Introduction
Ovarian cancer is the fifth most common cause of
cancer-associated mortality in females worldwide according to the
global cancer statistics for 2013 (1). The most common histological sub-type
of ovarian cancer is serous ovarian cancer (SOC). It is the most
lethal type of gynecological cancer and, in spite of advances in
the detection of SOC and cytotoxic therapies, has a five-year
survival rate of <30% (2). The
main reason for the high mortality of patients with SOC may be due
to the fact that >70% of patients are diagnosed at an advanced
stage of the disease. Although a large number of biomarkers with
potential use for diagnosis and drug development have been reported
(3–5), the detailed underlying mechanisms of
the development of metastasis of this neoplasia have remained
elusive.
Numerous studies have indicated that the adenosine
triphosphate (ATP)-binding cassette (ABC) transporter family, which
is an ancient family of transmembrane proteins, is frequently
upregulated in metastatic cancer types and leads to
chemotherapeutic resistance (6).
The ABCC sub-family of ABC transporters includes cystic fibrosis
transmembrane conductance regulator (CFTR) and the multidrug
resistance-associated proteins, which are active drug exporters
(7). CFTR, a ~170-kDa glycosylated
protein, is known to be a cyclid adenosine monophosphate-dependent
chloride anion-conducting channel (8). CFTR is expressed in epithelial cells
of the human female reproductive tract (9) and overexpression of CFTR is
associated with human cervical cancer as well as its progression
and prognosis (10).
Previous studies by our group reported high
expression of CFTR in SOC tissues, while knockdown of CFTR
suppressed the proliferation of ovarian cancer in vitro and
in vivo (11). Thus, it was
hypothesized that overexpression of CFTR may be involved in the
progression of SOC. In order to verify this hypothesis, the present
study constructed an adenoviral vector for the forced
overexpression of the CFTR gene in target cells. SOC cells were
then transfected with this adenovirus to induce CFTR
overexpression, and the effects of CFTR on cancer progression were
studied using Transwell and wound healing assays. Furthermore, the
present study assessed the mechanisms of the effects of CFTR on the
migratory and invasive malignant properties of SOC cells.
Materials and methods
Materials
Shuttle plasmid pAdTrace-TOX, E1/E3-deleted
replication-defective adenoviral backbone plasmid pAdEasy1 and the
BJ5183 Escherichia coli (E. coli) strain were kindly
provided by Dr Tongchuan He (Molecular Oncology Laboratory,
Department of Surgery, The University of Chicago Medical Center,
Chicago, IL, USA). HEK293 cells were obtained from the American
Type Culture Collection (Manassas, VA, USA). The pcDNA3.1(+) vector
containing the full-length purified human CFTR gene was kindly
provided by Dr WenMing Xu (Key Laboratory of Obstetric, Gynecologic
and Pediatric Diseases and Birth Defects of Ministry of Education,
West China Second University Hospital, Sichuan University, Chengdu,
China). DH5α and A2780 cells were purchased from the China Center
for Type Culture Collection (Wuhan, China). Cells used at passage
3–5. Dulbecco's modified Eagle's medium (DMEM) was purchased from
Hyclone. Plasmid Mini kit (cat no. DP103) was purchased from
Tiangen Biotek Inc. (Beijing, China). A Gel Extraction kit (cat no.
PD1601) was purchased from BioTeke Corp. (Beijing, China).
Restriction enzyme and T4 DNA Ligase were obtained from Thermo
Fisher Scientific (Waltham, MA, USA).
pAdTrace-CFTR-TOX construction
The adenovirus was constructed using the AdEasy
system (kindly provided by Dr Tongchuan He) (12). The human CFTR gene was digested
with EcoRV, SalI and KpnI (Thermo Fisher
Scientific) from pcDNA3.1-CFTR, which was directionally cloned into
the shuttle plasmid pAdTrace-TOX vector containing a red
fluorescent protein (RFP) reporter gene, cytomegalovirus (CMV)
promoters, as well as PmeI and PacI sites. The
ligation of purified CFTR fragment to pAdTrace-TOX at the molar
ratios of 1:1, 2:1 and 3:1 was achieved via incubation with T4 DNA
ligase (Thermo Fisher Scientific) at 22°C for 1 h. The plasmid was
extracted from clones resistant to 50 µg/ml kanamycin
(KeyGen Biotech, Nanjing, China) with sterile double-distilled
water and screened by digestion with restriction endonuclease
EcoRV and KpnI (Thermo Fisher Scientific).
Construction of recombinant adenoviral
plasmids by homologous recombination in E. coli BJ5183 cells
The correct recombinant pAdTrace-TOX-CFTR plasmid,
which was linearized by PmeI, was transfected into BJ5183
E. coli cells together with the adenoviral backbone vector
pAdEasy-1. The transformed cells were plated on LB agar medium
plates (Guduo Biotechnology, Shanghai, China) containing 100
µg/ml ampicillin and incubated at 37°C overnight. Plasmids
extracted from small colonies using the Plasmid Mini kit (cat no.
