Introduction
High oxygen levels may cause bronchial pulmonary
hypoplasia, which has a prevalence of 1.26% in China (1). It has been suggested that alterations
in the expression levels of aquaporin-1 (AQP1) may be involved in
the rapid transportation of alveolar fluid in the early stages of
pulmonary injury, due to previous observations that the expression
and activity levels of AQP1 are reduced in multiple pulmonary
injuries (2–4).
The present study aimed to investigate whether high
levels of oxygen affect the expression levels of AQP1 in newborn
rat lung epithelial cells, which may be one of the reasons for high
oxygen-induced pulmonary edema (5). At present, there is no direct
experimental evidence to support the definite involvement of a
downregulation in AQP1 in lung edema in newborn rats exposed to
hyperoxia, due to the fact that sodium-potassium ATPase and fluid
transportation systems in addition to AQP are also present in lung
tissues (6). In the present study,
the AQP1 gene was silenced using an RNA interference (RNAi)
technique in human pulmonary adenocarcinoma A549 cells. This was
performed in order to verify the direct correlation between AQP1
and cell fluid transportation at a cellular level, in addition to
further elucidating its association with the pathogenesis of lung
edema during hyperoxic pulmonary injury.
Oxygen is crucial for human beings, but long-term
inhalation of a high concentration of oxygen can lead to lung
injury in premature babies with bronchial pulmonary hypoplasia
(BPD). The pathogenesis of BPD is not entirely clear, however, the
pathological process includes early pulmonary edema and late
pulmonary fibrosis.
Materials and methods
Cell culture
Human pulmonary adenocarcinoma A549 cells (Central
Laboratory, China Medical University Affiliated Shenjing Hospital,
Shenyang, China) were cultured in RPMI 1640 culture medium
(Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine
serum (FBS; Clark, Seabrook, MD, USA) at 37°C and 5% CO2
in a cell incubator. The cell medium was refreshed every 1–2 days.
Trypsin (0.25%; Merck Millipore, Darmstadt, Germany) was used to
digest and passage the cells for 5–10 min every 2–3 days.
Experimental group assignment and
intervention
The A549 cells were randomized into two groups: Air
group and hyperoxia group. Each group was further divided into a
gene silencing group and negative control group. The cells in the
air group were cultured in a normal cell incubator (oxygen volume
fraction=0.21) and the cells in the hyperoxia group were cultured
in a hyperoxic incubator (oxygen volume fraction=0.90) for 24, 48
and 72 h prior to detection.
Preparation of the AQP1 gene-silencing
cell model
To silence the gene expression of AQP1, AQP1-siRNA
transient transfection using ON-TARGETplus Smart Pool siRNA (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and DharmaFECT 1
(T-2001-01; Thermo Fisher Scientific, Inc.) transfection reagent
was performed. The potential sequences in the target mRNAs of AQP1,
beginning with an AA dinucleotide were identified and compared with
the human genome database using the Basic Local Alignment Search
Tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast.cgi). Any target
sequences with base pairs of homology to other coding sequences
were eliminated from consideration. In total, four pairs of
sequences were designed and synthesized (Sangon Biotech Co., Ltd.,
Shanghai, China), as follows (5′-3′): siRNA1,
5′-GAACUCACUUGGCCGAAAU-3′; siRNA2, 5′-CCGUUAACCAUGUCGUGAA-3′;
siRNA3, 5′-CCCAAAUAGAGGAGGCUUG-3′; and siRNA4,
5′-UGACGUGUGUGUUUAUUAA-3′. Negative siRNA sequences were used as
negative controls. For knockdown of the expression of AQP1, the
A549 cells were plated at a concentration of 4×105
cells/well in 6-well plates and incubated in antibiotic-free media
overnight. A total of 100 µl siRNA (2 µmol/l) and 2
µl DharmaFECT were diluted with 100 µl and 198
µl serum-free medium, respectively, and set aside for 5 min
at room temperature. The two dilutions were mixed and left for 20
min at room temperature, prior to the addition of 1,600 µl
antibiotic-free and serum-free complete media, to produce a total
volume of 2 ml transfection medium. The cells were overlaid with
the transfection medium and were incubated for 6 h at 37°C. The
cells were then washed with phosphate-buffered saline (PBS) and
cultivated in RPMI 1640 medium containing 10% FBS. At 48 and 72 h
post-transfection, the cells were harvested and stored at −80°C for
further analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The A549 cells were harvested by centrifugation at
111.8 × g at 4°C for 5 min, and were randomized into a gene
silencing and negative control group (1×109
cells/group), and were cultured in a cell incubator (oxygen volume
fraction=0.21) for 48 h. Briefly, total RNA was extracted from the
harvested cells using an acid guanidinium-phenol-chloroform method
(TRIzol®; Invitrogen; Thermo Fisher Scientific, Inc.).
