Introduction
Ovarian cancer is one of the most common types of
malignant tumor of the female reproductive organs, with the third
highest incidence rate after cervical cancer and uterine cancer
(1). The mortality rate of
epithelial ovarian cancer is the highest of all gynecological
tumors (2). Among the different
types of ovarian cancer, epithelial tumors are the most common, and
the second most common are malignant germ cell tumors (3). Patients with epithelial ovarian
cancer with tumors that are confined to the ovaries at the time of
surgery only account for 30% of all cases, with the majority
experiencing spread to the uterus, bilateral accessory, and omental
and pelvic organs; therefore, there is a problem with early
diagnosis (4).
Peroxisome proliferator-activated receptor (PPAR) is
a type of nuclear transcription factor activated by ligands, and is
a member of the nuclear hormone receptor superfamily. Recent
research has demonstrated that PPARγ is highly expressed in a
number of types of cancer, including stomach, pancreatic, breast,
esophageal and lung cancer (5–9).
Certain studies have indicated that PPARγ can adjust the level of
matrix metalloproteinases (MMPs) and affect trophoblast invasion,
resulting in the restriction of fetal growth (10–12).
Thus, agonists of PPARγ can effect the activity of MMP-9 (13). A previous study showed that
following ligand activation in K562 and HL-60 human myeloid cell
leukemia cell lines, PPARγ can inhibit the adhesion and invasion of
cells by affecting the expression of MMP-2 and -9 (14).
Telmisartan is a common clinical antihypertensive
agent which is an angiotensin receptor inhibitor (15). Studies have demonstrated that
telmisartan not only protects against cerebral ischemia through
adjusting blood pressure, but also by activating PPARγ, which
results in antioxidant and anti-inflammatory effects reducing the
volume of cerebral infarction and protecting against cerebral
ischemia (16–17). Telmisartan can inhibit MMP-9 by
activating PPARγ, which significantly reduces ventricular
remodeling after myocardial infarction and retards atherosclerosis
(18). Therefore, the present
study was conducted to investigate the anticancer effects of
telmisartan.
In the current study, telmisartan was demonstrated
to decrease proliferation and promote the apoptosis of human
ovarian cancer cells by upregulating PPARγ and downregulating MMP-9
expression.
Materials and methods
Chemicals
Dulbecco's modified Eagle's Medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco-BRL (Rockville, MD,
USA). 3.3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium
bromide (MTT) was purchased from Promega Corporation (Madison, WI,
USA). The Caspase-3 Activity Assay kit was purchased from Beyotime
Institute of Biotechnology (Nanjing, China). The Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit was
purchased from BD Pharmingen (San Diego, CA, USA). RNeasy Plus Mini
kit was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China). Transcriptor First Strand cDNA Synthesis kit was purchased
from Sangon Biotech (Shanghai, China).
Cell culture
HEY human ovarian cancer cells were obtained from
the Animal Lab of the First Affiliated Hospital of Dalian Medical
University (Dalian, China). HEY cells were maintained in DMEM,
containing 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin
(Amresco LLC, Solon, OH, USA) at 37°C in 5% CO2.
MTT assay
HEY cells (1×104) were seeded in 96-well
plates and treated with telmisartan (Sigma-Aldrich, St. Louis, MO,
USA) (Fig. 1) at different
concentrations (0, 1, 10 and 100 µM) for 0, 24, 48 and 72 h
(19). The proliferation of HEY
cells was determined by an MTT assay. MTT (20 µl, 5 mg/ml)
was added to each well and incubated for 4 h. Then, the medium in
each well was removed. Approximately 150 µl
dimethylsulf-oxide (Amresco LLC) was added to each well and
incubated for 10 min at room temperature whilst being agitated. The
plates were read with a microplate reader (Model 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 570 nm.
Caspase-3 activity measurement
HEY cells (2.0×105/ml) were plated in
6-well plates and incubated for 24 h. After treatment with
telmisartan (0, 1, 10 or 100 µM), the activity of caspase-3
was detected using the caspase-3 colorimetric assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
instructions. Samples were measured with an ELISA reader (Model
680; Bio-Rad Laboratories, Inc.) at a wavelength of 405 nm.