DP103; Tiangen Biotek Inc.) were separated by 0.8% agarose gel
electrophoresis and examined by digestion with restriction
endonuclease HindIII (Thermo Fisher Scientific). Gene Ruler™
1-kb DNA ladder (cat no. SM1333; Thermo Fisher Scientific) was used
as a marker. The recombinant adenoviral plasmid was named as
pAd-CFTR and transformed into E. coli DH5α cells (cat. no.
CB101-02; Tiangen Biotek Inc.) for amplification.
Transfection of recombinant adenovirus
into HEK293 cells
HEK293 cells were cultured in DMEM supplemented with
10% fetal bovine serum (FBS; Invitrogen Life Technologies) at 37°C
in a humidified atmosphere containing 5% CO2. pAd-CFTR
plasmid was linearized using PacI and trans-fected into
HEK293 cells using Polyjet™ transfection reagent (SignaGen
Laboratories, Gaithersburg, MD, USA) to package the recombinant
adenoviral vector containing the CFTR gene according to the
manufacturer's instructions. After 24 and 48 h of culture, RFP
expression was observed by fluorescence microscopy (T-P2; Nikon,
Tokyo, Japan). At 10–14 days after transfection, the cells were
collected and subjected to three cycles of freezing at −80°C,
thawing in a 37°C-water bath and vigorous vortexing. The
recombinant adenovirus containing the CFTR gene was then
collected.
Adenovirus-mediated overexpression of
CFTR in A2780 cells
A2780 cells were seeded into six-well culture plates
at 50–60% confluence and the adenovirus was added to the medium.
After 48 h of transfection, RFP-positive cells were observed under
a microscope (T-P2; Nikon). Cells (1×106) were collected
and suspended in 1 ml 1% bovine serum albumin (BSA; KeyGen Biotech)
in phosphate-buffered saline. The ratio of RFP-positive to total
cells was acquired by flow cytometry using a FACSCanto™ II system
and Cell Quest Pro software (BD Biosciences, Franklin Lakes, NJ,
USA). Reverse-transcription quantitative polymerase chain reaction
(RT-qPCR), western blot analysis and immunofluorescence were
performed to detect the expression of CFTR. An empty adenovirus
(Ad-null) was used as an adenoviral control.
RT-qPCR
After 48 h of culture, total RNA was isolated from
cells using TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer's instructions. 1 µg
total RNA was used for reverse transcription in a 20-µl
reaction volume using the PrimeScript RT reagent kit (Takara Bio
Inc., Otsu, Japan). The following primers (all from Sangon Biotech,
Shanghai, China) were used: Human CFTR forward,
5′-TGCCCTTCGGCGATGTTT-3′ and reverse, 5′-GCGATAGAGCGTTCCTCCTTG-3′
(product length, 147 bp); GAPDH forward, 5′-CAGCGACACCCACTCCTC-3′
and reverse, 5′-TGAGGTCCACCACCCTGT-3′ (product length, 122 bp).
qPCR was performed using a CFX96 real-time system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). PCR was performed at 95°C
for 3 min followed by 40 cycles at 95°C for 3 sec and 60°C for 20
sec. Relative quantification of gene expression was performed using
the 2−ΔΔCT method (13). Real-time PCR analysis was performed
in at least three independent experiments.
Western blot analysis
Following transfection, cells were collected and
lysed with Lysis Buffer (Beyotime Institute of Biotechnology,
Haimen, China) containing phenylmethanesul-fonylfluoride. The
protein concentration was measured using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). 50–100
µg protein per lane was separated by 8% SDS-PAGE and
transferred onto a polyvinylidene difluoride membrane (Millipore
Corp., Billerica, MA, USA). After blocking with 5% skimmed milk at
37°C for 1 h, the membranes were incubated in mouse monoclonal
anti-human CFTR antibody (diluted at 1:1,000; cat no. ab2784;
Abcam, Cambridge, UK), and rabbit polyclonal GAPDH antibody
(sc-25778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight. After washing with Tris-buffered saline containing Tween
20, membranes were incubated with the appropriate horseradish
peroxidase-conjugated secondary antibodies (goat anti-mouse
IgG-HRP; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. Proteins were visualized using an enhanced
chemiluminescence kit (cat no. 34080; GE Healthcare, Little
Chalfont, UK) and images were captured using the ChemiDoc XRS
system (Bio-Rad Laboratories, Inc.).
Immunofluorescence staining
Transfected cells were fixed with methanol at −20°C
for 15 min and blocked with 1% BSA at room temperature for 30 min,
followed by incubation with CFTR primary antibody (diluted at
1:100; cat no. ab2784; Abcam) at 4°C overnight. After washing with
Tris-buffered saline containing Tween-20 twice, cells were
incubated with fluorescein isothiocyanate-conjugated secondary
antibody (Zhongshan Golden Bridge Inc., Zhongshan, China) at room
temperature for 1 h in the dark. DAPI (Invitrogen Life
Technologies, Inc.) was used to stain the nuclei. The presence of
the proteins was examined under an inverted fluorescence microscope
(T-P2; Nikon). Untreated SOC cells stained with mouse
isotype-specific immunoglobulin M (Rockland Immunochemicals,
Limerick, PA, USA) were used as negative controls.
