Introduction
Alzheimer's disease (AD), the most common form of
dementia, which currently has no effective available treatment, is
commonly diagnosed in individuals over 65 years old (1). There are numerous common symptoms
including confusion, irritability, aggression, mood swings, trouble
with language and long-term memory loss, which ultimately leads to
mortality. On average, the life expectancy following diagnosis is
3.2–6.6 years (2). β-amyloid (Aβ)
can aggregate to form flexible soluble oligomers, which may exist
in several forms. Senile plaques are extracellular deposits of Aβ
in the brain associated with degenerative neurons and an abundance
of microglia and astrocytes (3).
As a neurodegenerative disorder, AD is associated with the presence
of these neurotoxic senile plaques in the brain (4). Oxidative stress is another important
independent adverse factor in the development of AD (5).
Obesity may increase the risk of various diseases
such as heart disease, type 2 diabetes, obstructive sleep apnea,
certain types of cancer and osteoarthritis. Given that obesity is
associated with vascular and metabolic diseases, it is suggested
that it is associated with AD (6).
As a novel determinant of body weight and obesity, iroquois
homeobox protein 3 (IRX3) serves as a functional long-range target
of obesity-associated variants within fat mass and
obesity-associated (FTO) genes, influencing obesity (7). It is a member of the iroquois
homeobox gene family and serves a role in the early stages of
neural development (8).
Obesity-associated single nucleotide polymorphisms have been
demonstrated to be associated with IRX3 instead of fat mass and
obesity-associated protein in the human brain (7).
Catalpol is an iridoid glucoside, and large
quantities can be isolated from the genus Rehmannia
(Orobanchaceae). Previous studies have indicated that catalpol
serves a role in neuroprotection, however, the precise mechanisms
remain unclear (9,10). The current study predominantly
focuses on elucidating whether catalpol preserves neural function
and reduces the formation of senile plaques in AD using an AD mice
model. In addition, the effects of catalpol on IRX3 and
obesity-associated genes were investigated.
Materials and methods
Generation of AD mice
A total of 18 male 8-month-old Kunming mice provided
by the Animal Center of The Third Affiliated Hospital of Soochow
University (Changzhou, China). Mice were maintained under 50±5%
relative humidity at 22±2°C with a 12-h light/dark cycle and free
access to food and water. The mice were randomly divided into 3
groups with 6 mice/group: The AD group; AD mice treated with
catalpol group (AD+C); and the control group (CON). For generation
of the AD mouse model, 12 mice (6 each from the AD and AD+C groups)
were injected intraperitoneally with D-(+)-galactose (120 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA) and sodium nitrite (90 mg/kg;
Sigma-Aldrich), once daily continuously for 60 days. In order to
enhance the progression of senile dementia, a one-off injection of
2 µl Aβ1–42 (amino acid sequence:
DAEFGHDSGFEVRHQKLVFFAEDVGSNKGAIIGLMVGGVVIA; synthesized by Sangon
Biotech Co., Ltd., Shanghai, China) into the left lateral ventricle
of the 12 mice was conducted when the mice were 9-months old. At 10
months, the mice were considered to have AD. Catalpol (20 mg/kg;
Sigma-Aldrich) was administered at 10 months for 30 days by
intragastric administration in the AD+C group. No further treatment
was administered in the AD group. The CON group mice were 11-months
old without any chemical treatment. All mice used in the
experiments of the current study were 11 months old. All
experiments were approved by the Ethics Committee of Soochow
University (Suzhou, China).
Detection of reactive oxygen species
(ROS)-associated enzymes
Following sacrification of the mice by
CO2 inhalation, the cerebral cortex of the mouse brain
was dissected and centrifuged at a speed of 12,000 × g for 15 min
in Tris-buffered saline with Protease Inhibitor Cocktail (1:1,000;
Sigma-Aldrich) for 30 min at 4°C. The supernatant of the cerebral
cortex was extracted and transferred to a tube. ROS-associated
enzymes from homogenates were detected using the following kits
purchased from Beyotime Institute of Biotechnology (Haimen, China):
Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px3),
catalase (CAT) and malondialdehyde (MDH).
