Introduction
As the most common malignant tumor type of the
urinary tract, bladder cancer remains one of the major causes of
cancer-associated mortality worldwide (1). Due to its high recurrence rate and
requirement of costly lifelong follow-ups, the development of novel
and effective therapies for bladder cancer is necessary, for which
the elucidation of the underlying mechanisms of oncogenic
urothelial cell transformation is vital.
Mammalian target of rapamycin (mTOR) has a central
role in regulating cellular catabolism and anabolism and is
important in tumorigenesis and cancer progression (2). mTOR kinase exists as mTOR complex
(mTORC)1 and 2, two distinctive cellular protein complexes, which
each have a unique molecular composition, substrates and mechanisms
of activation (3). mTORC1
regulates autonomous cell growth in dependent on the availability
of growth factors and nutrients, whereas mTORC2 regulates cell
survival and proliferation (4).
Upon interaction of raptor with mTOR, mTORC1 is formed, which
represents the sensitive target of rapamycin that phosphorylates
downstream targets of eukaryotic initiation factor 4E binding
protein-1 (4E-BP1) and S6 kinase 1 (S6K1). The mTORC1 signaling
pathway has a central role in regulating cell functions, including
proliferation, growth, survival and mobility (5–7).
Aberrant expression of mTORC1 has been observed in numerous types
of cancer, including colorectal cancer (8), hepatocellular carcinoma (9), renal cell carcinoma (10), breast cancer (11), acute myeloid leukemia (12) and non-small cell lung cancer
(13). Although mTOR research
largely focuses on mTORC1, mTORC2 is emerging as a crucial
signaling complex in numerous cancer types. It has been reported
that mTORC2 directly phosphorylates AKT on serine (ser)473, which
leads to the activation of this anti-apoptotic kinase and
ultimately results in increased cell survival, proliferation and
migration (14).
The first-generation mTOR inhibitor rapamycin
partially suppresses mTORC1 activity and reduces the proliferation
of cancer cells (15); however,
rapamycin is not sufficiently potent for the effective treatment of
cancer. For this reason, efforts have been made to develop
selective small-molecule competitors of adenosine triphosphate
(ATP) as mTOR inhibitors with the ability to completely block
mTORC1 effectors in addition to mTORC2 substrates (16). The present study examined the
anti-proliferative and anti-migratory effects of PP242, an
ATP-competitive inhibitor that binds the mTOR catalytic site, in
bladder cancer cells. It was indicated that PP242 reduced the
viability and proliferation of bladder cancer cells and inhibited
their migratory potential. In addition, PP242 reduced the
mTORC2/AKT1 activity, however did not effect mTORC1/S6K1 activity
in bladder cancer cells.
Materials and methods
Cell lines and reagents
The SV-HUC-1, RT4, BIU-87, 5637 and T24 bladder
cancer cell lines were purchased from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). All cell lines were
maintained in a humidified atmosphere with 5% CO2 at
37°C. The cell lines were grown in RPMI-1640 (GE Healthcare Life
Sciences, Logan, UT, USA) media containing fetal bovine serum (GE
Healthcare Life Sciences) according to the manufacturer's
instructions.
PP242 was purchased from Selleck Chemicals Company
(Houston, TX, USA). For in-vitro experiments, PP242 was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA).
The rabbit polyclonal mTOR (ab2732), rabbit
monoclonal raptor (ab40768) and rabbit polyclonal rictor (ab70374)
antibodies were purchased from Abcam (Cambridge, MA, USA). Rabbit
polyclonal phosphorylated (p)-mTOR (Ser2448) (#2971), rabbit
polyclonal p-mTOR (Ser2481) (#2974), rabbit monoclonal p-S6K1
(Thr389) (#9234), rabbit monoclonal p-AKT1 (Ser473) (#4058) and
rabbit monoclonal GAPDH (#2118) antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Mouse monoclonal
AKT1 (sc-5298) and rabbit polyclonal S6K1 (sc-230) antibodies were
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell growth and proliferation assays
The Cell Counting Kit-8 assay (CCK-8, KeyGen Biotech
Co., Nanjing, China) was used to assess effects of PP242 on bladder
cancer cell growth according to the manufacturer's instructions.