The total RNA was reverse transcribed into cDNA in a 20 µl
reaction containing 8 µl RNA, 2 µl oligo(dT)15
(Tiangen Biotech Co., Ltd., Beijing, China), 2 µl dNTP (2.5
mM; Tiangen Biotech Co., Ltd.), 1.5 µl RNase-free
ddH2O, 4 µl 5X First-Strand Buffer (Tiangen
Biotech Co., Ltd.), 1 µl (0.1 M) DTT (Tiangen Biotech Co.,
Ltd.), 0.5 µl RNasin (Tiangen Biotech Co., Ltd.) and 1
µl (200 M) TIANScript M-MLV (Tiangen Biotech Co., Ltd.). The
mixture was incubated for 50 min at 42°C and the reaction was
terminated by heating to 95°C for 5 min. The cDNA synthesized by
reverse transcription was used as templates for quantitative
fluorescent analysis using a fluorescent quantitator (Exicycler™
96; Bioneer Corporation, Daejeon, Korea). An equal volume of cDNA
was amplified in a reaction mixture consisting of 10 µl Taq
2X PCR Master Mix (Tiangen Biotech Co., Ltd.), 7.5 µl
ddH2O, 9 µl SYBR Green (Tiangen Biotech Co.,
Ltd.), 1.5 µl cDNA and 1 µl gene-specific forward and
reverse AQP1 primers. The primer sequences (Sangon Biotech Co.,
Ltd.) were as follows: Forward, 5′-ACCCGCAACTTCTCAAAC-3′ and
reverse, 5′-CAGGTCATACTCCTCCACTT-3′. The initial denaturation was
at 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec, 55°C
for 30 sec and 68°C for 40 sec. Melt curves were used to determine
the formation of a single product. The quantification cycle (Cq)
was recorded for each sample to reflect the mRNA expression levels.
The Cq values and data were analyzed using the 2−∆∆Cq
method (7). The hGAPDH gene
(forward, GAAGGTCGGAGTCAACGGAT, and reverse, CCTGGAAGATGGTGATGGGAT)
was used as an internal control. All experiments were repeated
three times.
Western blot analysis
Total protein was extracted from the cells using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China), and were quantified using a
standard curve. The cells were randomized into a gene silencing and
a negative control group and were cultured in a cell incubator
(oxygen volume fraction=0.21) for 72 h. Proteins were extracted
from the harvested cells for quantitation. The proteins (9
µg) were loaded onto a 5% SDS-PAGE gel (Beyotime Institute
of Biotechnology) for electrophoresis and transferred onto
cellulose membranes (Merck Millipore) prior to blocking in buffer
containing 5% non-fat milk powder for 2 h. The membrane was
incubated with mouse anti-human monoclonal AQP1 primary antibody
(cat. no. sc25287; 1:400; Santa Cruz Biotechnology, Inc., Danvers,
MA, USA) at 37°C for 1 h, and washed with Tris-buffered saline with
Tween 20 prior to hybridization with alkaline phosphatase-labeled
goat anti-mouse monoclonal secondary antibody (cat. no. A0216;
1:10,000; Beyotime Institute of Biotechnology) at 37°C for 40 min.
Subsequently, images of the film (WD-9413B; Beijng Liuyi
Biotechnology Co., Ltd., Beijing, China) were captured using an
imaging system (WD-9413B; Beijing Six One Company) following
electrochemiluminescence (Merck Millipore) exposure, and analyzed
using Exicycler 96 (Bioneer Corporation). The results were
presented as the relative grey value of the AQP1 band/grey value of
the GAPDH band.
Determination of cell volume following
AQP1-silencing
The cells in each group were cultured for 24, 48 and
72 h prior to washing with PBS and digesting with 0.25% trypsin for
5–10 min. Equal volumes of Dulbecco's modified Eagle's medium
containing 10% FBS were used to terminate digestion. Subsequently,
the cells were centrifuged at 75.55 × g for 5 min at 4°C and washed
twice with PBS. The resuspended cells were agitated to form a
homogenous single cell suspension and were adjusted to
109 cells/l. Subsequently, 10,000 cells were obtained
from 0.5 ml cell suspension and analyzed using flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, CA, USA), and the
average intensity of forward scatter (FSC) was calculated (8). Light scattering was generated when a
single line of cells ejected from the nozzle of the flow cytometer
was exposed to laser irradiation. The channel numbers of the
average intensity of FSC were used to represent cell volume, as
cell volume increased with the intensity of scattering light, or
FSC intensity was proportional to cell volume.
Determination of cell osmotic fluid
permeability (Pf) following gene silencing
The osmotic Pf of the cell represents the water
trans-membrane transport capability driven by an osmotic gradient,
and can directly reflect cell osmotic water transport function
(9).
The Pf values were calculated using the following
formula: Pf = [V0xd (V/V0)/dt] /
[SxVWx(Osmin−Osmout)]; V = 4/3 ×
(area) × (area/π)½.
The area denotes the cell surface area, Osm denotes
osmotic pressure, Osmin denotes intracellular osmotic
pressure, Osmout denotes extracellular osmotic pressure.
The Pf value was determined by the initial V/V0 time
course ratio, d (V/V0)/dt; initial cell volume,
V0 (cm3); initial cell surface area, S
(cm2); and water molar volume, VW (18
mol/cm3).
The cell group assignment and harvesting methods
were, as described above. The cells were transferred between PBS of
200 mOsm and 70 mOsm (diluted with deionized water) prior to
microscopic image capture (IX53; Olympus Corporation, Tokyo, Japan)
every 30 sec for 3 min. The cell area was calculated using
Image-Pro 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA) for imaging analysis. The cell volume and Pf values were
calculated using the above formula.
Statistical analysis
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. The independent t-test
was used for comparisons of means between two samples. P<0.05
was considered to indicate a statistically significant
difference.
Results
mRNA expression of AQP1 is decreased in
A549 cells following gene silencing
The RT-qPCR analysis performed in the present study
revealed a significant downregulation in the mRNA expression of
AQP1 in the gene silencing group (P<0.01), when compared with
that of the negative control group. The AQP1 mRNA inhibition rate
was 75.5% (Fig. 1).