Detection of soluble Aβ40 and
Aβ42
The above-mentioned supernatant containing soluble
Aβ was homogenized in 5 M guanidine HCl and 50 mM Tris-HCl (pH 8.0)
buffer, and aliquots of the homogenates were diluted 40 fold. The
supernatant was used for the determination of the concentrations of
Aβ40 and Aβ42 peptides using Mouse ELISA kits
(Thermo Fisher Scientific, Waltham, MA, USA). The optical density
value was detected at λ=495 nm using the PR-310 ELISA reader
(Shenzhen Procan Electronics Inc., Shenzhen, China).
Thioflavin-S staining
Mouse brains in the three groups were dissected and
embedded in optimum cutting temperature compound (Sakura Finetek
USA Inc., Torrance, CA, USA) and rapidly frozen on dry ice. Coronal
sections (10 µm) were cut and deparaffinized in 4%
paraformaldehyde for 20 min. Slides were placed in 1% thioflavin-S
solution (catalog no. T1892; Sigma-Aldrich) for 12 min, then were
differentiated twice in 70% fresh alcohol for 10 min. Subsequent to
rinsing in distilled water, the slides were mounted in glycerin
jelly. Images were captured using a confocal microscope (A1R+;
Nikon Corporation, Tokyo, Japan) and analyzed by the relative area
(%) of senile plaques (white spot as indicated by the arrows) using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Detection of IRX3 and obesity-associated
genes
The expression of obesity-associated genes and IRX3
at the gene level was conducted using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
using the ABI 7500 cycler (Applied Biosystems; Thermo Fisher
Scientific). Total RNA was isolated from three groups using
TRIzol® reagent (Ambion; Thermo Fisher Scientific)
according to the manufacturer's instructions. For each group, 2.5
µg RNA in a 20-µl reaction mixture was
reverse-transcribed into cDNA using the SuperScript®
VILO™ cDNA Synthesis kit (Invitrogen; Thermo Fisher Scientific)
according to the manufacturer's instructions. Briefly, tube
contents were mixed and incubated at 25°C for 10 min, then
incubated at 42°C for 1 h and terminated at 85°C for 5 min. The
products of cDNA were used as templates in the following qPCR. The
following cycling conditions were used: Stage 1, 1 cycle of 95°C
for 30 sec; stage 2, 40 cycles of 95°C for 5 sec and 60°C for 20
sec; and stage 3, 1 cycle of 65°C for 15 sec. The Obesity PCR Array
was performed using a 96-well plate (SABiosciences; Qiagen Inc.,
Valencia, CA, USA) to detect the obesity-associated genes, which
predominantly include orexigenic genes, anorectic genes and genes
associated with energy expenditure. The PCR array was a set of
optimized primer assays in a 96-well plate and was used for gene
expression analysis. cDNA template was mixed with the PCR master
mix, and then run the PCR program The 102 bp primer sequence for
IRX3 (Sangon Biotech Co., Ltd.) was as follows: Forward,
5′-GAAAACTTAGACAGCGCGGCAG-3′ and reverse,
5′-AGTTTTGCAGTCCGAAATGGGT-3′. cDNA in these groups was reverse
transcribed and used as templates with the SYBR® Premix
Ex Taq™ II (Perfect Real Time) kit (Takara Biotechnology Co., Ltd.,
Dalian, China). Gene expression values were determined relative to
that of the control group, which was set as 1. 2−ΔΔCT
was used for analysis (11)
Western blot analysis
A total of 30 µg protein isolated from the
mouse cortex was boiled for 5 min then cooled on ice and injected
into 10% SDS-PAGE for electrophoresis. The proteins were then
transferred onto a Immobilon-PFL polyvinylidene fluoride membrane
(Millipore, Billerica, MA, USA) and blocked in 5% fat-free milk for
1 h. Membranes were incubated overnight at 4°C with rabbit
polyclonal immunoglobulin (Ig)G anti-BACE1 (70 kDa; 1:1,000; cat.