Cells were incubated with PP242 at 100, 200, 500 or 1,000 nM or
with DMSO (<0.5%) alone for 48 h. The absorbance was measured at
a wavelength of 450 nm using a plate reader (model 680; Bio-Rad
Laboratories, Hercules, CA, USA). In addition, the
5-ethynyl-2′-deoxyuridine (EdU) incorporation assay with the
Cell-Light™ EdU DNA Cell Proliferation kit (Ruibo Biotech,
Guangzhou, China) was used to assess the effects of PP242 at 500 nm
for 48 h on the proliferation of bladder cancer cells according to
the manufacturer's instructions. All cells were treated with 50
µmol/l of EdU for 24 h at 37°C. Following being fixed with
4% paraformaldehyde for 15 min, the cells were treated with 0.5%
Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 20 min and
rinsed with PBS three times. Thereafter, the cells were exposed to
100 µl of 1X Apollo® reaction cocktail for 30 min
and incubated with 5 µg/ml of Hoechst 33342 (Ruibo Biotech,
Guangzhou, China) to stain the cell nuclei for 30 min. EdU
incorporation of the cells was observed under an Olympus IX-71
inverted fluorescence microscope (Olympus, Tokyo, Japan) and images
were captured.
Wound healing migration assay
Cells were incubated in the presence or absence of
PP242 (500 nM) or with DMSO alone for 48 h. Following serum
starvation of confluent bladder cancer cells for 2 h in 24-well
plates, the media were replaced with RPMI-1640 medium without
supplementation and line-shaped wounds were generated across the
cell monolayers using a sterile pipette tip. At 0 and 24 h, images
of the wounded cell layers were captured using an Olympus IX-71
inverted fluorescence microscope (Olympus). ImageJ software
(National Institutes of Health, Bethesda, MD, USA) was used to
quantify the amount of wound closure, which resembled the migratory
potential of the cells.
Transwell assay
The lower chambers of a Transwell plate (with 8.0
µm-pore polycarbonate membranes; Corning Life Science,
Corning, NY, USA) were filled with 0.6 ml RPMI-1640 medium
containing 5% FBS. Cells were incubated in the presence or absence
of PP242 (500 nM) or with DMSO alone for 48 h. Cells were
re-suspended in RPMI-1640 containing 1% FBS and 200 µl cell
suspension (1×106 cells/100 ml) were added to the upper
chamber. After 24 h of incubation at 37°C with 5% CO2,
cells which had not migrated were removed using cotton swabs,
whereas migrated cells were fixed in 4% paraformaldehyde (Shanghai
Haochen Biological Technology Co., Ltd., Shanghai, China) for 10
min at room temperature, stained with 0.1% crystal violet (Santa
Cruz Biotechnology, Inc.) and counted under an Olympus IX-71
inverted fluorescence microscope. Experiments were performed in
triplicate.
Western blot analysis
The cells were lysed in radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Shanghai, China)
mixed with protease inhibitor (phenylmethanesulfonyl fluoride;
Beyotime Institute of Biotechnology) and equal quantities of
protein were subjected to western blot analysis as described
previously (17). Protein content
was determined by the Bicinchoninic Acid kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). The primary antibodies were incubated on
the membranes overnight at 4°C in Tris-buffered saline with Tween
20. The expression of mTOR, p-mTOR, AKT1, p-AKT1, S6K1, p-S6K1 was
detected using specific antibodies and GAPDH was used as the
loading control.
Statistical analysis
Values are expressed as the mean ± standard
deviation of three independent experiments. SPSS 17.0 (SPSS Inc.,
Chicago, IL, USA) was used for statistical analysis. The paired
t-test was used to analyze the results of the western blot
analysis. Groups with or without treatment were compared by using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference between values.