Flow cytometric analysis for detecting
cellular apoptosis
In accordance with the manufacturer's instructions,
apoptosis was detected using an Annexin V-FITC/PI kit. Briefly, HEY
cells were stained with 5 µl Annexin V-FITC and 5 µl
PI for 15 min at room temperature in the dark. Cell apoptosis was
determined using a flow cytometer (Gallios; Beckman Coulter, Inc.,
Brea, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of PPARγ
expression
In accordance with the manufacturer's instructions
of the RNeasy Plus Mini kit, total RNA (1 µg) was isolated
from HEY cells. cDNA was produced by reverse transcription using
the Transcriptor First Strand cDNA Synthesis kit. The synthesized
cDNA was used as a template to estimate the quantity of gene
transcription by qPCR. The amplification conditions were as
follows: 94°C for 10 sec, 58°C for 30 sec, and 72°C for 10 sec for
35 cycles. The following primer sequences were used: PPARγ,
forward, 5′-GCGGAAGCCCTTTGGTGA-3′ and reverse
5′-TGCAGCAGGTTGTCTTGGATG-3′; GAPDH, forward,
5′-CCCCCAATGTATCCGTTGTG-3′ and reverse 5′-TGCAGCAGGTTGTCTTGGATG-3′.
The Ct was obtained using the Sequence Detection System software
(Applied Biosystems; Thermo Fisher Scientific, Waltham, MA,
USA).
Analysis of MMP-9 expression
A gelatin zymography assay was performed to analyze
the expression of MMP-9. Briefly, the medium of each well was
collected, combined with an equal volume of sodium dodecyl sulfate
(SDS) sample buffer and resolved in 10% polyacrylamide gels
containing 0.1% gelatin (Beyotime Institute of Biotechnology).
After electrophoresis, the gel was washed with 2.5% Triton X-100
(Beyotime Institute of Biotechnology) for 0.5–1 h, and incubated in
a reaction buffer at 37°C for 12 h. Following incubation, the gel
was stained with 0.05% Coomassie brilliant blue R-250 (Bio-Rad
Laboratories, Inc.). A gelatin zymography assay was performed to
analyze the expression of MMP-9 as previously described (20).
Silencing of PPARγ
PPARγ small interfering (si)RNA was obtained from
Sangon Biotech. The following primers were used: S1 forward primer:
5′-AGAUAAAGCUUCUGGAUUUdTdT-3′ and reverse primer:
5′-dTdTUCUAUUUCGAAGACCUAAA-3′; S2 forward primer:
5′-AGGAAAGACAACAGACAAAdTdT-3′ and reverse primer:
5′-dTdTUCCUUUCUGUUGUCUGUUU-3′; and S3 forward primer:
5′-GUACCAAAGUGCAAUCAAAdTdT-3′ and reverse primer:
5′-dTdTCAUGGUUUCACGUUAGUUU-3′. PPARγ siRNA (100 mmol/l) was
transfected into HEY cells using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific.
Statistical analysis
Data were analyzed using SPSS 13.0 (Chicago, IL,
USA). Values are expressed as the mean ± standard deviation of
independent experiments. Differences were analyzed using analysis
of variance or Student's paired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
MTT analysis of cell proliferation
To evaluate the effect of telmisartan on HEY cells,
cell proliferation was measured using an MTT assay. The cells were
treated with telmisartan (0, 1, 10 and 100 µM) for 0, 24, 48
and 72 h, which resulted in significantly inhibited growth of HEY
cells in a time- and dose-dependent manner (Fig. 2).
Flow cytometric analysis for detecting
cellular apoptosis
As telmisartan significantly inhibited the growth of
HEY cells, it was further explored whether telmisartan may have an
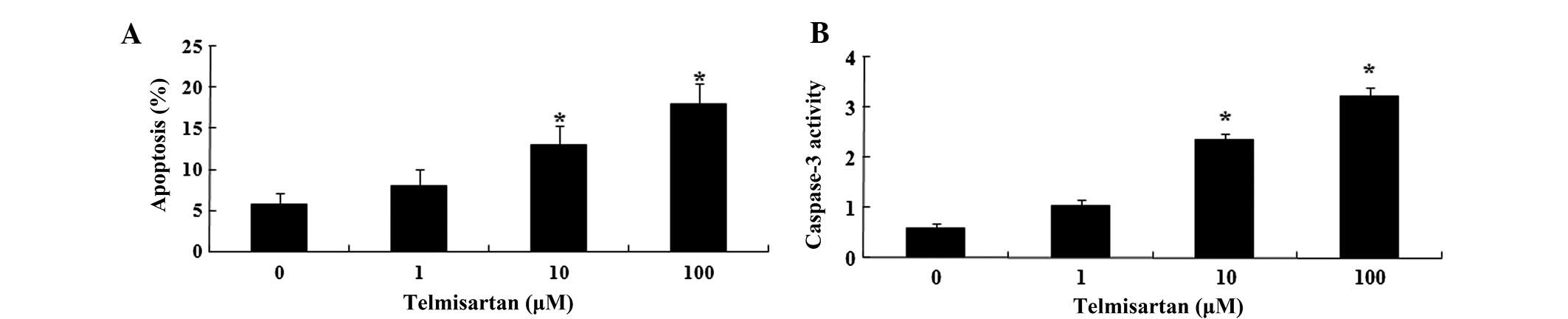
effect on apoptosis. Flow cytometric analysis showed that
telmisartan treatment for 48 h resulted in concentration-dependent
apoptosis of HEY cells (Fig. 3A).