no. ab2077; Abcam, Cambridge, UK), rabbit polyclonal IgG anti-IDE
(118 kDa; 1:1,000; catalog no. ab32216; Abcam), rabbit polyclonal
IgG anti-NEP (86 kDa; catalog no. AB5458; Chemicon; EMD Millipore)
and rabbit monoclonal IgG anti-IRX3 (52 kDa; 1:1,500; cat. no.
ab174307; Abcam) primary antibodies, and for 1 h at room
temperature followed by three washes with phosphate-buffered saline
for 10 min each. Subsequently, the membranes were incubated with
the alkaline phosphatase-conjugated goat anti-rabbit IgG secondary
antibody (1:2,000; cat. no. 65-6122; Thermo Fisher Scientific). A
polyclonal rabbit anti-glyceraldehyde 3-phosphate dehydrogenase
antibody (cat. no. G9545; Sigma-Aldrich) was used as an internal
control. Immunoreactive bands were visualized using Pierce™ ECL
Substrate (Thermo Fisher Scientific) and then scanned (Fujifilm LAS
4000; Fujifilm, Tokyo, Japan).
Morris water maze test
The Morris water maze was used to detect the
abilities of learning and memory of the spatial location via
training mice to find a hidden platform. The swimming pool (120 cm
in diameter, 50 cm in height, 30 cm depth of water made opaque with
milk) was divided into 4 equal quadrants. A submerged platform (10
cm in diameter, 29 cm in height) was placed inside the target
quadrant and 1 cm below the surface of the water while other three
quadrants were entry points of the mice. Mice were trained to swim
and find the hidden platform during the Morris water maze test for
five consecutive days, including four days of place navigation with
three consecutive trials per day, and the probe trial performed on
the last day. On the testing day (day 5), the platform was removed
and mice were allowed to explore the pool for 90 sec. The time
(sec) spent in the target quadrant and the number of crossings of
the former location of the hidden platform were recorded and
considered as an index of spatial learning and memory.
Statistical analysis
Data are presented as the mean ± standard deviation.
Calculations were performed using SPSS software, version 16.0
(SPSS, Inc., Chicago, IL, USA). The results were evaluated by
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effects of catalpol on ROS-associated
enzymes
The activities of the ROS-associated enzymes, SOD,
GSH-Px and CAT, and the concentration of MDA were detected
separately using the kits mentioned above. As shown in Fig. 1, the activity of SOD, GSH-Px and
CAT was significantly reduced in the AD mice (P<0.01), which
suggested the suppressive capacities of ROS clearance in the AD
group. Subsequent to treatment with catalpol, the activity of these
enzymes was increased significantly although were still lower than
those of the CON group. The concentration of MDA was increased
significantly in the AD group, suggesting the enhanced induction of
ROS, however, no clear effect on MDA levels was detected following
treatment with catalpol.
 | Figure 1Detection of reactive oxygen
species-associated enzymes in the supernatant of the cerebral
cortex of mouse brains in 3 groups. (A) SOD; (B) GSH-Px; (C) CAT;
and (D) MDA. Data are presented as the mean ± standard deviation.
##P<0.01, vs. CON; *P<0.05, vs. AD;
**P<0.01, vs. AD. SOD, superoxide dismutase; GSH-Px,
glutathione peroxidase; CAT, catalase; MDA, malondialdehyde; AD,
Alzheimer's disease; CON, control; C, catalpol. |
Effects of catalpol on reduction of
Aβ40 and Aβ42
As presented in Fig.
2, the concentration of Aβ40 (Fig. 2A) and Aβ42 (Fig. 2B) in the cortex of the brain was
increased in AD mice as expected. Subsequent to treatment with
catalpol, the level of Aβ40 and Aβ42,
particularly Aβ40, in the AD+C group reduced
significantly but remained higher than the level in the CON
group.