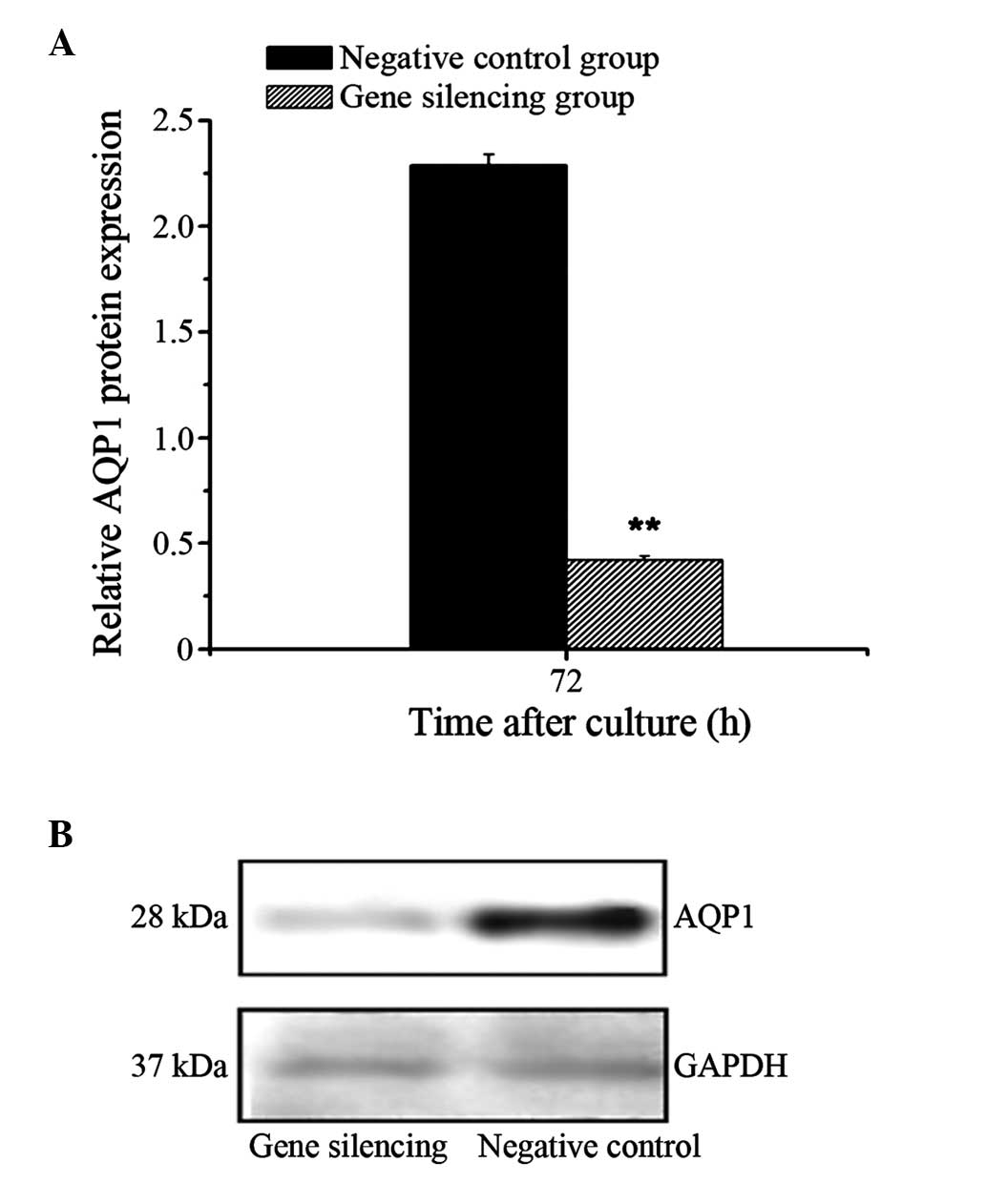
Protein expression of AQP1 decreases
following A549 cell gene silencing
The results of the western blotting revealed a
significant downregulation in the protein expression of AQP1 in the
gene silencing group (P<0.01), compared with that of the
negative control group. The inhibition rate of AQP1 protein was
81.5% (Fig. 2).
Changes in A549 cell volume following
gene silencing
No significant difference in cell volume was
observed at 24 or 48 h between the gene silencing group and
negative control group when exposed to air (P>0.05), whereas the
cell volume in the gene silencing group was significantly reduced,
compared with that in the negative control group at 72 h
(P<0.01; Fig. 3).