Cell invasion assay
In the present study, cell invasion was determined
by a Transwell assay using the Cell Invasion Assay kit (8 µm
pore size; Cell Biolabs, Inc., San Diego, CA, USA) according to the
manufacturer's instructions. Approximately 1×105 cells
were seeded into the upper chamber of the Transwell insert with
serum-free media, and the bottom chambers were filled with medium
containing 10% FBS as a chemoattractant. After incubation for 24 h
in a humidified incubator at 37°C, cells on the upper side of the
Transwell membrane were wiped off. Any cells that had transgressed
through the membrane and were located at the lower side of the
insert were fixed with 4% formaldehyde and stained in 0.5% crystal
violet solution (KeyGen Biotech). Images of the cells were captured
using a light microscope (TS100; Nikon, Tokyo, Japan) under ×100
magnification and cells in random fields were counted. The
procedure was repeated independently three times with triplicate
inserts in each group.
Wound healing assay
Cell migration was evaluated in vitro using
the scratch wound healing assay. In brief, ~5×105 cells
were seeded onto a six-well plate and grown to a confluent
monolayer. A line-shaped wound of the surface of confluent cells
was generated using a pipette tip. The cells were washed with
growth medium to remove any cell debris, and to smooth the edges of
the scratch wound. The cells were then incubated at 37°C; cellular
migration was evaluated and images were captured using a
phase-contrast microscope. The width of the same area of the wound
was observed at 0 and 24 h, and the relative width of the scratch
was measured quantitatively using Adobe Photoshop 7.0 (Adobe
Systems, San Jose, CA, USA). The extent of gap closure was
determined as the rate of cell migration. Each assay was performed
in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Values are expressed as the
mean ± standard deviation. A two-tailed Student's t-test assuming
equal variances was performed to determine significant differences
between two groups. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Construction and identification of
recombinant pAd-CFTR adenoviral plasmid
The plasmid pcDNA3.1-CFTR was digested by enzymes
EcoRV, SalI and KpnI, and subsequently
subjected to agarose gel electrophoresis. As shown in Fig. 1A, the products included four
fragments with sizes of 4.7, 2.4, 2.0 and 1.0 kb, respectively. The
fragment of 4.7 kb resembled CFTR and the other fragments were
pcDNA3.1 fragments. The CFTR fragment from pcDNA3.1-CFTR digestion
was inserted into the RFP-labeled adenoviral shuttle vector
pAdTrace-TOX. Digestion of the recombinant pAdTrace-TOX-CFTR