Effects of catalpol on the formation of
senile plaques
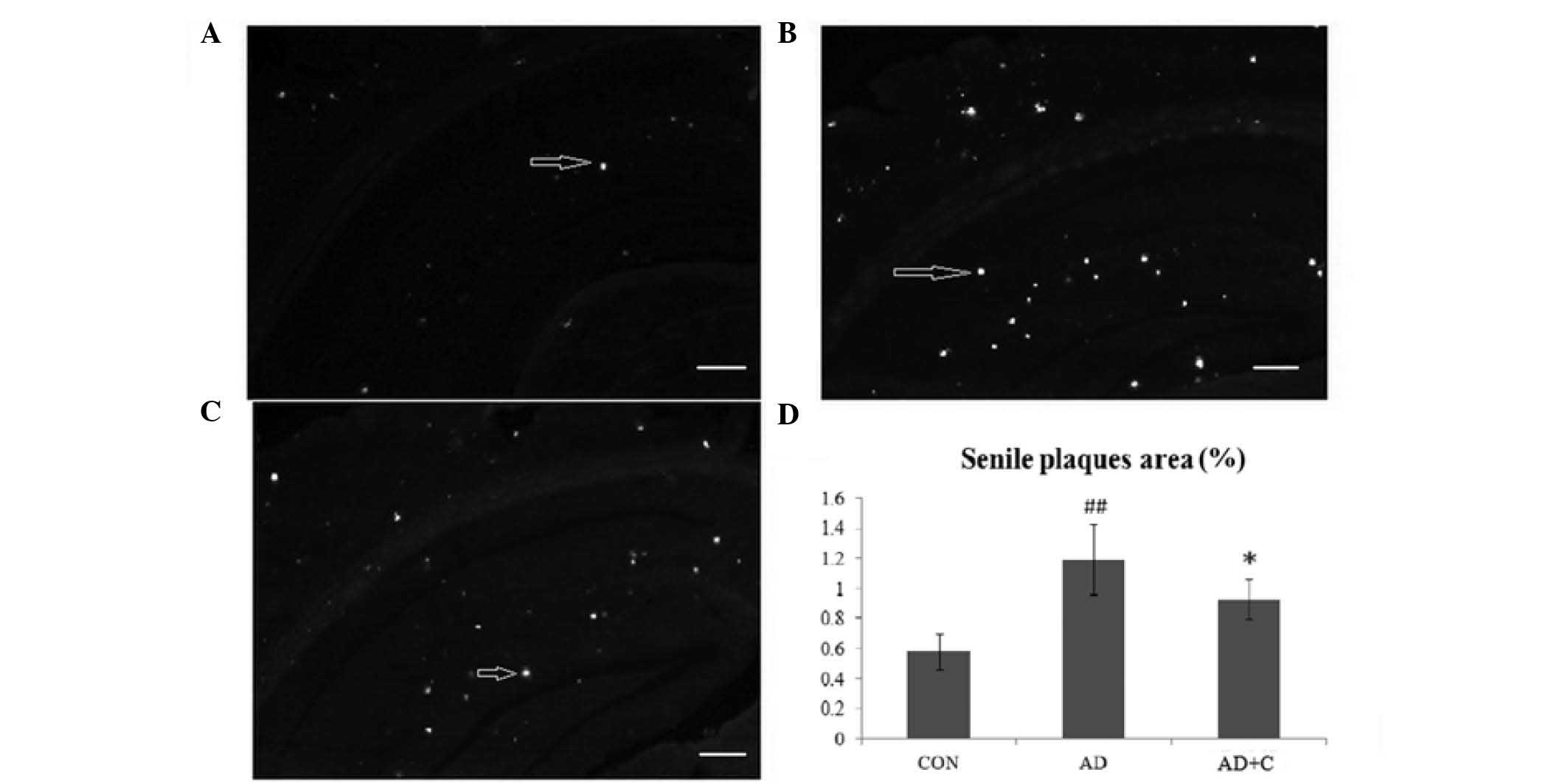
Senile plaques in the hippocampus and cortex of mice
were detected using thioflavin-S staining. As presented in Fig. 3A, the senile plaques (white spots
indicated by arrows) were seldom detected in the hippocampus and
cortex of mice in the CON group. Senile plaques were observed to be
significantly increased in the AD group (Fig. 3B). Subsequent to treatment with
catalpol, the number of senile plaques in the hippocampus and
cortex were observed to be significantly reduced in the AD+C group
(Fig. 3C), but remained higher
than that in the CON group. The result indicated that catalpol
reduced the formation of senile plaques in the hippocampus and
cortex. The quantification of the plaque area is presented in
Fig. 3D.