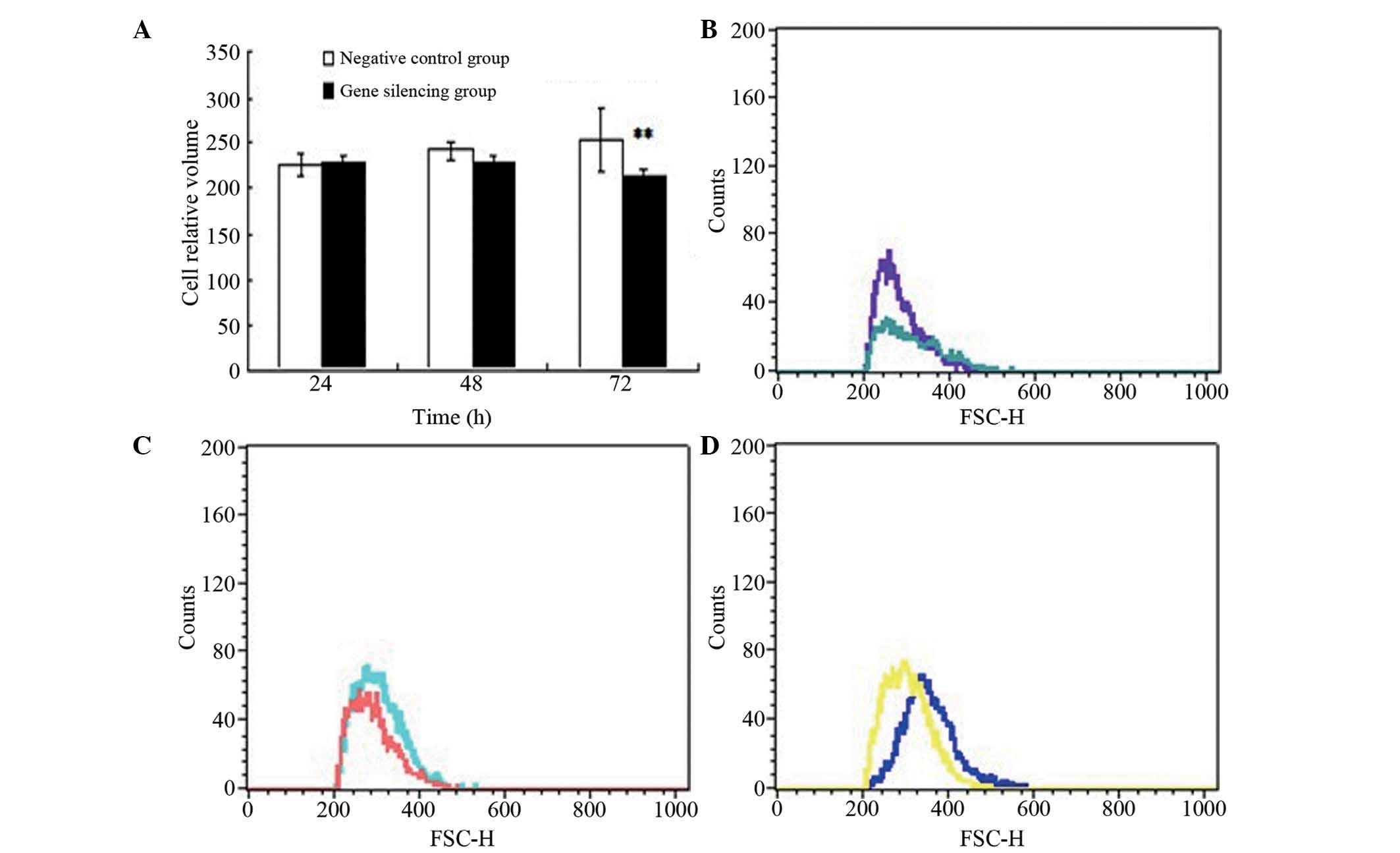 | Figure 3Changes in cell volume. (A) Cell
volume of the two groups following gene silencing. Data are
presented as means ± standard deviation. **P<0.01,
vs. negative control group. Determination cell volumes in the AQP1
gene-silencing group exposed to air were determined using flow
cytometry at (B) 24 h (purple, silencing group; blue, negative
control group), (C) 48 h (pink, silencing group; blue, negative
control group) and (D) 72 h (yellow, silencing group; blue,
negative control group) following transfection. AQP1, aquaporin-1;
FSC-H, forward scatter height. |
Cell volume in the gene silencing group exposed to
hyperoxia was significantly reduced, compared with the negative
control group at 48 and 72 h (P<0.01), although no significant
differences in cell volume were observed at 24 h (P>0.05;
Fig. 4).
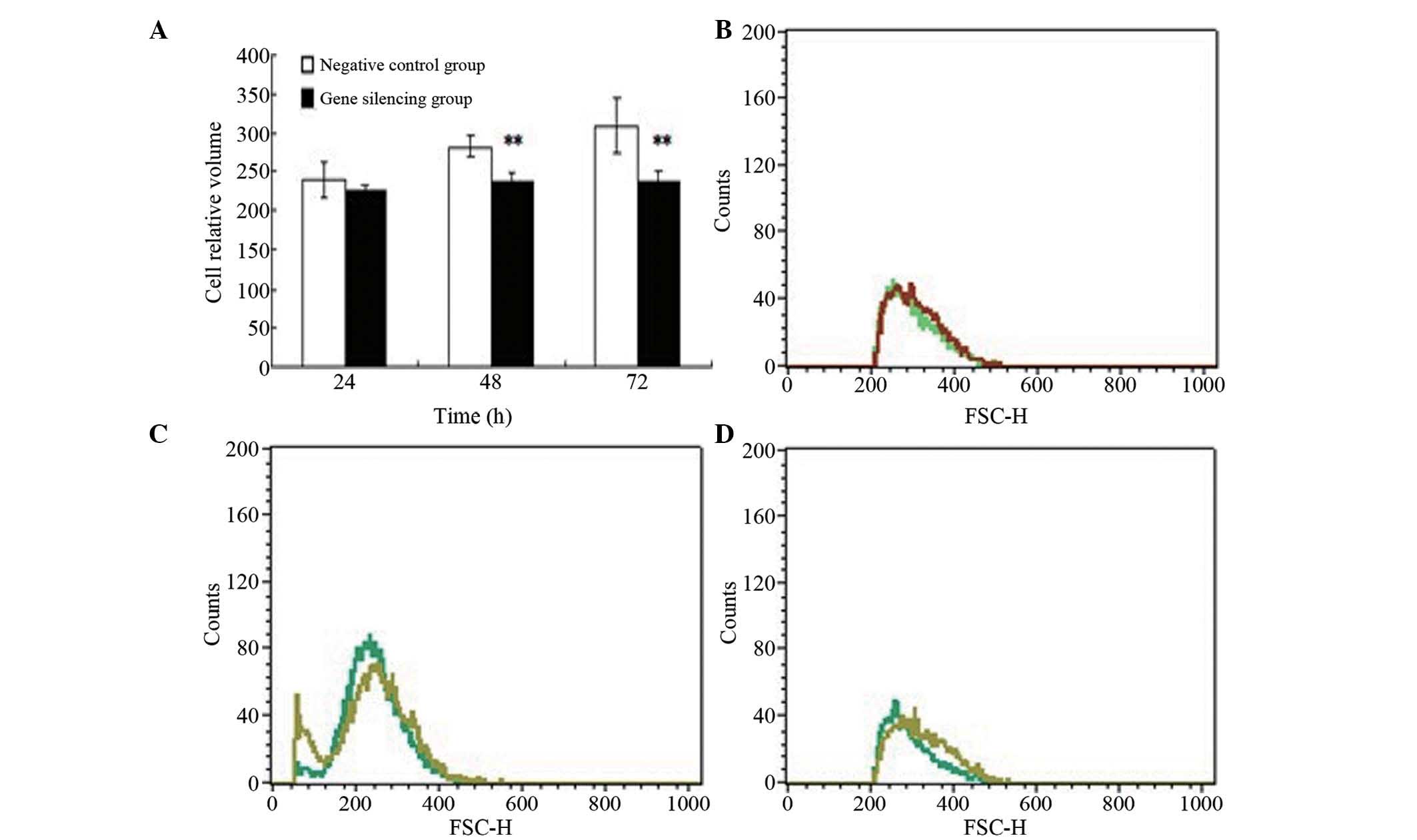 | Figure 4Changes in cell volume. (A) Cell
volumes in the two hyperoxic groups following gene silencing. Data
are presented as means ± standard deviation.
