Introduction
Diabetes mellitus (DM) is a metabolic disorder and
can cause several complications, such as cardiomyopathy, peripheral
neuropathy and nephropathy. Evidence has demonstrated that DM can
also cause dysfunction in the brain termed diabetic encephalopathy
(DE), which is characterized by brain neurophysiological changes,
neurobehavioral deficits and neuron loss (1–4).
Studies regarding streptozotocin (STZ)-induced type-1 diabetes in
mice have provided evidence demonstrating that apoptosis is
associated with neuron loss (5,6).
Glial cell line-derived neurotrophic factor (GDNF)
is one of the most potent growth and survival factors for neuronal
cells and dopaminergic neurons (7–10). A
previous study demonstrated that GDNF administration ameliorated
cell apoptosis in neurodegeneration (11). One recent study indicated that GDNF
could rescue retinal cells from neurodegeneration at an early stage
of diabetes (12). GDNF-epithelial
growth factor receptor 1 (EGR1) pathway activation is critical in
renal epithelial cell apoptosis and migration in diabetic renal
embryopathy (13). In addition,
GDNF, nestin and glial fibrillary acidic protein (GFAP)-positive
reactive astrocytes appeared at an early stage in
6-hydroxydopamine-induced Parkinsonism in rats, which suggested the
existence of endogenous neuroprotective mechanisms that act via the
release of BDNF and GDNF from GFAP-positive immunoreactive
astrocytes (14). However, one
recent study indicated that GDNF treatment did not affect neuronal
survival but another neurotrophic factor, neurotrophin 3, was able
to enhance the survival of enteric ganglion cells in
H2O2-treated cultures (15).
A series of studies have explored the signal
transduction processes of GDNF, which underlie the neuroprotective
effects (16,17). A recent study showed that the
GDNF/mitogen-activated proten kinase (MAPK)/EGR-1 pathway is
critical in renal epithelial cell apoptosis and migration in
diabetic renal embryopathy (13).
The pleiotropic function of GDNF has been found to control
migration and neuronal differentiation of enteric ganglion
precursors (18). High
intracellular activation of GDNF downstream pathways triggers
neuronal differentiation, while low-level activation of GDNF
signaling is crucial for the maintenance of the progenitor state
(18). In addition, the ERK/MAPK
pathway, instead of the phosphoinositide 3-kinase/Akt (PI3K/Akt)
pathway, was proposed to be the key endogenous neuroprotective
mechanism, acting via the release of bone-derived neurotrophic
factor (BDNF) and GDNF from nestin and GFAP-positive reactive
astrocytes in the 6-hydroxydopamine-induced rat model of
Parkinson's (14). Furthermore, a
recent study demonstrated that mechanical and thermal
hypersensitivity was correlated with the structural changes and
loss of GDNF/Akt signaling in injured limbs 7 days after chronic
constrictive injury was induced. Intramuscular injection of
adenovirus encoding GDNF could restore the protein level and
activity of the GDNF/Akt signaling pathway in the sciatic nerve
(19). Notably, GDNF increased Akt
phosphorylation, lowered β-cell death, and raised glucose-induced
insulin secretion in cultured human islets (20).
Although it was previously demonstrated that GDNF is
important in decreasing apoptosis in cultured Muller cells during
the early stages of diabetic retinopathy (21), the possible effect of GDNF and
downstream PI3K/Akt signaling on apoptosis in diabetic
encephalopathy remains unclear. In the present study, it was
revealed that administration of GDNF markedly increased p-Akt
levels and decreased Bax and DNA fragmentation levels in the
hippocampus of rats with STZ-induced DE. These effects of GDNF most
likely occurred via the activation of the PI3K/Akt signaling
pathway.
Materials and methods
Induction of diabetes and DE
A total of 45 male Sprague-Dawley rats (age, ~60
days; weight, 250 g) were obtained from the Research Animal Center
of Henan Province (Zhengzhou, China). The animals were provided
with ad libitum access to water and food under a 12:12 h
light-dark cycle at 24°C. All of the animal experiments were
conducted in accordance with the National Institute of Health
guidelines for the Care and Use of Laboratory Animals (22). The present study was approved by
the ethics committee of the Xinxiang Medical University (Xinxiang,
China). Type 1 diabetes was induced by a single intraperitoneal
injection of STZ (60 mg/kg body weight; Sigma-Aldrich, St. Louis,
MO, USA) or 0.1 M citrate buffer vehicle (Beyotime Institute of
Biotechnology, Shanghai, China) as previously described (23,24).