plasmid with KpnI and EcoRV resulted in two
fragments: CFTR (4.7 kb) and the adenoviral shuttle vector (~9.1
kb; Fig. 1B). Sequence analysis
also confirmed that the fragment inserted into pAdTrace-TOX was the
human CFTR cDNA fragment obtained by PCR only. These results
confirmed that the pAdTrace-TOX-CFTR containing the CFTR gene was
successfully constructed. After homologous recombination with
pAd-easy1 in BJ5183 E. coli cells, recombinant adenoviral
vector pAd-CFTR containing the CFTR fragment was obtained and
confirmed by HindIII endonuclease analysis. As shown in
Fig. 1C, digestion of pAd-CFTR
with HindIII produced eight fragments. Compared with the
negative control (Fig. 1C; lane
2), the largest fragment of pAd-CFTR was ~12.1 kb (Fig. 1C; lane 3) which was larger than
that of pAdEasy-1 bone vector (~4,700 kb), indicating the
successful construction of the recombinant adenoviral expression
vector pAd-CFTR. The target genes were confirmed to be correctly
cloned into the adenoviral vector by gene sequencing and matched to
the CFTR sequence in GenBank (www.ncbi.nlm.nih.gov/gene/1080).
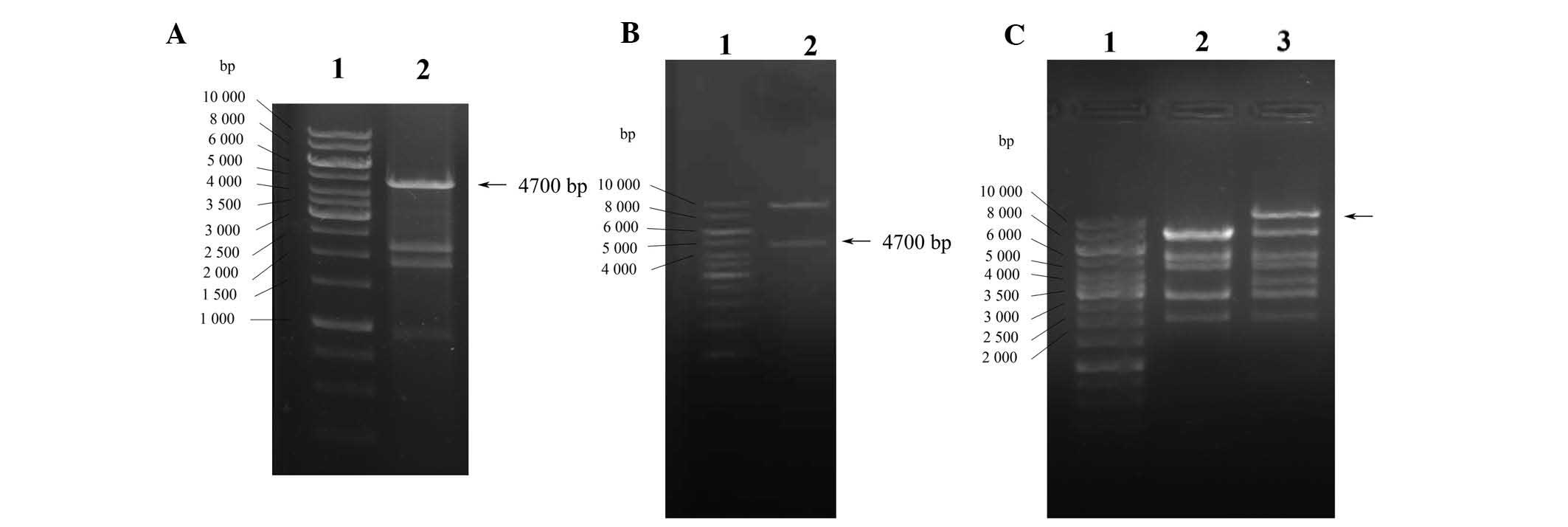 | Figure 1Construction and identification of
Ad-CFTR vector. (A) Identification of pcDNA3.1-CFTR plasmid by
enzyme digestion. Lanes: 1, Gene Ruler™ 1-kb DNA Ladder; 2,
pcDNA3.1-CFTR digested by EcoRV, SalI and KpnI
enzymes to liberate the CFTR gene (4.7 kb). (B) Identification of
recombinant pAdTrace-TOX-CFTR plasmid by enzyme digestion. Lanes:
1, Gene Ruler™ 1 kb DNA Ladder; 2, pAdTrace-TOX-CFTR plasmid
digested by KpnI and EcoRV enzymes, indicating the
human CFTR gene was cloned into the shuttle plasmid. (C)
Identification of recombinant pAd-CFTR by enzyme digestion. Lanes:
1, Gene Ruler™ 1 kb DNA Ladder; 2, pAdEasy-1 bone vector digestion
with HindIII as the negative control; 3, digestion products
of pAd-CFTR by HindIII. The largest fragment of pAd-CFTR was
larger than the pAdEasy-1 bone vector (~4,700 kb), indicating the
successful construction of the recombinant adenoviral expression
vector. CFTR, cystic fibrosis transmembrane conductance regulator;
Ad, adenovirus. |
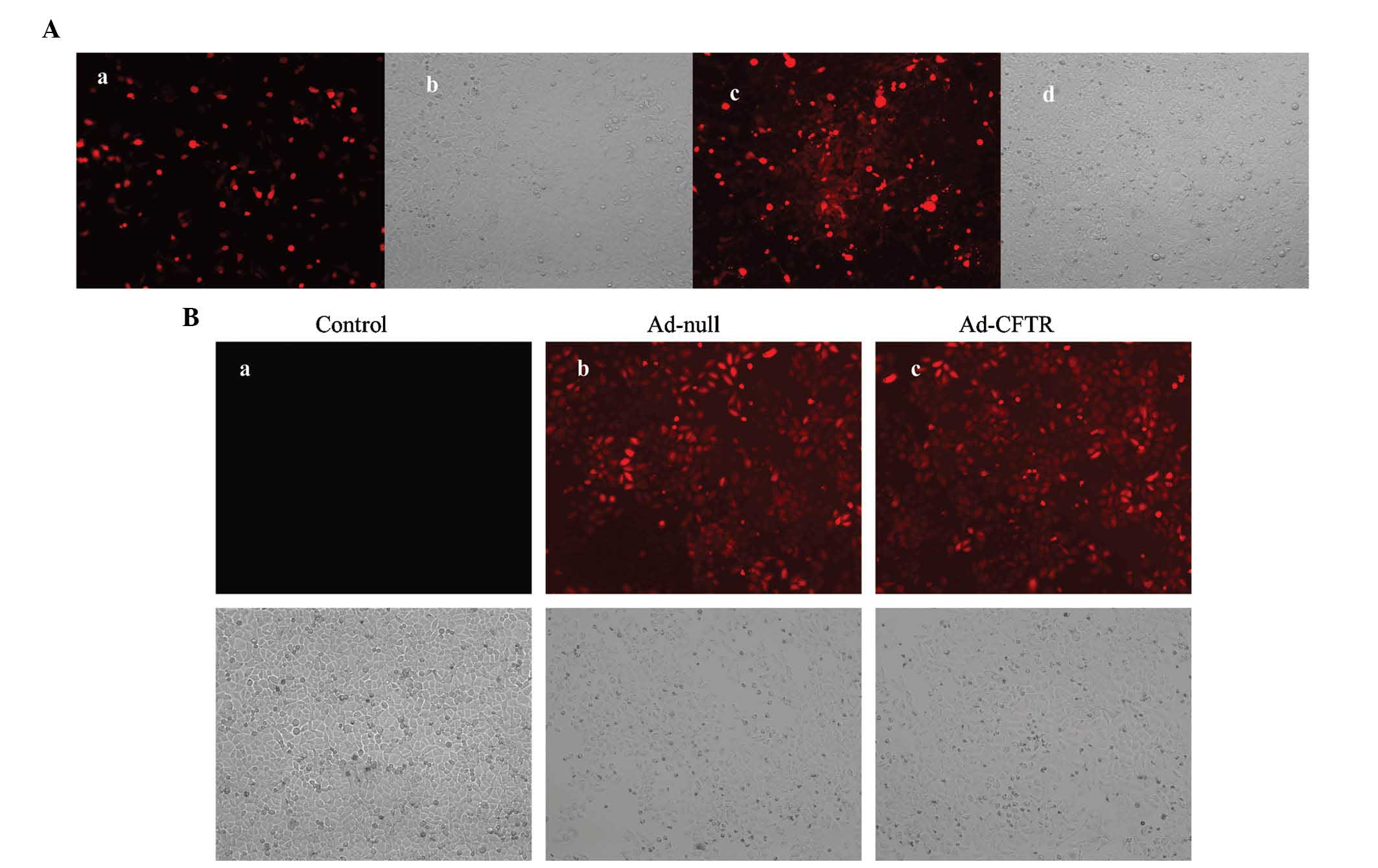
Generation of adenoviral recombinants in
HEK293 cells
The pAd-CFTR vector contains an RFP coding region
driven by the CMV promoter; thus, the levels of red fluorescence
are a measure of the transfection efficiency. At 24 h post
-transfection with PacI-linearized pAd-CFTR plasmid, >10%
of HEK293 cells were RFP-positive (Fig. 2Aa and Ab). Amplification of Ad-CFTR
produced a cloudy appearance of red fluorescence in the HEK293 cell
line at day 10 during packaging (Fig.