Effects of catalpol on the expression
levels of IRX3 and obesity-associated genes
As presented in Fig.
4A, no significant alterations in the expression levels of IRX3
were detected at the gene level. Additionally, no significant
alterations in the expression levels of obesity-associated genes
were observed. In addition, no alterations in the protein
expression levels of IRX3 were observed (Fig. 4B). These results indicated that
catalpol was not able to regulate the levels of IRX3. The
alterations were considered significant if the fold change was
>2 fold (increased) or <0.5 fold (reduced).
 | Figure 4(A) The expression of IRX3 at the gene
level in the mouse brain in 3 groups. Data are presented as the
mean ± standard deviation. (B) The expression of IRX3, BACE-1, IDE
and NEP at the protein level in the mouse brains in 3 groups as
observed by western blotting. GAPDH was used as an internal
control. Lane 1, CON group; lane 2, AD group; lane 3, AD+C group.
IRX3, iroquois homeobox protein 3; BACE-1, β-secretase 1; NEP,
neprilysin; IDE, insulin-degrading enzyme; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; CON, control; AD, Alzheimer's disease;
C, catalpol. |
Effects of catalpol on enzyme
regulation
BACE-1, IDE and NEP are three key enzymes regulating
the formation of senile plaques during the progression of AD
(12). As presented in Fig. 4B, compared with the CON group, the
expression of IDE was downregulated in the AD group. Subsequent to
treatment with catalpol, the expression of IDE was observed to be
significantly upregulated in the AD+C group. However, no
alterations to the expression of BACE-1 and NEP were detected among
these groups. These results indicated that catalpol protected the
brain by upregulating the expression of IDE.
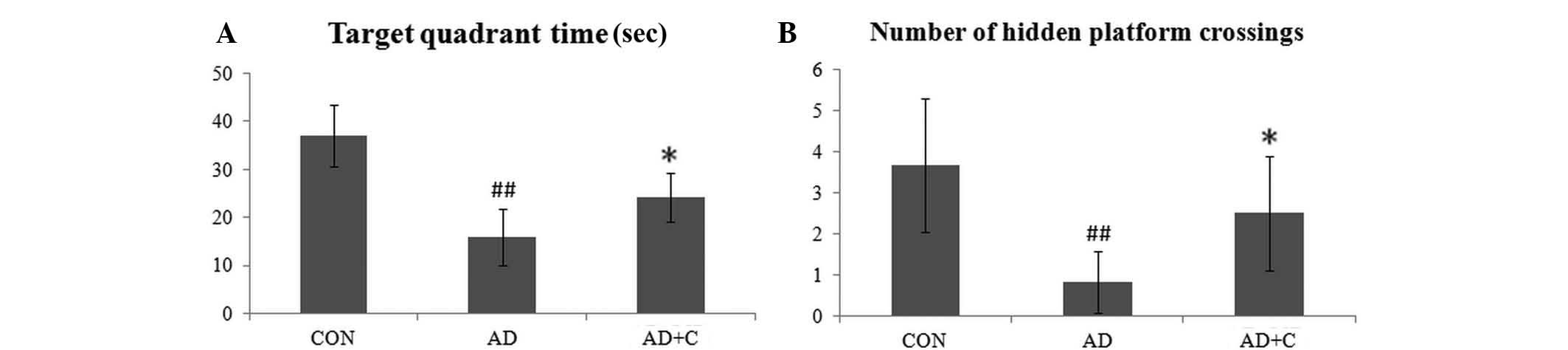
Effects of catalpol on learning and
memory promotion
The target quadrant time and the average number of
hidden platform crossings in the CON, AD and AD+C groups were
recorded. As presented in Fig. 5,
the time spent in the target quadrant determined by the
representative swim paths in the pool was longer in the CON group,
however was significantly reduced in the AD group (P<0.01). The
number of hidden platform crossings in the AD mice was
significantly reduced when compared with that of the CON group
(P<0.01). Following catalpol treatment, these data were
increased compared with the AD group (P<0.05), but remained
lower than that in the CON group. These results indicate the
effects on learning and spatial memory in the AD mice, which may be
ameliorated and by catalpol.