Fasting blood glucose concentrations were measured 72 h after STZ
injection. Rats (n=35) with blood glucose levels >11.1 mmol/l
were considered to be diabetic (23). Rats (n=5) with blood sugar levels
<11.1 mmol/l were excluded from the study. The 35 diabetic rats
were divided into seven groups: Group 1 was untreated; group 2 was
treated with GDNF; group 3 with vehicle (10 µl of 0.1 M
phosphate-buffered saline (PBS) administered to the left lateral
cerebral ventricle); group 4 with GDNF + Wortmannin (Calbiochem;
Merck Millipore, Darmstadt, Germany); group 5 with Wortmannin;
group 6 with GDNF + SB203580 (Calbiochem); and group 7 with
SB203580. A total of 60 days following injection of STZ, diabetic
rat brain structural abnormalities and cognitive impairment was
assessed using a Y-maze as previously described (24).
Intracerebroventricular administration of
GDNF
Beginning 60 days after STZ injection, the animals
were anesthetized using ketamine/xylazine (10:6.5 solution; 1.0
ml/kg intraperitoneally; Sigma-Aldrich), A total of three 100
µg intracerebroventricular injections of human recombinant
GDNF (Peprotech, Inc., Rocky Hill, NJ, USA) in 10 µl of 0.1
M PBS or an equal volume of vehicle were administered to the left
lateral cerebral ventricle (AP-1.0, LM-2.0, DV-3.4) at a rate of 1
µl/min every 7 days according to the methods of a previous
study (25). To evaluate the
signaling pathway involved in the effect of GDNF on apoptosis in
the hippocampus, 1 h prior to GDNF injection rats received an
intracerebroventricular injection of the PI3K/Akt inhibitor,
Wortmannin (2.5 µM, 10 µl), p38 MAPK inhibitor,
SB203580 (50 µM, 10 µl), or vehicle.
GDNF ELISA detection
The rats were sacrificed with an overdose of
barbiturates 3 days after the final injection, and the hippocampus
was removed immediately. The preparation of hippocampus homogenates
for GDNF detection was conducted as previously described (26). Briefly, the hippocampus was
homogenized in the lysate buffer containing 0.1% Tween-20, 0.5%
bovine serum albumin, 2mM EDTA in 1X PBS, and protease inhibitors
(all Sigma-Aldrich) and centrifuged at 14,000 × g for 30 min at
4°C. After centrifugation, the supernatant was collected. The
levels of GDNF were quantified in duplicate using a GDNF ELISA kit
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer's instructions.
Preparation of tissue lysates and western
blot analysis
The hippocampal tissue samples were lysed in
ice-cold radioimmunoprecipitation assay buffer (Roche Diagnostics,
GmbH, Mannheim, Germany) containing 1X PBS, 0.1% SDS, 1% Nonidet
P-40, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate and
protease inhibitor cocktail (Sigma-Aldrich) to extract the protein.
Samples were incubated for 30 min on ice, and then the protein
lysates were centrifugated at 12,000 × g for 30 min to remove the
pellets. For western blot analysis, equal quantities of protein
lysates were separated on 5–15% Bis-Tris gels (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and proteins were
transferred onto 0.22-µm polyvinylidene difluoride membranes
(Invitrogen; Thermo Fisher Scientific, Inc.) electrophoretically.
The membrane was blocked with 5% non-fat milk for 2 h at room
temperature then incubated with the specific antibodies at 4°C
overnight: Rabbit monoclonal anti-rat p-Akt (1:1,000; cat. no.
13038; Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
monoclonal anti-rat Akt (1:1,000; cat. no. 46855; Cell Signaling
Technology, Inc.) and rabbit polyclonal anti-rat Bax (1:1,000; cat.
no. ab7977; Abcam, Cambridge, MA, USA). After washing with 1X
Tris-buffered saline with 0.05% Tween-20, membranes were incubated
with horseradish peroxidase-conjugated secondary antibody (Pierce,
Rockford, IL, USA) for 1 h at room temperature and immunoreactivity
was visualized by enhanced chemiluminescence (Pierce).
