyama H, Shibata K, Nawa A and Kikkawa F: High
expression of N-acetylglucosaminyltransferase V in mucinous tumors
of the ovary. Oncol Rep. 22:1027–1032. 2009.PubMed/NCBI
|
|
11
|
Huang B, Sun L, Cao J, Zhang Y, Wu Q,
Zhang J, Ge Y, Fu L and Wang Z: Downregulation of the GnT-V gene
inhibits metastasis and invasion of BGC823 gastric cancer cells.
Oncol Rep. 29:2392–2400. 2013.PubMed/NCBI
|
|
12
|
Seto K, Uchida F, Baba O, Yamatoji M,
Karube R, Warabi E, Sakai S, Hasegawa S, Yamagata K, Yanagawa T, et
al: Negative expression of N-acetylglucosaminyltransferase V in
oral squamous cell carcinoma correlates with poor prognosis.
Springerplus. 2:6572013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang H, Chen W, Liu Q, Wei T, Zhu W, Meng
H, Guo L and Zhang J: Inhibition of N-acetylglucosaminyltransferase
V enhances sensitivity of radiotherapy in human prostate cancer.
Biochem Biophys Res Commun. 451:345–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo HB, Liu F, Zhao JH and Chen HL:
Down-regulation of N-acetylglucosaminyltransferase V by
tumorigenesis- or metastasis-suppressor gene and its relation to
metastatic potential of human hepatocarcinoma cells. J Cell
Biochem. 79:370–385. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taniguchi N, Ihara S, Saito T, Miyoshi E,
Ikeda Y and Honke K: Implication of GnT-V in cancer metastasis: A
glycomic approach for identification of a target protein and its
unique function as an angiogenic cofactor. Glycoconj J. 18:859–865.
2001. View Article : Google Scholar
|
|
16
|
Yang JY, Luo HY, Lin QY, Liu ZM, Yan LN,
Lin P, Zhang J and Lei S: Subcellular daunorubicin distribution and
its relation to multidrug resistance phenotype in drug-resistant
cell line SMMC-7721/R. World J Gastroenterol. 8:644–649. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li GP, Chen XP and Ye L: The role of BCRP
in hepatocellular carcinoma multidrug resistance and mechanism. J
Abdom Surg. 5:242006.
|
|
18
|
Albermann N, Schmitz-Winnenthal FH,
Z'graggen K, Volk C, Hoffmann MM, Haefeli WE and Weiss J:
Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1,
MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear
cells and their relationship with the expression in intestine and
liver. Biochem Pharmacol. 70:949–958. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yanagi M, Aoyagi Y, Suda T, Mita Y and
Asakura H: N-Acetylglucosaminyltransferase V as a possible aid for
the evaluation of tumor invasiveness in patients with
hepatocellular carcinoma. J Gastroenterol Hepatol. 16:1282–1289.
2001. View Article : Google Scholar
|
|
20
|
Dosaka-Akita H, Miyoshi E, Suzuki O, Itoh
T, Katoh H and Taniguchi N: Expression of
N-acetylglucosaminyltransferase v is associated with prognosis and
histology in non-small cell lung cancers. Clin Cancer Res.
10:1773–1779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo P, Chen HJ, Wang QY and Chen HL: Down
regulation of N-acetylglucosaminyltransferase V facilitates
all-transretinoic acid to induce apoptosis of human hepatocarcinoma
cells. Mol Cell Biochem. 284:103–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyoshi E, Terao M and Kamada Y:
Physiological roles of N-ac etylglucosaminyltransferase V (GnT-V)
in mice. BMB Rep. 45:554–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beerheide W, Tan YJ, Teng E, Ting AE,
Jedpiyawongse A and Srivatanakul P: Downregulation of proapoptotic
proteins Bax and Bcl-X(S) in p53 overexpressing hepatocellular
carcinomas. Biochem Biophys Res Commun. 273:54–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng B, Zhang XF, Zhu XC, Huang H, Jia HL,
Ye QH, Dong QZ and Qin LX: Correlation and prognostic value of
osteopontin and Bcl-2 in hepatocellular carcinoma patients after
curative resection. Oncol Rep. 30:2795–2803. 2013.PubMed/NCBI
|
|
26
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmalfeldt B, Prechtel D, Härting K,
Späthe K, Rutke S, Konik E, Fridman R, Berger U, Schmitt M, Kuhn W,
et al: Increased expression of matrix metalloproteinases (MMP)-2,
MMP-9, and the urokinase-type plasminogen activator is associated
with progression from benign to advanced ovarian cancer. Clin
Cancer Res. 7:2396–2404. 2001.PubMed/NCBI
|
|
28
|
Karahan N, Güney M, Baspinar S, Oral B,
Kapucuoglu N and Mungan T: Expression of gelatinase (MMP-2 and
MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinoma. Eur J
Gynaecol Oncol. 28:184–188. 2007.PubMed/NCBI
|
|
29
|
Määttä M, Soini Y, Liakka A and
Autio-Harmainen H: Differential expression of matrix
metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in
hepatocellular and pancreatic adenocarcinoma: Implications for
tumor progression and clinical prognosis. Clin Cancer Res.
6:2726–2734. 2000.PubMed/NCBI
|
|
30
|
Arii S, Mise M, Harada T, Furutani M,
Ishigami S, Niwano M, Mizumoto M, Fukumoto M and Imamura M:
Overexpression of matrix metalloproteinase 9 gene in hepatocellular
carcinoma with invasive potential. Hepatology. 24:316–322. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
32
|
Gillet JP, Efferth T and Remacle J:
Chemotherapy-induced resistance by ATP-binding cassette transporter
genes. Biochim Biophys Acta. 1775:237–262. 2007.PubMed/NCBI
|
|
33
|
Haimeur A, Conseil G, Deeley RG and Cole
SP: The MRP-related and BCRP/ABCG2 multidrug resistance proteins:
Biology, substrate specificity and regulation. Curr Drug Metab.
5:21–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leslie EM, Deeley RG and Cole SP:
Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2,
and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol.
204:216–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Natarajan K, Xie Y, Baer MR and Ross DD:
Role of breast cancer resistance protein (BCRP/ABCG2) in cancer
drug resistance. Biochem Pharmacol. 83:1084–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maliepaard M, van Gastelen MA, de Jong LA,
Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, Floot BG and
Schellens JH: Overexpression of the BCRP/MXR/ABCP gene in a
topotecan-selected ovarian tumor cell line. Cancer Res.
59:4559–4563. 1999.PubMed/NCBI
|
|
37
|
Robey RW, Medina-Pérez WY, Nishiyama K,
Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD and Bates
SE: Overexpression of the ATP-binding cassette half-transporter,
ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast
cancer cells. Clin Cancer Res. 7:145–152. 2001.PubMed/NCBI
|
|
38
|
Duan Z, Brakora KA and Seiden MV:
Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small
interfering RNA and reversal of paclitaxel resistance in human
ovarian cancer cells. Mol Cancer Ther. 3:833–838. 2004.PubMed/NCBI
|
|
39
|
Sukowati CH, Rosso N, Pascut D, Anfuso B,
Torre G, Francalanci P, Crocè LS and Tiribelli C: Gene and
functional up-regulation of the BCRP/ABCG2 transporter in
hepatocellular carcinoma. BMC Gastroenterol. 12:1602012. View Article : Google Scholar : PubMed/NCBI
|