2Ac and Ad). This result demonstrated that the recombinant
adenoviral vector containing the human CFTR gene was successfully
constructed.
Transfection of Ad-CFTR leads to
overexpression of CFTR in A2780 cells
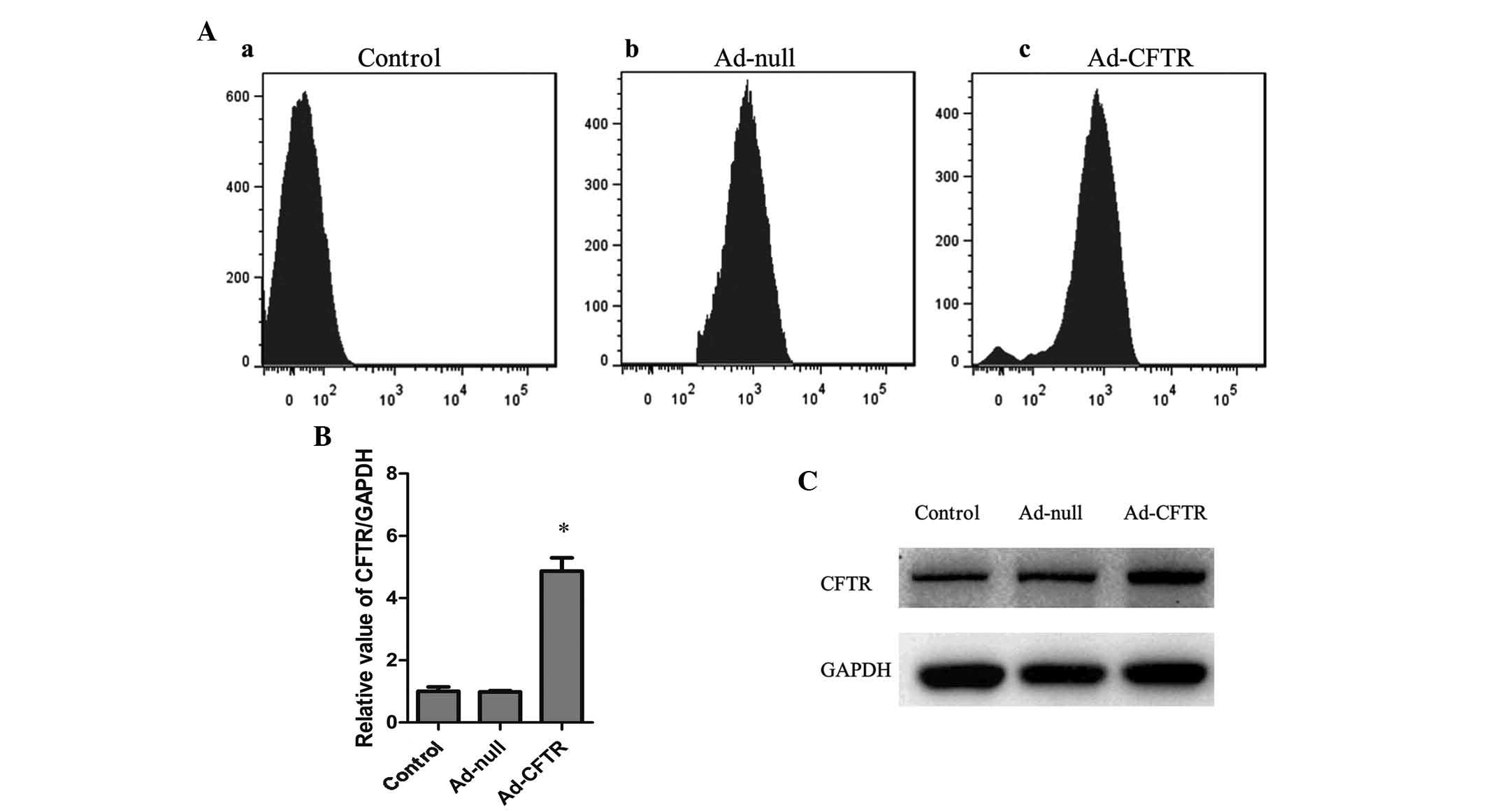
A2780 cells were transfected with Ad-CFTR and
RFP-positive cells were observed by fluorescence microscopy and
flow cytometry. After 48 h of transfection, 88.21% of A2780 cells
were observed to be RFP-positive, while 90.15% of A2780 were
RFP-positive following transfection with the empty Ad-null control
vector (Figs. 2B and 3A). RT-qPCR and western blot analysis
showed that native A2780 cells expressed relatively low levels of
endogenous CFTR, which was not affected by transfection with
Ad-null vector, while transfection with Ad-CFTR significantly
induced the expression of CFTR in A2780 cells by ~5-fold (Fig. 3B and C). The immunofluorescence
images indicated localization of exogenous CFTR in the cytoplasm
and cell membrane of A2780 cells (Fig.