Discussion
Catalpol is an iridoid glucoside isolated from the
genus Rehmannia (Orobanchaceae). Previous experiments by our
group suggested that catalpol can stimulate adrenocortical
hormones, increasing the production of androgens (unpublished
data). Catalpol has additionally been identified to produce
anti-inflammatory (13,14), anticancer (15,16),
diuretic and hypoglycemic effects (17,18).
Previously, catalpol was reported to exhibit neuroprotective
effects, including protection against neurodegeneration (19). However, the mechanisms remain
unclear.
Oxidative stress serves a key role in AD, which is
the most common form of dementia and currently has no cure. It
reflects an imbalance in ROS, which is regulated by numerous
enzymes, and damages the neurons in the brain. ROS are regulated by
many enzymes. The activity of ROS-associated enzymes, SOD, GSH-Px
and CAT, and the concentration of MDA are important in the process
of AD (20). SOD catalyzes the
dismutation of the toxic superoxide (O2−)
radical into O2 or H2O2. It is an
important antioxidant in the majority of living cells exposed to
oxygen. GSH-Px reduces lipid hydroperoxides to their corresponding
alcohols and reduces free hydrogen peroxide to H2O. CAT
catalyzes the decomposition of hydrogen peroxide to water and
oxygen, and is an important enzyme in protecting the cell from
oxidative damage. MDA is a marker of oxidative stress as ROS
degrades polyunsaturated lipids, forming MDA. The activities of
these enzymes were observed to be significantly reduced in AD mice
following the progression of disease. Subsequent to treatment with
catalpol, the activities of these enzymes were increased
significantly but remained lower than those of the CON group. These
results suggested that catalpol may partially ameliorate the
effects on the levels of these enzymes. Notably, there was no clear
inhibition on MDA detected following treatment with catalpol,
however this requires further investigation in order to be
verified.
Previous studies suggest that Aβ, which is a peptide
of 36–43 amino acids in length, serves a central role in the
pathological development of AD; increases in Aβ40 and
Aβ42 have been implicated in the pathogenesis of AD
(21,22). Levels of Aβ40 and
Aβ42, which aggregate to form flexible soluble oligomers
that are toxic to neurons, were observed to be significantly
elevated in the brains of patients with AD (23). Senile plaques, the presence of
which are an important criterion of the neurohistopathological
verification of AD, contain Aβ40 and Aβ42
(24). In the current study, the
concentrations of Aβ40 and Aβ42 in the cortex
of the brain were increased in AD mice as hypothesized. Following
treatment with catalpol, the levels of Aβ40 and
Aβ42, particularly Aβ40, in the AD+C group
reduced significantly but remained higher than the levels in the
CON group. Thus, catalpol was shown to reduce the formation of
senile plaques in the hippocampus and cortex, which are increased
in AD. Aβ is removed and cleaved by several amyloid-degrading
enzymes, including BACE-1, IDE and NEP (12). The results of the current study
indicated that catalpol protected the brain by upregulating IDE,
rather than the other two enzymes.