Following treatment with telmisartan (10 and 100 µM) for 48
h, the level of apoptosis was significantly increased. In addition,
the activity of caspase-3 in HEY cells was significantly increased
(Fig. 3B).
Effect of telmisartan on MMP-9
expression
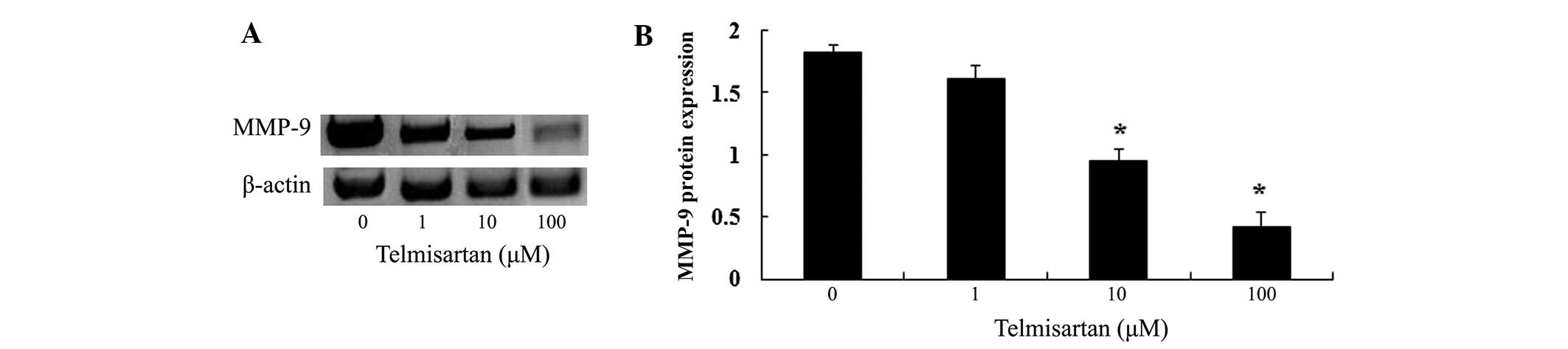
Following treatment (48 h) with telmisartan (0, 1,
10 and 100 µM), the expression of MMP-9 protein in HEY cells
was analyzed using gelatin zymography assays (Fig. 4A). Treatment with telmisartan at
concentrations of 10 and 100 µM significantly decreased the
expression of MMP-9 protein (Fig.
4B).
Telmisartan activates PPARγ
expression
Quantitative analysis of PPARγ expression in HEY
cells showed that treatment with telmisartan (10 and 100 µM)
for 48 h could significantly increase the expression of PPARγ
(Fig. 5).
PPARγ siRNA can reverse the effects of
telmisartan
PPARγ siRNA was transfected into HEY cells. The
results indicated that transfection of PPARγ siRNA significantly
reduced the expression of PPARγ in HEY cells (Fig. 6A). Notably, it was observed that
downregulating the expression of PPARγ could prevent the effects of
telmisartan on cell proliferation (Fig. 6B) and the activity of caspase-3
(Fig. 6C). It also increased the
expression of MMP-9 protein in HEY cells (Fig. 6D) at 48 h.