Results
PP242 dose-dependently inhibits the
proliferation of bladder cancer cells
To determine the potency of PP242 against bladder
cancer, its ability to inhibit the proliferation of four bladder
cancer cell lines, RT4, BIU-87, 5637 and T24, was assessed using
the CCK-8 and EdU incorporation assays. As shown in Fig. 1A, treatment with 100–1,000 nM PP242
for 48 h reduced the viability of the four bladder cancer cell
lines in a dose-dependent manner, with 5637 and T24 showing higher
sensitivity to PP242 than RT4 and BIU-87 (P<0.05). Therefore,
the 200-nM concentration of PP242, which was close to the
IC50 on the 5637 and T24 cell lines in the presence of
serum, was used in the subsequent experiments, which were performed
on 5637 and T24 cells.
 | Figure 1PP242 inhibits growth and
proliferation of four bladder cancer cell lines. (A) A cell
counting kit-8 assay was used to examine the viability of RT4,
BIU-87, 5637 and T24 cells following treatment with PP242 (0, 100,
200, 500 or 1,000 nM for 48 h). *P<0.05 vs. 0, 100
and 200 nM. (B) Proliferation of 5637 and T24 cells was assessed by
EdU incorporation with or without the treatment of PP242 (200 nM,
48 h). EdU (red) was used to stain the proliferative cells, and
Hoechst 33324 (blue) was used to stain the nuclei. Images are
representative of three individual experiments (magnification,
×100). (C) Quantification of EdU incorporation in 5637 and T24
cells with or without the treatment of PP242 (200 nM, 48 h).
*P<0.05 vs. Mock and DMSO. Values are expressed as
the mean ± standard deviation of three experiments. EdU,
5-ethynyl-2′-deoxyuridine; DMSO, dimethyl sulfoxide; Mock,
untreated control. |
The EdU incorporation assay showed that following 48
h of incubation, 200 nM PP242 decreased the mean percentage of
proliferating cells by 39% for 5637 cells and by 43% for T24 cells
(Fig. 1B and C; P<0.05). All of
these results indicated that PP242 exerts anti-proliferative
effects on bladder cancer cells.
PP242 inhibits the migration of bladder
cancer cells
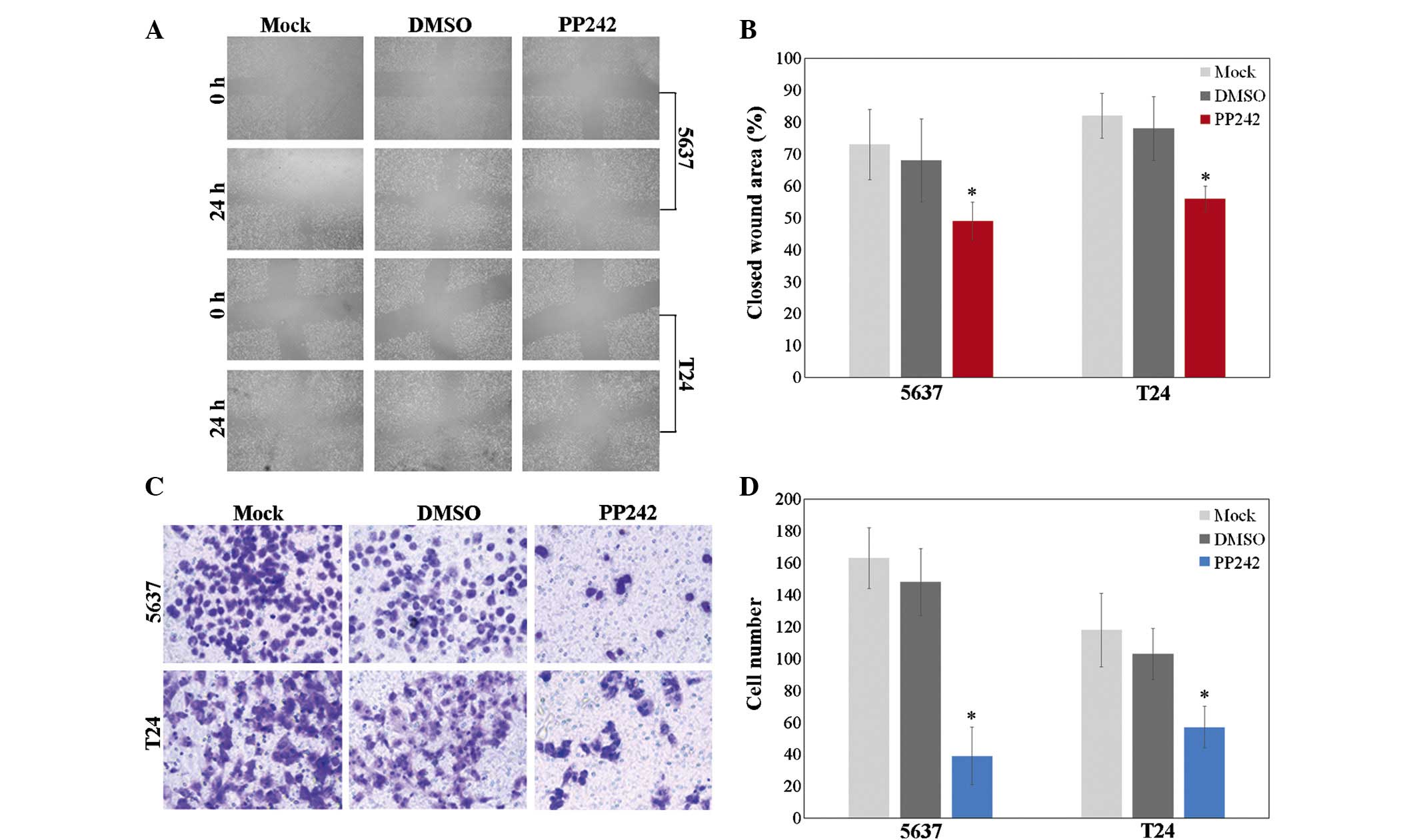