Measurement of DNA fragmentation
DNA fragmentation was evaluated using the Cell Death
Detection ELISAPLUS kit (Roche Diagnostics) according to the
manufacturer's instructions. Briefly, the tissues were washed with
D-Hanks solution (Sigma-Aldrich), incubated with 200 µl
lysis buffer for 30 min at 37°C and centrifuged at 200 × g for 10
min at 4°C. The supernatant from each tube was incubated in a
microplate coated with streptavidin containing anti-DNA-peroxidase
(1:50; clone MCA-33) and anti-histone-biotin (1:50; clone H11-4) to
capture the apoptotic nucleosomes. Biotinylated mouse monoclonal
anti-rat histone antibody bound via the histone component and the
peroxidase-conjugated mouse monoclonal anti-rat DNA antibody bound
to the DNA of the nucleosomes. The unbound antibodies were removed
by washing with PBS, the amount of peroxidase retained within the
immunocomplex was determined using
2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid
(Sigma-Aldrich). The absorbance was read at 405 nm using a
microplate reader (CSL Behring, King of Prussia, PA, USA). The DNA
fragmentation was defined as a percentage of the vehicle-treated
group.
Statistical analysis
All data are presented as the mean ± standard error
of the mean, and statistical analysis was performed using one-way
analysis of variance. The exact F statistic value and degrees of
freedom were used to calculate probabilities. SPSS 16.0 (SPSS,
Inc., Chicago, IL, USA) was used to conduct the statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
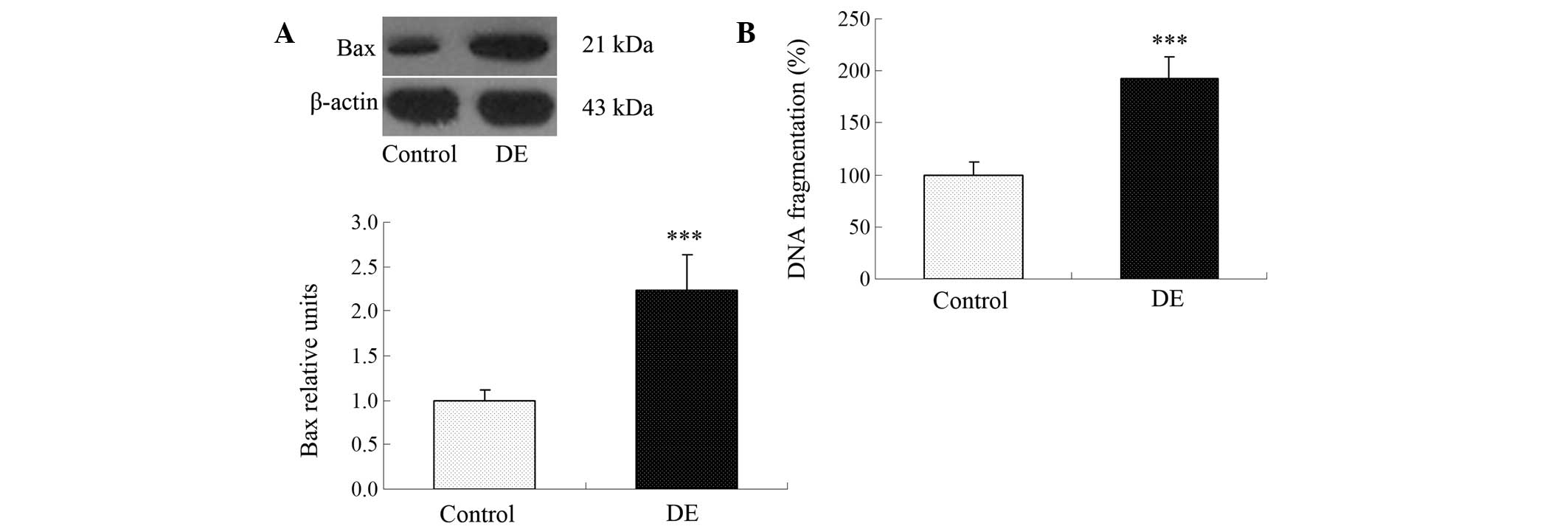
Diabetes increases apoptosis in the
hippocampus
In order to investigate whether or not apoptosis is
induced in the hyppocampus, the level of Bax and DNA fragmentation
in the hippocampus was examined. Following the induction of
diabetes (60 days), a significant increase in apoptosis was
observed in the rat hippocampi as compared with the control group.
The data clearly shows that the levels of Bax (Fig. 1A) and DNA fragmentation (Fig. 1B) were significantly increased in
the rats with DE compared with the control group.