4A). These results demonstrated that Ad-CFTR was able to
effectively transfect A2780 cells and increase the mRNA and protein
expression of CFTR.
Overexpression of CFTR enhances cell
invasion and motility of A2780 cells in vitro
As shown in Fig. 4B and
C, Ad-CFTR cells displayed a 1.69-fold increase in invasive
capacity compared with that of the control cells. (P<0.05). In
parallel with this, the migratory capacity of the Ad-CFTR was
increased by 1.73-fold of that of the control cells (P<0.05)
(Fig. 4D and E).
Overexpression of CFTR affects the
activation of c-Src signaling
To further elucidate the underlying mechanisms of
CFTR-induced cell motility and invasion, the protein expression of
Src kinase was evaluated in Ad-CFTR-transfected and control cells.
There were no significant differences in the levels of total Src
and phospho-Src (Tyr419) between any of the groups. However,
Ad-CFTR cells showed decreased levels of phospho-Src (Tyr530),
suggesting that CFTR may affect the invasion and motility of A2780
cells mainly through dephosphorylation of critical tyrosine
residues at 530 bp of Src (Fig. 5A and
B). Further loss/gain of function studies are necessary to
verify these results.
Discussion
CFTR, by controlling ion and protein transport, is
thought to aid in the maintenance of cellular homeostasis in most
human cells (14). Accumulating
evidence suggested that CFTR has a key role in the progression and
metastasis of cancer. In the female reproductive system, it has
been reported that overexpression of CFTR is closely associated
with the development of cervical cancer, aggressiveness of tumor
cells and poor prognosis of patients (10). CFTR has a tumor-suppressing role in
the development of prostate cancer, which is mediated via
microRNA-193b targeting urokinase plasminogen activator (15). Another study indicated that CFTR
expression is significantly downregulated in breast cancer, which
promotes epithelial-to-mesenchymal transition and is associated
with poor prognosis (16). CFTR is
also known to be involved in modulating signaling pathways in cell
inflammation and apoptosis (17,18).
The present study constructed a vector to
overexpress exogenous CFTR in SOC cells and determine its role in
tumor progression. Viruses can be used for efficient gene transfer,
and are able to survive and replicate in mammalian hosts (19). Adenoviral vectors are commonly used
for gene transfection as the transferred DNA does not integrate
into the host's genome (20). The
present study successfully constructed an adenoviral vector
carrying the CFTR gene by using the replication-defective AdEasy
adenovirus system. Ad-CFTR was confirmed to transfect SOC cells
with an efficacy of >80%, and significantly enhanced the mRNA
and protein expression of CFTR. Immunofluorescence analysis
indicated localization of CFTR in the cytomembrane and cytoplasm of
SOC cells, suggesting that Ad-CFTR induced the expression of
biologically active CFTR. Further experiments were performed to
assess the effects of CFTR on the aggressiveness of ovarian cancer
cells. Ad-CFTR-transfected SOC cells showed an increased cell
motility and adhesion to Matrigel compared with that of the control
cells. Metastasis is a complex biological process during which
tumor cells acquire invasive and migratory abilities, leading to
the dissemination from a primary tumor to distant secondary organs
or tissues (21). Overexpression
of CFTR in ovarian cancer cells enhanced their
metastasis-associated migratory and invasive abilities, indicating
that CFTR has a significant role in ovarian cancer metastasis and
may represent a potential target for the treatment of ovarian
cancer.
Studies have shown that CFTR binds to
ezrin/radixin/moesin (ERM)-binding phosphoprotein of 50 kDa
(EBP50), which interacts with the ERM-family proteins via its
C-terminal domain. ERM-family proteins bind to the actin
cytoskeleton, which is essential in the apical polarization of CFTR
in epithelial cells (22,23). The present study hypothesized that
the CFTR may enhance the invasive and migratory abilities of cancer
cells by connecting membrane rafts to the actin cytoskeleton.