**P<0.01, vs. negative control group. Determination
of cell volumes in the AQP1 gene silencing group exposed to
hyperoxia were determined using flow cytometry (B) 24 h (green,
gene silencing group; red, negative control group), (C) 48 h (dark
green, gene silencing group; light green, negative control group)
and (D) 72 h (dark green, gene silencing group; light green,
negative control group) following transfection. AQP1, aquaporin-1;
FSC-H, forward scatter height. |
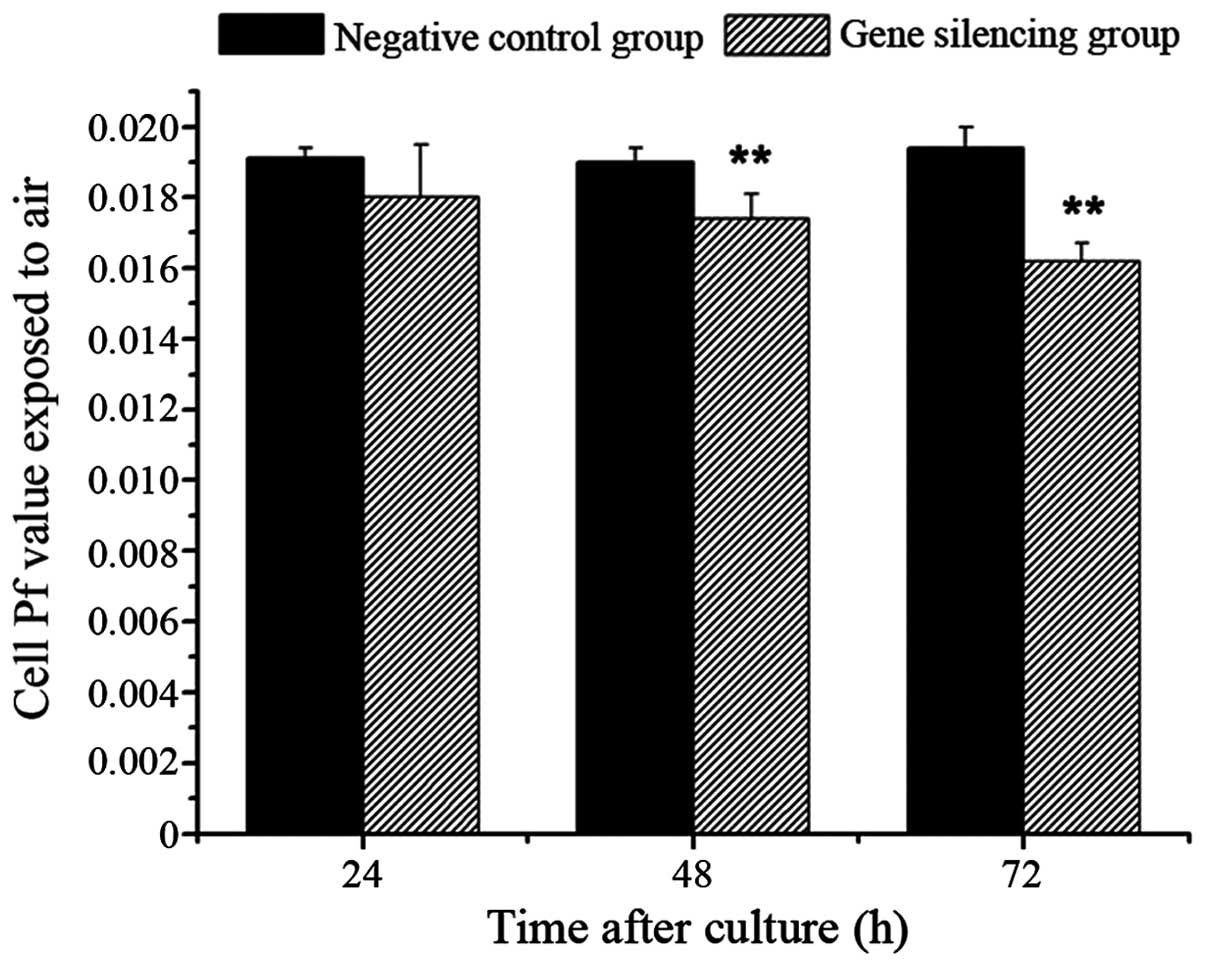
Osmotic Pf following gene silencing
No significant differences were observed in the Pf
values between the gene silencing group and negative control group
exposed to air at 24 h (P>0.05). However, the Pf values of the
gene silencing group were significantly reduced, compared with
those of the negative control group at 48 and 72 h (P<0.01,
Fig. 5).