Discussion
At present, the mortality rate of ovarian cancer is
the highest of all gynecological malignant tumors. Its treatment
utilizes the combination of surgery and chemotherapy (21). In the chemotherapy of ovarian
cancer, platinum drugs, such as cisplatinum, are important, while
the emergence of drug resistance to cisplatinum in ovarian cancer
restricts the clinical efficacy (22). Thus, enhancing the sensitivity to
chemotherapeutic drugs is key in the treatment of ovarian cancer
and is a focus of research. Overall, in the present study it was
demonstrated that treatment with telmisartan significantly
inhibited the growth of HEY cells in a time- and dose-dependent
manner. In addition, telmisartan could also induce apoptosis of HEY
cells and decrease the activity of caspase-3 in HEY cells. In a
previous study, it was determined that telmisartan increased the
apoptosis of human renal cell carcinoma cells via downregulation of
Bcl-2 and involvement of caspase-3 (23). In addition, telmisartan has been
previously demonstrated to significantly inhibit growth of human
endometrial cancer cells (24).
Through the adjustment of the expression of the
relevant genes, PPARγ is important in regulating fat formation,
lipid metabolism, energy metabolism and the immune system. In
addition, it is associated with the generation and development of
numerous diseases, such as diabetes, obesity, metabolic syndrome
and hypertension (25). PPARγ is
predominantly expressed on the surface of granule cells of
follicles during the growth period in ovarian tissue, however,
there is only low expression in theca and luteal cells (26). PPARγ participates in the regulation
of normal physiology of the ovary, luteinizing hormone peak
reduction and the secretion of estrogen and progesterone, which can
affect the growth of follicles, ovulation and the quality of ovum
(21). In the present study,
treatment with telmisartan could significantly increase the
expression of PPARγ. In addition, a previous study has shown that
telmisartan activates PPARγ and does not affect osteoblast
differentiation or bone mass (27). Telmisartan can also protect the
nutrient deprivation-induced apoptosis of cerebellar granule cells
in vitro through activation of the PPARγ pathway (28).
Studies have also demonstrated that in the renal
cortex or fiber cells, PPARγ agonists can improve renal
tubulointerstitial fibrosis process by preventing cell growth and
adjusting the levels of MMP-9, TIMP-1 and TIMP-2 (29,30).
In early pregnancy chorionic villi, the expression of PPARγ is
negatively correlated with the levels of MMP-2 and MMP-9, which
suggests that PPARγ regulates the expression of MMP-2 and MMP-9,
affecting the invasion of trophoblast cells (31). The results of the present study
showed that treatment with telmisartan could significantly decrease
the expression of MMP-9 protein. Araújo et al (32) reported that telmisartan can reduce
COX-2, MMP-2, MMP-9 and RANKL/RANK in ligature-induced
periodontitis in rats. Telmisartan can weaken acute myocardial
infarction through downregulation of MMP-2 and MMP-9 (33). These results suggest that
downregulating the expression of PPARγ could restrain the effect of
telmisartan on anti-proliferative and apoptotic effects, and
up-regulation the expression of MMP-9 protein level in HEY cells.
Telmisartan is a novel and specific Ang II receptor antagonist with
long term efficiency, which not only effectively decreases blood
pressure, but also has anti-inflammatory, anti-thrombotic and other
non-antihypertensive effects (30,34).
Telmisartan has antihypertensive effects and can affect the
expression of PPARγ in muscle. Overall, in addition to inhibiting
cell growth by reducing proliferation and inducing apoptosis,
telmisartan may have therapeutic potential in ovarian cancer by
upregulating PPARγ and downregulating the MMP-9 expression
signaling pathway. Further studies are required to clarify
additional mechanisms underlying the effects of telmisartan on
ovarian cancer cells.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Des Jarlais G, Kaplan CP, Haas JS,
Gregorich SE, Pérez-Stable EJ and Kerlikowske K: Factors affecting
participation in a breast cancer risk reduction telephone survey
among women from four racial/ethnic groups. Prev Med. 41:720–727.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salehi F, Dunfield L, Phillips KP, Krewski
D and Vanderhyden BC: Risk factors for ovarian cancer: An overview
with emphasis on hormonal factors. J Toxicol Environ Health B Crit
Rev. 11:301–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vo C and Carney ME: Ovarian cancer
hormonal and environmental risk effect. Obstet Gynecol Clin North
Am. 34:687–700. viii2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie Y, Zhu J, Zhou XJ, Chen J, Lu NH and
Wang CW: Expression of peroxisome proliferators-activated
receptor-gamma and cyclooxygenase-2 in Helicobacter pylori
infection-associated diseases and significance thereof. Zhonghua Yi
Xue Za Zhi. 86:2683–2689. 2006.In Chinese.
|
|
6
|
DuBois RN, Gupta R, Brockman J, Reddy BS,
Krakow SL and Lazar MA: The nuclear eicosanoid receptor, PPARgamma,
is aberrantly expressed in colonic cancers. Carcinogenesis.