STZ reduces the expression of GDNF and
p-Akt/Akt level in the hippocampus of diabetic rats
To investigate whether GDNF signals are involved in
DE, the protein level of GDNF was analyzed by ELISA 2 months after
STZ administration. The expression of GDNF in the hippocampus was
evidently lower in the STZ-induced group as compared with the
control group (Fig. 2A). The
expression of p-Akt/Akt, a downstream target of GDNF signals, was
also significantly decreased in the hippocampus at day 60 in the
rats with DE as compared with the control group (Fig. 2B).
GDNF delivery changes the expression of
p-Akt/Akt and the apoptotic status in rats with DE
Effects of human recombinant GDNF administration on
Akt levels in rats with DE were evaluated by western blot analysis.
As shown in Fig. 3, the p-Akt/Akt
level in the GDNF-treated rats was significantly increased compared
with that of the control diabetic group, while the levels of Bax
and DNA fragmentation in the GDNF-treated diabetic rats were
largely reduced (Fig. 3).
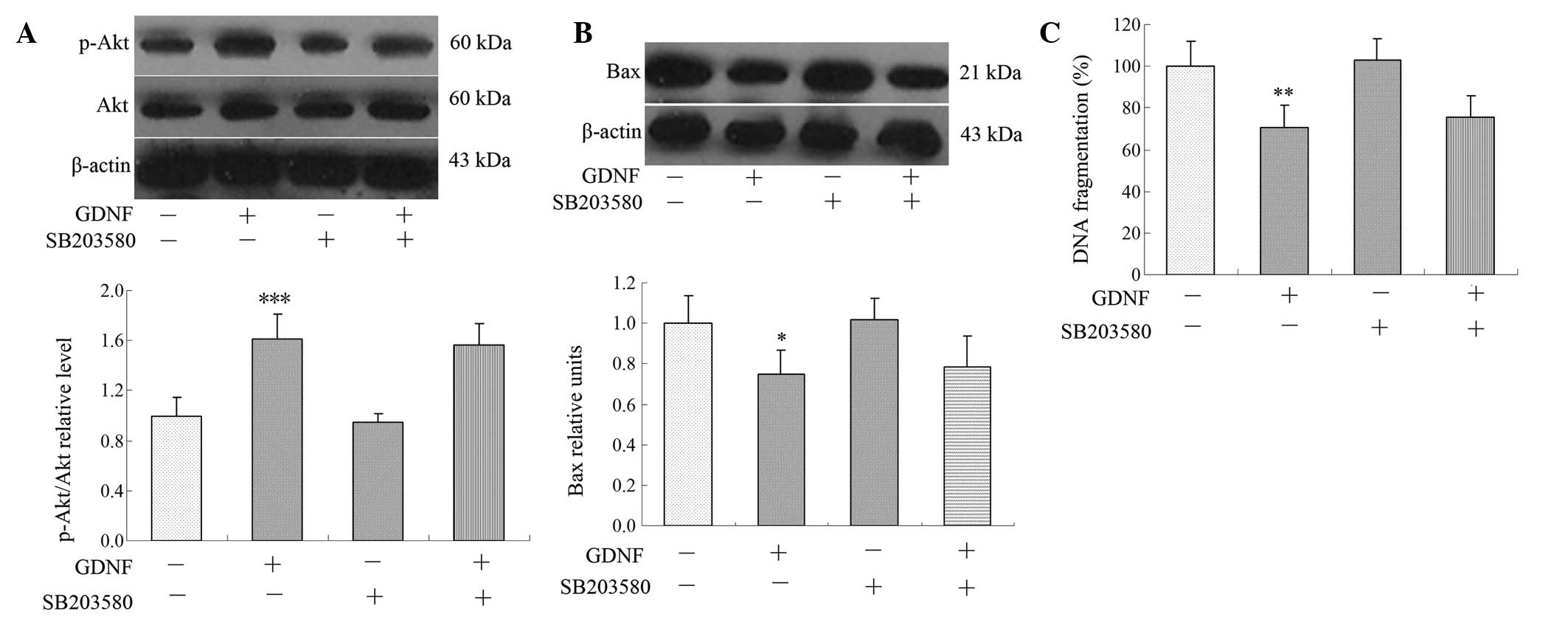
PI3K/Akt inhibitor reverses GDNF-induced
activation of p-Akt
The signaling pathway involved in GDNF-induced
anti-apoptosis was then analyzed in the hippocampi of rats with DE.