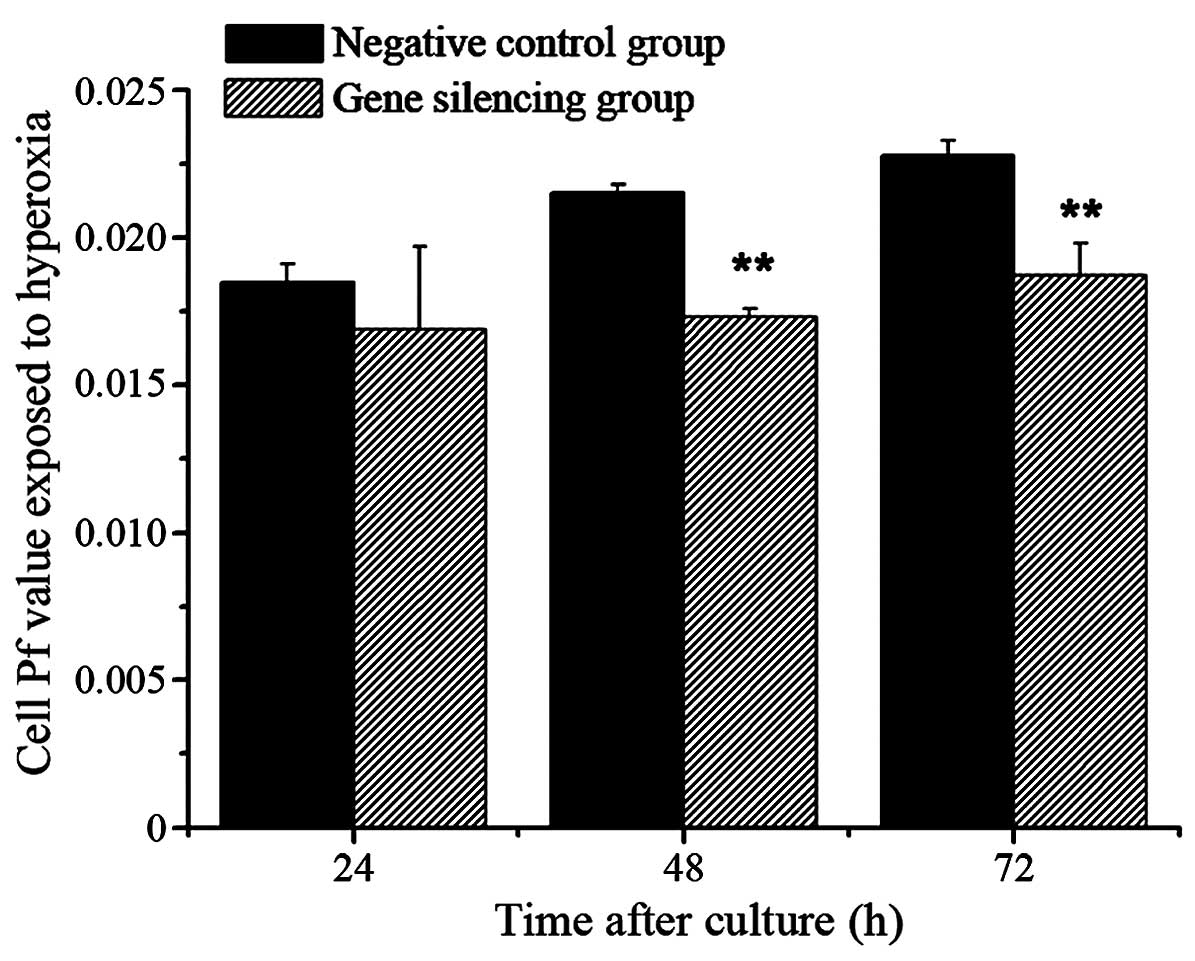
No significant difference were observed in the Pf
values between the gene silencing group and negative control group
exposed to hyperoxia at 24 h (P>0.05), however, the Pf values of
the gene silencing group were reduced, compared with those of the
negative control group at 48 and 72 h (P<0.01; Fig. 6).
Discussion
The AQP family is a group of small, hydrophobic
trans-membrane proteins, of which the monomers are 26–34 kDa in
size. AQPs are selectively permeable to water at a velocity of
100–200×10−6 m.s−1, which is
109-fold higher than normal diffusion velocity (10). At present, 13 AQPs (AQP0-12) have
been identified, which are observed in all life forms, including
humans, animals, plants, bacteria and yeasts (11). Currently, the expression of six
AQPs, including AQP1, AQP3, AQP4, AQP5, AQP8 and AQP9, have been
identified in lung tissues (12).