To further elucidate the underlying mechanisms of
CFTR-induced cell motility and invasion, protein expression of Src
kinase was evaluated in Ad-CFTR-transfected and control cells. The
non-receptor tyrosine kinase Src has important roles in numerous
aspects of cell physiology and is associated with signalling
pathways by which cell surface receptors regulate diverse
processes, including cell division, motility, adhesion,
angiogenesis and survival, as well as the function of modular
domains that mediate protein-protein interactions (24). Validation studies showed that Src
family kinases, most notably c-Src, are frequently overexpressed
and/or aberrantly activated in a variety of human cancers.
Activation of c-Src is common in human ovarian cancer (25,26)
and is involved in ovarian cancer cell survival and resistance
against chemotherapy (27). Wiener
et al (28) reported that
reduction in Src activity affected cellular morphology, inhibited
anchorage-independent growth and diminished tumor growth in
vivo. The findings of the present study indicated that
upregulation of CFTR reduced the levels of phospho-Src (Tyr530),
while levels of total Src and phospho-Src (Tyr419) were similar
between Ad-CFTR and control groups.
The phosphorylation of the conserved tyrosine
residues Tyr-416/419 and Tyr-527/530 (in chicken/human c-Src) is
crucial for the regulation of c-Src, which is mostly phosphorylated
at Tyr-527/530 and adopts the inactive conformation (29). The phosphorylation of Tyr-416/419
in the activation loop locks the catalytic domain in its active
conformation (30). However, the
conserved Tyr-527/530 in the C-terminal tail was identified as the
site of inhibitory phosphorylation (31). It is known that carboxyl-terminal
Src kinase (Csk), a cytoplasmic tyrosine kinase, can phosphorylate
the C-terminal regulatory sites Tyr-527/530 of c-Src to inhibit its
activity (29). Furthermore,
Csk-binding protein (Cbp), a transmembrane protein, can recruit Csk
to the membrane where c-Src is located. The Cbp-mediated
re-location of Csk to the membrane may be involved in blocking Src
signaling events (32). Of note,
EBP50 is a specific Cbp-binding partner interacting with the
C-terminal sequence of Cbp. The Cbp-EBP50 interaction may be
important for Cbp to recruit Csk to the membrane, which may
facilitate the repressive function of Csk on c-Src (22). Therefore, the present study
hypothesized that CFTR may indirectly affect the phosphorylation of
c-Src at its Tyr-527/530 sites through interacting with EBP50, Cbp
and Csk, while not affecting the Tyr-416/419 positions of Src.
Furthermore, it is inferred that, as SOC cells express relatively
high levels of CFTR, the induction of the progression of ovarian
cancer, including migration and invasiveness, may be facilitated by
de-phosphorylation of c-Src at its Tyr-527/530 sites. In line with
the results of the present study, a recent study by our group
performed small hairpin RNA-mediated knockdown of CFTR, which
suppressed the aggressive malignant biological behavior of ovarian
cancer cells in vitro and in vivo (11).
In conclusion, the present study demonstrated that
adenovirus-mediated CFTR overexpression in SOC cells enhanced the
migratory and invasive abilities of SOC cells in vitro. As
an ion channel protein, CFTR may also be a critical candidate
molecule for c-Src signal activation. Further studies on CFTR
should be performed to elucidate the biochemical mechanism of the
c-Src signaling pathway in SOC development.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172492), the Key
Project of Chongqing Science and Technology Commission (grant no.
CSTC 2012JJB10030) and the Key Project of Chongqing Municipal
Health Bureau (grant no. 2011-1-056).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rauh-Hain JA, Krivak TC, Del Carmen MG and
Olawaiye AB: Ovarian cancer screening and early detection in the
general population. Rev Obstet Gynecol. 4:15–21. 2011.PubMed/NCBI
|
|
3
|
Lee JM, Trepel JB, Choyke P, et al: CECs
and IL-8 have prognostic and predictive utility in patients with
recurrent platinum-sensitive ovarian cancer: Biomarker correlates
from the randomized phase-2 trial of olaparib and cediranib
compared with olaparib in recurrent platinum-sensitive ovarian
cancer. Front Oncol. 5:1232015. View Article : Google Scholar
|
|
4
|
Chambers SK, Clouser MC, Baker AF, et al:
Overexpression of tumor vascular endothelial growth factor A may
portend an increased likelihood of progression in a phase II trial
of bevacizumab and erlotinib in resistant ovarian cancer. Clin
Cancer Res. 16:5320–5328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rankin EB, Fuh KC, Taylor TE, Krieg AJ,
Musser M, Yuan J, et al: AXL is an essential factor and therapeutic
target for metastatic ovarian cancer. Cancer Res. 70:7570–7579.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Higgins CF: ABC transporters: From
microorganisms to man. Annu Rev Cell Biol. 8:67–113. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bear CE, Li CH, Kartner N, Bridges RJ,
Jensen TJ, Ramjeesingh M and Riordan JR: Purification and
functional reconstitution of the cystic fibrosis transmembrane
conductance regulator (CFTR). Cell. 68:809–818. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tizzano EF, Silver MM, Chitayat D,
Benichou JC and Buchwald M: Differential cellular expression of
cystic fibrosis transmembrane regulator in human reproductive
tissues. Clues for the infertility in patients with cystic
fibrosis. Am J Pathol. 144:906–914. 1994.PubMed/NCBI
|
|
10
|
Peng X, Wu Z, Yu L, Li J, Xu W, Chan HC,
Zhang Y and Hu L: Overexpression of cystic fibrosis transmembrane
conductance regulator (CFTR) is associated with human cervical
cancer malignancy, progression and prognosis. Gynecol Oncol.