19:49–53. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang WG, Redfern A, Bryce RP and Mansel
RE: Peroxisome proliferator activated receptor-gamma (PPAR-gamma)
mediates the action of gamma linolenic acid in breast cancer cells.
Prostaglandins Leukot Essent Fatty Acids. 62:119–127. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wijnhoven BP, Lindstedt EW, Abbou M,
Ijzendoorn Y, de Krijger RR, Tilanus HW and Dinjens WN; Rotterdam
Esophageal Tumor Study Group: Molecular genetic analysis of the von
Hippel-Lindau and human peroxisome proliferator-activated receptor
gamma tumor-suppressor genes in adenocarcinomas of the
gastroesophageal junction. Int J Cancer. 94:891–895. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang TH and Szabo E: Induction of
differentiation and apoptosis by ligands of peroxisome
proliferator-activated receptor gamma in non-small cell lung
cancer. Cancer Res. 60:1129–1138. 2000.PubMed/NCBI
|
|
10
|
Papi A, De Carolis S, Bertoni S, Storci G,
Sceberras V, Santini D, Ceccarelli C, Taffurelli M, Orlandi M and
Bonafé M: PPARγ and RXR ligands disrupt the inflammatory cross-talk
in the hypoxic breast cancer stem cells niche. J Cell Physiol.
229:1595–1606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chatterjee A, Kusunoki H, Taniyama Y,
Rakugi H and Morishita R: Improvement of metabolic syndrome by
irbesartan via the PPARγ/HGF pathway in apolipoprotein E knockout
mice. Biomed Rep. 1:65–70. 2013.PubMed/NCBI
|
|
12
|
Łukaszewicz-Zając M, Mroczko B,
Guzińska-Ustymowicz K, Pryczynicz A, Gryko M, Kemona A, Kędra B and
Szmitkowski M: Matrix metalloproteinase 2 (MMP-2) and their tissue
inhibitor 2 (TIMP-2) in gastric cancer patients. Adv Med Sci.
58:235–243. 2013. View Article : Google Scholar
|
|
13
|
Waite LL, Louie RE and Taylor RN:
Circulating activators of peroxisome proliferator-activated
receptors are reduced in preeclamptic pregnancy. J Clin Endocrinol
Metab. 90:620–626. 2005. View Article : Google Scholar
|
|
14
|
Liu J, Lu H, Huang R, Lin D, Wu X, Lin Q,
Wu X, Zheng J, Pan X, Peng J, et al: Peroxisome proliferator
activated receptor-gamma ligands induced cell growth inhibition and
its influence on matrix metalloproteinase activity in human myeloid
leukemia cells. Cancer Chemother Pharmacol. 56:400–408. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balakumar P, Bishnoi HK and Mahadevan N:
Telmisartan in the management of diabetic nephropathy: A
contemporary view. Curr Diabetes Rev. 8:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takagi H, Mizuno Y, Yamamoto H, Goto SN
and Umemoto T; All-Literature Investigation of Cardiovascular
Evidence Group: Effects of telmisartan therapy on interleukin-6 and
tumor necrosis factor-alpha levels: A meta-analysis of randomized
controlled trials. Hypertens Res. 36:368–373. 2013. View Article : Google Scholar
|
|
17
|
Lacourcière Y: Telmisartan or valsartan
alone or in combination with hydrochlorothiazide: A review. Clin
Exp Hypertens. 35:50–60. 2013. View Article : Google Scholar
|
|
18
|
Maejima Y, Okada H, Haraguchi G, Onai Y,
Kosuge H, Suzuki J and Isobe M: Telmisartan, a unique ARB, improves
left ventricular remodeling of infarcted heart by activating PPAR
gamma. Lab Invest. 91:932–944. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Chen L, Yu P, Liu B, Zhu J and Yang
Y: Telmisartan exerts anti-tumor effects by activating peroxisome
proliferator-activated receptor-γ in human lung adenocarcinoma A549
cells. Molecules. 19:2862–2876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi M, Cao M, Song J, Liu Q, Li H, Meng F,
Pan Z, Bai J and Zheng J: PinX1 inhibits the invasion and
metastasis of human breast cancer via suppressing NF-κB/MMP-9
signaling pathway. Mol Cancer. 14:662015. View Article : Google Scholar
|
|
21
|
Mozzetti S, Ferlini C, Concolino P,
Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E,
Ranelletti FO, Ferrandina G and Scambia G: Class III beta-tubulin
overexpression is a prominent mechanism of paclitaxel resistance in
ovarian cancer patients. Clin Cancer Res. 11:298–305.