AQPs are important in maintaining the water
equilibrium between alveoli and capillaries. AQP1 is expressed in
lung endothelial and epithelial cells and regulates water
transport, being important in a number of edema-associated lung
diseases (13,14). Lung injury resulting from multiple
factors is accompanied by reductions in the expression and
activities of AQP1. Towne et al (14) demonstrated that adenovirus
infection resulted in pneumonia, pulmonary edema and downregulation
of AQP1 in lung tissues. It has also been reported that the
trans-membrane fluid transportation capacity mediated by AQP in
lung tissues is reduced by 43% in mice following exposure to 100%
oxygen for 5 days (15), with
reductions in the expression or activity of AQP1 observed. The mRNA
expression levels of AQP1 and AQP4 have been found to be
downregulated in allergen-induced mouse models of asthma (16). In addition, reduced expression
levels of lung AQP1 and AQP5 may be associated with the development
of pulmonary edema and increased severity of lung injury and
pulmonary edema, which provides an additional mechanism for
pancreatitis-associated lung injury (17). It has also been observed that
impaired alveolar fluid clearance rate is the predominant
manifestation in lipopolysaccharide-induced acute lung injury, and
symptoms can be relieved through dopamine-induced upregulation in
the expression levels of AQP1 and AQP5, and subsequent enhancement
of alveolar fluid reabsorption (18). Qi et al (2) reported that limb
ischemia-reperfusion-induced lung injury was accompanied by
upregulation of the toll-like receptor 4-myeloid differentiation
primary response protein 88-nuclear factor κB pathway and
downregulation of the expression of AQP1/AQP5. Ventilation with a
large tidal volume results in the production of inflammatory
mediators and the downregulation of AQP1, which can be attenuated
by the inhibition of cyclooxygenase-2 (19). Gao et al (20) observed that the Chinese medicine,
Qing Yi Tang, protects the lungs from injury induced by severe
acute pancreatitis via the upregulation of AQP1, which suppresses
the expression of tumor necrosis factor α.
Experiments have revealed that, compared with
wild-type mice, water permeability between the alveoli and the
capillaries in AQP1-knockout mice was reduced 10-fold (21). King et al (22) reported that the airway wall was
unchanged following saline perfusion in the congenital absence of
AQP1, whereas the wall was thickened by 50% in normal airways.
However, upregulation in the expression of AQP1 in
lung injury has been observed in previous studies. In a study by Li
et al (23), results from
RT-qPCR and western blotting indicated that intratracheal
installation of seawater upregulated the mRNA and protein levels of
AQP1 and AQP5 in lung tissues. Lai et al (24) observed that inflammatory factors
were able to stimulate the expression of AQP1 in cell models, and
Song et al (25) used
transgenic technology and observed that AQP1-, AQP4- and
AQP5-knockout in mice had no effects on pulmonary edema or pleural
effusion formation, or on the clearance of lung fluid in acute lung
injury of neonatal mice. The underlying pathology of early phase
pulmonary edema in hyperoxic lung injury had been confirmed
(26). The present study
hypothesized that hyperoxia results in the downregulation of AQP1
on the type II alveolar epithelial cell (AEC-II) membrane,
affecting alveolar water transport and clearance. In order to
confirm this, an RNAi technique was used to determine the roles of
AQP1 in extracellular water transport by silencing the gene
expression of AQP1, and to assist in elucidating the mechanism of
impaired alveolar fluid clearance in lung edema. As A549 cells and
AEC-II share similar structure and function (27), in the present study A549 cells were
used as they multiply more rapidly and are easy to obtain, rather
than pulmonary epithelial cells.
RNAi refers to the phenomenon of specific
degradation of homologous mRNA, resulting from exogenous or
endogenous small double-stranded RNA and subsequent
post-transcriptional gene silencing in an organism (28). RNAi as a novel gene disruption
technique, which has become an important tool in investigating gene
function due to its advantages, including high specificity, high
efficacy, high stability and low toxicity (29).
In the present study, the extracellular to
intracellular fluid transportation capacity was attenuated
following AQP1-silencing, as the cell volume was reduced in the
transfection group exposed to air and hyperoxia, compared with that
in the negative control group at 72 h. Reduced osmotic water
permeability and impaired water transport function following AQP1
gene silencing were also observed. In addition, the Pf values were
reduced in the gene silencing group, compared with the negative
control group at 48 and 72 h, in exposure to air or hyperoxia.
These results indicated that AQP1 is important in fluid transport
in A549 cells. Combined with the findings of previous studies,
these changes may be correlated with the downregulation of AQP1
induced by hyperoxia, and weakened fluid transportation function
(5,18). Therefore, the downregulation of
AQP1 induced by hyperoxia in A549 cells impaired the fluid
transportation function of the cell membrane and reduced alveolar
fluid clearance, which may be one of the important causal factors
responsible for lung edema during the acute phase of hyperoxic lung
injury.
Acknowledgments
The authors would like to thank the Experimental
Center of China Medical University Affiliated Shengjing Hospital
for the guidance and assistance provided. The present study was
supported by the National Natural Science Foundation of China
(grant no. 30872781) and the Scientific Research Fund of the First
Affiliated Hospital of Harbin Medical University (grant no.
2013B18).
References
|
1
|
Chang LW: Part of China's urban premature
incidence of bronchial pulmonary dysplasia and risk factors. In:
The 9th national neonatal meeting; 2009
|
|
2
|
Qi QY, Chen W, Li XL, Wang YW and Xie XH:
H2S protecting against lung injury following limb
ischemia-reperfusion by alleviating inflammation and water
transport abnormality in rats. Biomed Environ Sci. 27:410–418.
2014.PubMed/NCBI
|
|
3
|
Elias AS, Oliveira GP, Ornellas DS,
Morales MM, Capelozzi VL, Haddad R, Pelosi P, Rocco PR and Garcia
CS: Effects of early and late pneumothorax drainage on the
development of pulmonary oedema. Respir Physiol Neurobiol.