The PI3K/Akt inhibitor Wortmannin antagonized GDNF-induced p-Akt
expression, and the levels of Bax and DNA fragmentation (Fig. 4A–C), while the p38 MAPK inhibitor
SB203580 did not affect the level of p-Akt/Akt, Bax or DNA
fragmentation (Fig. 5A–C). This
suggested that the PI3K/Akt pathway but not the MAPK pathway, is
involved in GDNF-induced Akt expression in the hippocampus.
 | Figure 4PI3K/Akt pathway was involved in
GDNF-mediated effects on the level of p-Akt/Akt, Bax and DNA
fragmentation. The PI3K/Akt inhibitor Wortmannin diminished the
GDNF-induced change in (A) the level of p-Akt/Akt, (B) Bax
expression and (C) DNA fragmentation following treatment with (+)
or without (−) GDNF, Wortmannin, or both. Data are expressed as the
mean ± standard error of the mean, n=5 for each group,
*P<0.05, **P<0.01 and
***P<0.001 compared with the vehicle-treated control
group; #P<0.05, ##P<0.01 and
###P<0.001 compared with GDNF-treated group. PI3K,
phosphoinositide 3-kinase; GDNF, glial cell line-derived
neurotrophic factor. |
Discussion
Neurons in the central nervous system are
particularly vulnerable to hyperglycemia and GDNF has been shown to
be protective against STZ-induced neural retina apoptosis in
diabetic rats (12). Our previous
study showed that exogenous GDNF decreased apoptosis in cultured
Muller cells under high glucose conditions (21). The present study investigated the
effect of supplementation of GDNF on apoptosis in the hippocampus
and the mechanisms underlying this effect in rats with DE.
STZ-induced diabetes caused notable apoptosis with markedly
decreased levels of p-Akt/Akt in the brain. Treatment with GDNF
significantly ameliorated apoptosis by upregulating p-Akt/Akt. The
GDNF-induced decrease in the level of Bax and increase in the
p-Akt/Akt level were associated with the PI3K/Akt pathway.
A single high dose injection of STZ can induce
diabetes mellitus (27). This type
of animal model has been widely used in the study of the
pathophysiology of diabetes and its complications. The cognitive
function of diabetic rats has been shown to be impaired in
STZ-induced diabetes mellitus (3).
Elevated blood glucose levels have been reported to cause
degeneration of neurons in patients with diabetes (28). Type 2 diabetes has been found to
induce changes in the cellular composition of the brain during
aging (29). In the present study,
it was demonstrated that Bax protein levels significantly increased
(Fig. 3A and B) 2 months following
induction of diabetes, which indicated that STZ induced apoptosis
in DE.
It has been shown that the hippocampus is involved
in learning/memory processes; and the neuronal cell apoptosis in
the hippocampus has been shown to be associated with learning and
memory or performance. The occurrence of neuropathy in diabetes,
not only in diabetic patients but also in animal models, is well
known. In the STZ model of diabetes, the persistence of increased
apoptosis in the neural retina has been demonstrated (12). However, the evidence of neuronal
apoptosis and its underlying mechanism in diabetes has not been
fully elucidated. In the present study, it was demonstrated that
hippocampus neuronal apoptosis is associated with diabetic
neuropathy, as shown by the expression of Bax and by DNA
fragmentation. A deficiency in the neurotrophic factor GDNF was
also detected in the hippocampus. This is consistent with previous
studies which reported that the levels of GDNF were downregulated
in the colon of diabetic rats (30) and in the sciatic nerves of diabetic
rats (31).
In vivo, administration of GDNF into the
striatum or the lateral ventricle has been found to protect nigral
dopaminergic neurons in a partial lesion model of Parkinson's
disease (32). In addition,
intracerebroventricular GDNF administration improved motor ability
in bilaterally 6-hydroxydopamine lesioned rats (33). The above studies suggest that
delivery of GDNF to cerebral ventricles may prove to be a possible
method to treat DE.
The mechanisms involved in the effect of GDNF on
neuronal apoptosis and survival have not been fully explored. GDNF
has been shown to reduce apoptosis and enhance the survival of
retinal ganglion cells (34).