IRX3, a member of the iroquois homeobox gene family,
serves a key role in early stages of neural development. A previous
study demonstrated tha IRX3 is involved in neural formation and
growth. IRX3 was previously identified to influence obesity
(7). However, no alterations on
the expression of IRX3 were detected at the gene and protein
levels, thus suggesting that catalpol was not able to regulate
IRX3. In addition, no significant alterations in the expression of
obesity-associated genes were observed following treatment with
catalpol. Although obesity has an important role in AD due to
vascular defects, impaired insulin metabolism and defects in
glucose transport in the brain, the results indicated that catalpol
is not capable of regulating obesity.
The Morris water maze test is widely used to
investigate spatial learning and memory, whilst it has been
additionally used to measure the effect of drugs on neurocognitive
disorders (25). The current study
identified that catalpol was able to increase the time spent in the
target quadrant and the average number of hidden platform
crossings, suggesting that the effects on learning and spatial
memory in the AD mice were partially ameliorated by catalpol.
In conclusion, the present study indicates that
catalpol reduces the levels of oxidative stress in the cerebral
cortex of the hippocampus by regulating SOD, GSH-Px and CAT.
Catalpol is additionally suggested to reduce the levels of soluble
Aβ40 and Aβ42 in the cerebral cortex of the
hippocampus and thus inhibit the formation of senile plaques, which
are regulated by IDE. Catalpol is not able to directly regulate the
expression of IRX3 and obesity-associated genes. The learning and
memory impairments were additionally identified to be relieved
following treatment with catalpol. Thus, catalpol may be a
potential drug for the treatment of neurodegenerative diseases such
as AD.
Acknowledgments
The current study was supported by the Third
Affiliated Hospital of Soochow University.
References
|
1
|
Forsyth E and Ritzline PD: An overview of
the etiology, diagnosis, and treatment of Alzheimer disease. Phys
Ther. 78:1325–1331. 1998.PubMed/NCBI
|
|
2
|
Todd S, Barr S, Roberts M and Passmore AP:
Survival in dementia and predictors of mortality: A review. Int J
Geriatr Psychiatry. 28:1109–1124. 2013.PubMed/NCBI
|
|
3
|
Jang BG, In S, Choi B and Kim MJ:
Beta-amyloid oligomers induce early loss of presynaptic proteins in
primary neurons by caspase-dependent and proteasome-dependent
mechanisms. Neuroreport. 25:1281–1288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henriques AG, Oliveira JM, Gomes B, Ruivo
R, da Cruz e Silva EF and da Cruz e Silva OA: Complexing Aβ
prevents the cellular anomalies induced by the Peptide alone. J Mol
Neurosci. 53:661–668. 2014.PubMed/NCBI
|
|
5
|
Shichiri M: The role of lipid peroxidation
in neurological disorders. J Clin Biochem Nutr. 54:151–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spielman LJ, Little JP and Klegeris A:
Inflammation and insulin/IGF-1 resistance as the possible link
between obesity and neurodegeneration. J Neuroimmunol. 273:8–21.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smemo S, Tena JJ, Kim KH, Gamazon ER,
Sakabe NJ, Gómez-Marín C, Aneas I, Credidio FL, Sobreira DR,
Wasserman NF, et al: Obesity-associated variants within FTO form
long-range functional connections with IRX3. Nature. 507:371–375.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodríguez-Seguel E, Alarcón P and
Gómez-Skarmeta JL: The Xenopus Irx genes are essential for neural
patterning and define the border between prethalamus and thalamus
through mutual antagonism with the anterior repressors Fezf and
Arx. Dev Biol. 329:258–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai QY, Chen XS, Zhan XL and Yao ZX:
Protective effects of catalpol on oligodendrocyte death and myelin
breakdown in a rat model of chronic cerebral hypoperfusion.