195:27–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Q, Ishikawa T, Michiue T, Zhu BL,
Guan DW and Maeda H: Molecular pathology of pulmonary edema after
injury in forensic autopsy cases. Int J Legal Med. 126:875–882.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang QY, Fu JH and Xue XD: Expression and
function of aquaporin-1 in hyperoxia-exposed alveolar epithelial
type II cells. Exp Ther Med. 8:493–498. 2014.PubMed/NCBI
|
|
6
|
Lecuona E, Trejo HE and Sznajder JI:
Regulation of Na, K-ATPase during acute lung injury. J Bioenerg
Biomembr. 39:391–395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winer J, Jung CK, Shackel I and Williams
PM: Development and validation of real-time quantitative reverse
transcriptase- polymerase chain reaction for monitoring gene
expression in cardiac myocytes in vitro. Anal Biochem. 270:41–49.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jordan CT, Yamasaki G and Minamoto D:
High-resolution cell cycle analysis of defined phenotypic subsets
within primitive human hematopoietic cell populations. Exp Hematol.
24:1347–1355. 1996.PubMed/NCBI
|
|
9
|
Preston GM, Carroll TP, Guggino WB and
Agre P: Appearance of water channels in Xenopus oocytes expressing
red cell CHIP28 protein. Science. 256:385–387. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verkman AS: Physiological importance of
aquaporin water channels. Ann Med. 34:192–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itoh T, Rai T, Kuwahara M, Ko SB, Uchida
S, Sasaki S and Ishibashi K: Identification of a novel aquaporin,
AQP12, expressed in pancreatic acinar cells. Biochem Biophys Res
Commun. 330:832–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borok Z and Verkman AS: Lung edema
clearance: 20 years of progress: Invited review: Role of aquaporin
water channls in fluid transport in lung and airways. J Appl
Physiol (1985). 93:2199–2206. 2002. View Article : Google Scholar
|
|
13
|
Jiao GY, Li ER and Yu R: Decreased
expression of AQP1 and AQP5 in acute injured lungs in rats. Chin
Med J (Engl). 155:963–967. 2002.
|
|
14
|
Towne JE, Harrod KS, Krane CM and Menon
AG: Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung
after acute viral infection. Am J Respir Cell Mol Biol. 22:34–44.
2000. View Article : Google Scholar
|
|
15
|
Matthay MA, Folkesson HG and Clerici C:
Lung epithelial fluid transport and the resolution of pulmonary
edema. Physiol Rev. 82:569–600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krane CM, Deng B, Mutyam V, McDonald CA,
Pazdziorko S, Mason L, Goldman S, Kasaian M, Chaudhary D, Williams
C and Ho MW: Altered regulation of aquaporin gene expression in
allergen and IL-13-induced mouse models of asthma. Cytokine.
46:111–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Huang H, Lu F and Chen Y: Acute
lung injury and change in expression of aquaporins 1 and 5 in a rat
model of acute pancreatitis. Hepatogastroenterology. 57:1553–1562.
2010.
|
|
18
|
Wu XM, Wang HY, Li GF, Zang B and Chen WM:
Dobutamine enhances alveolar fluid clearance in a rat model of
acute lung injury. Lung. 187:225–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin LD, Wang LR, Wu LQ, Shan YL, Zhao XY,
Xiong XQ, Zhou JH, Lin LN and Jin LL: Effects of COX-2 inhibitor on
ventilator-induced lung injury in rats. Int Immunopharmacol.
16:288–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Z, Xu J, Sun D, Zhang R, Liang R, Wang
L and Fan R: Traditional Chinese medicine, Qing Ying Tang,
ameliorates the severity of acute lung injury induced by severe
acute pancreatitis in rats via the upregulation of aquaporin-1. Exp
Ther Med. 8:1819–1824. 2014.PubMed/NCBI
|
|
21
|
Song Y, Ma T, Mathay MA and Verkman AS:
Role of aquaporin-4 in airspace-to-capillary water permeability in
intact mouse lung measured by a novel gravimetric method. J Gen
Physol. 155:17–27. 2000.
|
|
22
|
King LS, Nielsen S, Agre P and Brown RH:
Decreased pulmonary vascular permeability in aquaporin-1-null
humans. Proc Natl Acad Sci USA. 99:1059–1063. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Xu M, Fan Q, Xie X, Zhang Y, Mu D,
Zhao P, Zhang B, Cao F, Wang Y, et al: Tanshinone IIA ameliorates
seawater exposure-induced lung injury by inhibiting aquaporins
(AQP) 1 and AQP5 expression in lung. Respir Physiol Neurobiol.
176:39–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai KN, Leung JC, Metz CN, Lai FM, Bucala
R and Lan HY: Role for macrophage migration inhibitory factor in
acute respiratory distress syndrome. J Pathol. 199:496–508. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song Y, Fukuda N, Bai C, Ma T, Matthay MA
and Verkman AS: Role of aquaporins in alveolar fluid clearance in
neonatal and adult lung and in oedema formation following acute
lung injury: Studies in transgenic aquaporin null mice. J Physiol.
525:771–779. 2000. View Article : Google Scholar
|
|
26
|
Modi N: Clinical implications of postnatal
alterations in body water distribution. Semin Neonatol. 8:301–306.
2003. View Article : Google Scholar
|
|
27
|
Chen F, Zhang C, Jia X, Wang S, Wang J,
Chen Y, Zhao J, Tian S, Han X and Han L: Transcriptome profiles of
human lung epithelial cells A549 interacting with aspergillus
fumigatus by RNA-Seq. PLoS One. 10:e01357202015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and spectific genetic interference
by double-stranded RNA in Caenorhabditis elegans. Nature.
391:806–811. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aagaard L and Rossi JJ: RNAi therapeutics:
Principles, prospects and chanllenges. Adv Drug Deliv Rev.
59:75–86. 2007. View Article : Google Scholar : PubMed/NCBI
|