Previously it was demonstrated that exogenous GDNF not only
decreased apoptosis under high glucose conditions but also
accelerated the speed of synthesis of GDNF and GFRα1 proteins in
Muller cells (21). In this study,
it was observed that GDNF delivery significantly increased the
level of Bax. The GDNF-mediated effect of preventing apoptosis was
further confirmed by the decrease in DNA fragmentation in the
hippocampus of rats with DE. GDNF signaling has been found to
promote β-cell survival and improve glucose tolerance through the
PI3K/Akt signaling pathway (35).
In line with these observations, in the present study, GDNF
enhanced the expression of p-Akt/Akt in the hippocampus and
protected against apoptosis induced by diabetes mellitus. In
another study, GDNF has also been shown to enhance human islet
posttransplantation survival (20).
Intracellular signaling pathways, including P38 MAPK
and PI3K/Akt signaling pathways were involved in the regulation of
cell survival. It was then investigated whether GDNF administration
enhanced the activation of the PI3K/Akt pathway. GDNF-induced
upregulation of p-Akt/Akt was reversed by treatment with the
PI3K/Akt inhibitor Wortmannin, which is a specific inhibitor of
PI3K in the nanomolar range (36)
and 2.5 µM Wortmannin has been shown to inhibit the
expression of long-term potentiation in the dentate gyrus of rats
(37). In addition, the PI3K/Akt
inhibitor, Wortmannin also inhibited the GDNF-decrease of Bax and
DNA fragmentation. However, treatment of diabetic rats with the p38
MAPK inhibitor SB203580 did not abolish the GDNF-mediated increase
of p-Akt and the decrease of Bax and DNA fragmentation. PI3K/Akt is
the only enzyme known to be inhibited by Wortmannin (38), which suggests that GDNF has the
ability to reduce the level of Bax and the expression of DNA
fragmentation is dependent on the activation of the PI3K/Akt
pathway.
In conclusion, the present study demonstrates the
involvement of GDNF loss in STZ-induced DE. It also demonstrated a
protective effect of administration of GDNF, which was associated
with its antiapoptotic effects. It was demonstrated that this
function may be dependent on the activation of the PI3K/Akt
pathway. These results provide evidence for the role of GDNF in
preventing the progression of DE.
Acknowledgments
This study was supported partly by the key research
areas of Xinxiang Medical University (grant no. ZD2011-28), the
Science and Technology Project of Department of Education of Henan
Province (grant no. 13A310862), the Doctoral Scientific Research
Activation Foundation of Xinxiang Medical University, and the
National Natural Science Foundation of China (grant no.
81301174).
References
|
1
|
Cui H, Lee JH, Kim JY, Koo BN and Lee JE:
The neuroprotective effect of agmatine after focal cerebral
ischemia in diabetic rats. J Neurosurg Anesthesiol. 24:39–50. 2012.
View Article : Google Scholar
|
|
2
|
Kuhad A, Bishnoi M, Tiwari V and Chopra K:
Suppression of NF-kappabeta signaling pathway by tocotrienol can
prevent diabetes associated cognitive deficits. Pharmacol Biochem
Behav. 92:251–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Luo Y and Dai J: Axonal and
dendritic changes are associated with diabetic encephalopathy in
rats: An important risk factor for Alzheimer's disease. J
Alzheimers Dis. 34:937–947. 2013.PubMed/NCBI
|
|
4
|
Ola MS, Aleisa AM, Al-Rejaie SS,
Abuohashish HM, Parmar MY, Alhomida AS and Ahmed MM: Flavonoid,
morin inhibits oxidative stress, inflammation and enhances
neurotrophic support in the brain of streptozotocin-induced
diabetic rats. Neurol Sci. 35:1003–1008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Feng L, Ma D, Zhang M, Gu J, Wang
S, Fu Q, Song Y, Lan Z, Qu R and Ma S: Neuroprotective effect of
paeonol on cognition deficits of diabetic encephalopathy in
streptozotocin-induced diabetic rat. Neurosci Lett. 549:63–68.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mastrocola R, Restivo F, Vercellinatto I,
Danni O, Brignardello E, Aragno M and Boccuzzi G: Oxidative and
nitrosative stress in brain mitochondria of diabetic rats. J
Endocrinol. 187:37–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Day JS, O'Neill E, Cawley C, Aretz NK,
Kilroy D, Gibney SM, Harkin A and Connor TJ: Noradrenaline acting
on astrocytic β2-adrenoceptors induces neurite outgrowth in primary
cortical neurons. Neuropharmacology. 77:234–248. 2014. View Article : Google Scholar
|
|
8
|
Saavedra A, Baltazar G and Duarte EP:
Interleukin-1beta mediates GDNF up-regulation upon dopaminergic
injury in ventral midbrain cell cultures. Neurobiol Dis. 25:92–104.