Neurosci Lett. 497:22–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li DQ, Li Y, Liu Y, Bao YM, Hu B and An
LJ: Catalpol prevents the loss of CA1 hippocampal neurons and
reduces working errors in gerbils after ischemia-reperfusion
injury. Toxicon. 46:845–851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miners JS, van Helmond Z, Kehoe PG and
Love S: Changes with age in the activities of beta-secretase and
the Abeta-degrading enzymes neprilysin, insulin-degrading enzyme
and angio-tensin-converting enzyme. Brain Pathol. 20:794–802. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi HJ, Jang HJ, Chung TW, Jeong SI, Cha
J, Choi JY, Han CW, Jang YS, Joo M, Jeong HS and Ha KT: Catalpol
suppresses advanced glycation end-products-induced inflammatory
responses through inhibition of reactive oxygen species in human
monocytic THP-1 cells. Fitoterapia. 86:19–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang A, Hao S, Bi J, Bao Y, Zhang X, An L
and Jiang B: Effects of catalpol on mitochondrial function and
working memory in mice after lipopolysaccharide-induced acute
systemic inflammation. Exp Toxicol Pathol. 61:461–469. 2009.
View Article : Google Scholar
|
|
14
|
Gao N, Tian JX, Shang YH, Zhao DY and Wu
T: Catalpol suppresses proliferation and facilitates apoptosis of
OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and
downregulating MMP-2 expression. Int J Mol Sci. 15:19394–19405.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pungitore CR, Ayub MJ, Borkowski EJ, Tonn
CE and Ciuffo GM: Inhibition of Taq DNA polymerase by catalpol.
Cell Mol Biol (Noisy-le-grand). 50:767–772. 2004.
|
|
16
|
Li X, Xu Z, Jiang Z, Sun L, Ji J, Miao J,
Zhang X, Huang S, Wang T and Zhang L: Hypoglycemic effect of
catalpol on high-fat diet/streptozotocin-induced diabetic mice by
increasing skeletal muscle mitochondrial biogenesis. Acta Biochim
Biophys Sin (Shanghai). 46:738–748. 2014. View Article : Google Scholar
|
|
17
|
Huang WJ, Niu HS, Lin MH, Cheng JT and Hsu
FL: Antihyperglycemic effect of catalpol in streptozotocin-induced
diabetic rats. J Nat Prod. 73:1170–1172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu G, Xiong Z, Yong Y, Wang Z, Ke Z, Xia Z
and Hu Y: Catalpol attenuates MPTP induced neuronal degeneration of
nigral-striatal dopaminergic pathway in mice through elevating
glial cell derived neurotrophic factor in striatum. Neuroscience.
167:174–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watson JB, Arnold MM, Ho YS and O'Dell TJ:
Age-dependent modulation of hippocampal long-term potentiation by
antioxidant enzymes. J Neurosci Res. 84:1564–1574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bondareff W: Age-related changes in brain
extracellular space affect processing of amyloid-β peptides in
Alzheimer's disease. J Alzheimers Dis. 35:1–6. 2013.
|
|
21
|
Hampel H, Shen Y, Walsh DM, Aisen P, Shaw
LM, Zetterberg H, Trojanowski JQ and Blennow K: Biological markers
of amyloid beta-related mechanisms in Alzheimer's disease. Exp
Neurol. 223:334–346. 2010. View Article : Google Scholar :
|
|
22
|
Hashimoto M, Bogdanovic N, Volkmann I,
Aoki M, Winblad B and Tjernberg LO: Analysis of microdissected
human neurons by a sensitive ELISA reveals a correlation between
elevated intracellular concentrations of Abeta42 and Alzheimer's
disease neuropathology. Acta Neuropathol. 119:543–554. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian J, Shi J and Mann DM: Cerebral
amyloid angiopathy and dementia. Panminerva Med. 46:253–264.
2004.
|
|
24
|
Bromley-Brits K, Deng Y and Song W: Morris
water maze test for learning and memory deficits in Alzheimer's
disease model mice. J Vis Exp. 53:pii29202011.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|