2005.PubMed/NCBI
|
|
22
|
Kamat AA, Kim TJ, Landen CN Jr, Lu C, Han
LY, Lin YG, Merritt WM, Thaker PH, Gershenson DM, Bischoff FZ, et
al: Metronomic chemotherapy enhances the efficacy of antivascular
therapy in ovarian cancer. Cancer Res. 67:281–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Araújo Júnior RF, Leitão Oliveira AL,
de Melo Silveira RF, de Oliveira Rocha HA, de França Cavalcanti P
and de Araújo AA: Telmisartan induces apoptosis and regulates Bcl-2
in human renal cancer cells. Exp Biol Med (Maywood). 240:34–44.
2015. View Article : Google Scholar
|
|
24
|
Koyama N, Nishida Y, Ishii T, Yoshida T,
Furukawa Y and Narahara H: Telmisartan induces growth inhibition,
DNA double-strand breaks and apoptosis in human endometrial cancer
cells. PLoS One. 9:e930502014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Auwerx J: PPARgamma, the ultimate thrifty
gene. Diabetologia. 42:1033–1049. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Komar CM: Peroxisome
proliferator-activated receptors (PPARs) and ovarian
function-implications for regulating steroidogenesis,
differentiation and tissue remodeling. Reprod Biol Endocrinol.
3:412005. View Article : Google Scholar
|
|
27
|
Kolli V, Stechschulte LA, Dowling AR,
Rahman S, Czernik PJ and Lecka-Czernik B: Partial agonist,
telmisartan, maintains PPARγ serine 112 phosphorylation, and does
not affect osteoblast differentiation and bone mass. PLoS One.
9:e963232014. View Article : Google Scholar
|
|
28
|
Pang T, Sun LX, Wang T, Jiang ZZ, Liao H
and Zhang LY: Telmisartan protects central neurons against nutrient
deprivation-induced apoptosis in vitro through activation of PPARγ
and the Akt/GSK-3 β pathway. Acta Pharmacol Sin. 35:727–737. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yiqin Y, Meilin X, Jie X and Keping Z:
Aspirin inhibits MMP-2 and MMP-9 expression and activity through
PPARalpha/gamma and TIMP-1-mediated mechanisms in cultured mouse
celiac macrophages. Inflammation. 32:233–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Funao K, Matsuyama M, Kawahito Y, Sano H,
Chargui J, Touraine JL, Nakatani T and Yoshimura R: Telmisartan as
a peroxisome proliferator-activated receptor-γ ligand is a new
target in the treatment of human renal cell carcinoma. Mol Med Rep.
2:193–198. 2009.PubMed/NCBI
|
|
31
|
Zafiriou S, Stanners SR, Saad S, Polhill
TS, Poronnik P and Pollock CA: Pioglitazone inhibits cell growth
and reduces matrix production in human kidney fibroblasts. J Am Soc
Nephrol. 16:638–645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Araújo AA, Souza TO, Moura LM, Brito GA,
Aragão KS, Araújo LS, Medeiros CA, Alves MS and Araújo RF Jr:
Effect of telmisartan on levels of IL-1, TNF-α, down-regulated
COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis
model. J Clin Periodontol. 40:1104–1111. 2013. View Article : Google Scholar
|
|
33
|
Yokota T, Osanai T, Hanada K, Kushibiki M,
Abe N, Oikawa K, Tomita H, Higuma T, Yokoyama J, Hanada H and
Okumura K: Effects of telmisartan on markers of ventricular
remodeling in patients with acute myocardial infarction: Comparison
with enalapril. Heart Vessels. 25:460–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu J, Lin H, Liu D, Liu J, Wang N, Mei X,
Sun J, Yang G and Zhang X: The protective effect of telmisartan in
Type 2 diabetes rat kidneys is related to the downregulation of
thioredoxin-interacting protein. J Endocrinol Invest. 36:453–459.
2013.
|