2007. View Article : Google Scholar
|
|
9
|
Shimizu F, Sano Y, Saito K, Abe MA, Maeda
T, Haruki H and Kanda T: Pericyte-derived glial cell line-derived
neurotrophic factor increase the expression of claudin-5 in the
blood-brain barrier and the blood-nerve barrier. Neurochem Res.
37:401–409. 2012. View Article : Google Scholar
|
|
10
|
Zuo T, Qin JY, Chen J, Shi Z, Liu M, Gao X
and Gao D: Involvement of N-cadherin in the protective effect of
glial cell line-derived neurotrophic factor on dopaminergic neuron
damage. Int J Mol Med. 31:561–568. 2013.PubMed/NCBI
|
|
11
|
Li F, Wang M, Zhu S, Li L, Xiong Y and Gao
DS: The potential neuroprotection mechanism of GDNF in the
6-OHDA-induced cellular models of Parkinson's disease. Cell Mol
Neurobiol. 33:907–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Deng QQ, Wu XH, Yu J, Yang XL and
Zhong YM: Upregulation of glutamate-aspartate transporter by glial
cell line-derived neurotrophic factor ameliorates cell apoptosis in
neural retina in streptozotocin-induced diabetic rats. CNS Neurosci
Ther. 19:945–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CY, Lin TY, Lee MC, Chen SC and Chang
JS: Hyperglycemia: GDNF-EGR1 pathway target renal epithelial cell
migration and apoptosis in diabetic renal embryopathy. PloS One.
8:e567312013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lui NP, Chen LW, Yung WH, Chan YS and Yung
KK: Endogenous repair by the activation of cell survival signalling
cascades during the early stages of rat Parkinsonism. PloS One.
7:e512942012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korsak K, Silva AT and Saffrey MJ:
Differing effects of NT-3 and GDNF on dissociated enteric ganglion
cells exposed to hydrogen peroxide in vitro. Neurosci lett.
517:102–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mwangi SM, Nezami BG, Obukwelu B, Anitha
M, Marri S, Fu P, Epperson MF, Le NA, Shanmugam M, Sitaraman SV, et
al: Glial cell line-derived neurotrophic factor protects against
high-fat diet-induced obesity. Am J Physiol Gastrointest Liver
Physiol. 306:G515–G525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeda M, Takahashi M, Hara N and
Matsumoto S: Glial cell line-derived neurotrophic factor modulates
the excitability of nociceptive trigeminal ganglion neurons via a
paracrine mechanism following inflammation. Brain Behav Immun.
28:100–107. 2013. View Article : Google Scholar
|
|
18
|
Uesaka T, Nagashimada M and Enomoto H:
GDNF signaling levels control migration and neuronal
differentiation of enteric ganglion precursors. J Neurosci.
33:16372–16382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi JY, Liu GS, Liu LF, Kuo SM, Ton CH,
Wen ZH, Tee R, Chen CH, Huang HT, Chen CL, et al: Glial cell
line-derived neurotrophic factor gene transfer exerts protective
effect on axons in sciatic nerve following constriction-induced
peripheral nerve injury. Hum Gene Ther. 22:721–731. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mwangi SM, Usta Y, Shahnavaz N, Joseph I,
Avila J, Cano J, Chetty VK, Larsen CP, Sitaraman SV and Srinivasan
S: Glial cell line-derived neurotrophic factor enhances human islet
posttransplantation survival. Transplantation. 92:745–751. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu X, Sun Y, Wang Z, Cui W, Peng Y and Li
R: Expression of glial cell line-derived neurotrophic factor and
its receptors in cultured retinal müller cells under high glucose
circumstance. Anat Rec (Hoboken). 295:532–539. 2012. View Article : Google Scholar
|
|
22
|
Maekawa M, Takashima N, Matsumata M,
Ikegami S, Kontani M, Hara Y, Kawashima H, Owada Y, Kiso Y,
Yoshikawa T, et al: Arachidonic acid drives postnatal neurogenesis
and elicits a beneficial effect on prepulse inhibition, a
biological trait of psychiatric illnesses. PloS One. 4:e50852009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez-Nölting R, Arnal E, Barcia JM,
Miranda M and Romero FJ: Protection by DHA of early hippocampal
changes in diabetes: Possible role of CREB and NF-kB. Neurochem
Res. 37:105–115. 2012. View Article : Google Scholar
|
|
24
|
Xue HY, Lu YN, Fang XM, Xu YP, Gao GZ and
Jin LJ: Neuroprotective properties of aucubin in diabetic rats and
diabetic encephalopathy rats. Mol Biol Rep. 39:9311–9318. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lapchak PA, Jiao S, Collins F and Miller
PJ: Glial cell line-derived neurotrophic factor: Distribution and
pharmacology in the rat following a bolus intraventricular
injection. Brain Res. 747:92–102. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gearhart DA, Middlemore ML and Terry AV:
ELISA methods to measure cholinergic markers and nerve growth
factor receptors in cortex, hippocampus, prefrontal cortex and
basal forebrain from rat brain. J Neurosci Methods. 150:159–173.