125:470–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J, Yong M, Li J, Dong X, Yu T, Fu X and
Hu L: High level of CFTR expression is associated with tumor
aggression and knockdown of CFTR suppresses proliferation of
ovarian cancer in vitro and in vivo. Oncol Rep. 33:2227–2234.
2015.PubMed/NCBI
|
|
12
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Schwiebert EM, Benos DJ, Egan ME, Stutts
MJ and Guggino WB: CFTR is a conductance regulator as well as a
chloride channel. Physiol Rev. 79(Suppl): S145–S166.
1999.PubMed/NCBI
|
|
15
|
Xie C, Jiang XH, Zhang JT, Sun TT, Dong
JD, Sanders AJ, Diao RY, Wang Y, Fok KL, Tsang LL, et al: CFTR
suppresses tumor progression through miR-193b targeting urokinase
plasminogen activator (uPA) in prostate cancer. Oncogene.
32:2282–2291. 2291 e1–7. 2013. View Article : Google Scholar
|
|
16
|
Zhang JT, Jiang XH, Xie C, Cheng H, Da
Dong J, Wang Y, Fok KL, Zhang XH, Sun TT, Tsang LL, et al:
Downregulation of CFTR promotes epithelial-to-mesenchymal
transition and is associated with poor prognosis of breast cancer.
Biochim Biophys Acta. 1833:2961–2969. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacquot J, Tabary O, Le Rouzic P and
Clement A: Airway epithelial cell inflammatory signalling in cystic
fibrosis. Int J Biochem Cell Biol. 40:1703–1715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Z, Peng X, Li J, Zhang Y and Hu L:
Constitutive activation of nuclear factor KB contributes to cystic
fibrosis transmembrane conductance regulator expression and
promotes human cervical cancer progression and poor prognosis. Int
J Gynecol Cancer. 23:906–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verma IM and Weitzman MD: Gene therapy:
Twenty-first century medicine. Annu Rev Biochem. 74:711–738. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Björklund A, Kirik D, Rosenblad C,
Georgievska B, Lundberg C and Mandel RJ: Towards a neuroprotective
gene therapy for Parkinson's disease: use of adenovirus, AAV and
lentivirus vectors for gene transfer of GDNF to the nigrostriatal
system in the rat Parkinson model. Brain Res. 886:82–98. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brdickova N, Brdicka T, Andera L, Spicka
J, Angelisová P, Milgram SL and Horejsí V: Interaction between two
adapter proteins, PAG and EBP50: A possible link between membrane
rafts and actin cytoskeleton. FEBS Lett. 507:133–136. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moyer BD, Denton J, Karlson KH, Reynolds
D, Wang S, Mickle JE, Milewski M, Cutting GR, Guggino WB, Li M and
Stanton BA: A PDZ-interacting domain in CFTR is an apical membrane
polarization signal. J Clin Invest. 104:1353–1361. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin GS: The hunting of the Src. Nat Rev
Mol Cell Biol. 2:467–475. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Summy JM and Gallick GE: Src family
kinases in tumor progression and metastasis. Cancer Metastasis Rev.
22:337–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wiener JR, Windham TC, Estrella VC, Parikh
NU, Thall PF, Deavers MT, Bast RC, Mills GB and Gallick GE:
Activated src protein tyrosine kinase is overexpressed in
late-stage human ovarian cancers. Gynecol Oncol. 88:73–79. 2003.
View Article : Google Scholar
|
|
27
|
Pengetnze Y, Steed M, Roby KF, Terranova
PF and Taylor CC: Src tyrosine kinase promotes survival and
resistance to chemotherapeutics in a mouse ovarian cancer cell
line. Biochem Biophys Res Commun. 309:377–383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wiener JR, Nakano K, Kruzelock RP, Bucana
CD, Bast RC Jr and Gallick GE: Decreased Src tyrosine kinase
activity inhibits malignant human ovarian cancer tumor growth in a
nude mouse model. Clin Cancer Res. 5:2164–2170. 1999.PubMed/NCBI
|
|
29
|
Okada M: Regulation of the SRC family
kinases by Csk. Int J Biol Sci. 8:1385–1397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knighton DR, Xuong NH, Taylor SS and
Sowadski JM: Crystallization studies of cAMP-dependent protein
kinase. Cocrystals of the catalytic subunit with a 20 amino acid
residue peptide inhibitor and MgATP diffract to 30 A resolution. J
Mol Biol. 220:217–220. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cooper JA, Gould KL, Cartwright CA and
Hunter T: Tyr527 is phosphorylated in pp60c-src: Implications for
regulation. Science. 231:1431–1434. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeuchi S, Takayama Y, Ogawa A, Tamura K
and Okada M: Transmembrane phosphoprotein Cbp positively regulates
the activity of the carboxyl-terminal Src kinase, Csk. J Biol Chem.
275:29183–29186. 2000. View Article : Google Scholar : PubMed/NCBI
|