2006. View Article : Google Scholar
|
|
27
|
Kwon MH, Ryu JK, Kim WJ, Jin HR, Song KM,
Kwon KD, Batbold D, Yin GN, Koh GY and Suh JK: Effect of
intracavernous administration of angiopoietin-4 on erectile
function in the strep-tozotocin-induced diabetic mouse. J Sex Med.
10:2912–2927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chandrasekharan B, Anitha M, Blatt R,
Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV
and Srinivasan S: Colonic motor dysfunction in human diabetes is
associated with enteric neuronal loss and increased oxidative
stress. Neurogastroenterol Motil. 23:131–138. e1262011. View Article : Google Scholar :
|
|
29
|
Hussain S, Mansouri S, Sjoholm A, Patrone
C and Darsalia V: Evidence for cortical neuronal loss in male type
2 diabetic goto-kakizaki rats. J Alzheimers Dis. 41:551–560.
2014.PubMed/NCBI
|
|
30
|
Liu W, Yue W and Wu R: Effects of diabetes
on expression of glial fibrillary acidic protein and neurotrophins
in rat colon. Auton Neurosci. 154:79–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu GS, Shi JY, Lai CL, Hong YR, Shin SJ,
Huang HT, Lam HC, Wen ZH, Hsu KS, Chen CH, et al: Peripheral gene
transfer of glial cell-derived neurotrophic factor ameliorates
neuropathic deficits in diabetic rats. Hum Gene Ther. 20:715–727.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosenblad C, Kirik D, Devaux B, Moffat B,
Phillips HS and Björklund A: Protection and regeneration of nigral
dopaminergic neurons by neurturin or GDNF in a partial lesion model
of Parkinson's disease after administration into the striatum or
the lateral ventricle. Eur J Neurosci. 11:1554–1566. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bowenkamp KE, Lapchak PA, Hoffer BJ,
Miller PJ and Bickford PC: Intracerebroventricular glial cell
line-derived neurotrophic factor improves motor function and
supports nigrostriatal dopamine neurons in bilaterally
6-hydroxydo-pamine lesioned rats. Exp Neurol. 145:104–117. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koeberle PD and Bähr M: The upregulation
of GLAST-1 is an indirect antiapoptotic mechanism of GDNF and
neurturin in the adult CNS. Cell Death Differ. 15:471–483. 2008.
View Article : Google Scholar
|
|
35
|
Mwangi S, Anitha M, Mallikarjun C, Ding X,
Hara M, Parsadanian A, Larsen CP, Thule P, Sitaraman SV, Anania F
and Srinivasan S: Glial cell line-derived neurotrophic factor
increases beta-cell mass and improves glucose tolerance.
Gastroenterology. 134:727–737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Divecha N and Irvine RF: Phospholipid
signaling. Cell. 80:269–278. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kelly A and Lynch MA: Long-term
potentiation in dentate gyrus of the rat is inhibited by the
phosphoinositide 3-kinase inhibitor, wortmannin. Neuropharmacology.
39:643–651. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Niswender KD, Morrison CD, Clegg DJ, Olson
R, Baskin DG, Myers MG Jr, Seeley RJ and Schwartz MW: Insulin
activation of phosphatidylinositol 3-kinase in the hypothalamic
arcuate nucleus: A key mediator of insulin-induced anorexia.
Diabetes. 52:227–231. 2003. View Article : Google Scholar : PubMed/NCBI
|