A wound-healing assay was performed to assess the
effect of PP242 (200 nM) on the migratory ability of bladder cancer
cells following 48 h of incubation. A wound healing assay
demonstrated that wound healing of 5637 and T24 cells treated with
PP242 was reduced compared to that of the negative control or
DMSO-treated cells (Fig. 2A and B;
P<0.05).
Next, a Transwell assay was used to test the
chemotactic motility of cells treated with 200 nM PP242 for 48 h.
The results showed a significant decrease in the number of 5637 and
T24 cells on the lower side of the Transwell membrane following
treatment with PP242 compared with that in the control or
DMSO-treated group (Fig. 2C and D;
P<0.05).
Hyper-activation of the mTORC1 and mTORC2
in bladder cancer cells
As PP242 is an ATP-competitive inhibitor which binds
the catalytic site of mTOR, the present study assessed whether it
curtails mTORC1/2 activity. The expression and activation of
signaling proteins of the mTOR pathway in 5637 and T24 cells was
therefore assessed using western blot analysis. The protein
expression of mTOR, raptor, rictor, AKT1 and S6K1 was shown to be
elevated in 5637 and T24 cells compared with that in SV-HUC-1, an
immortalized bladder epithelial cell line (Fig. 3A and B; P<0.05). Selective
phosphorylation of S6K1 on threonine 389 is indicative of TORC1
activity, while AKT1 phosphorylation at the ser473 residue is
characteristic for TORC2 activity (5,7).
Furthermore, phosphorylation of mTOR on ser2481 leads to the
generation of the active form of the TORC2 complex, whereas
phosphorylation of mTOR on ser2448 generates the activated form of
the TORC1 complex (18). In the
present study, western blot analysis showed that the
phosphorylation of mTOR at ser2481 and ser2448 as well as the
TORC-specific phosphorylation of S6K1 and AKT1 were all increased
in 5637 and T24 cells compared with those in the SV-HUC-1 normal
bladder epithelial cell line (Fig. 3C
and D; P<0.05). These results therefore indicated that
mTORC1 as well as mTORC2 were activated in bladder cancer
cells.
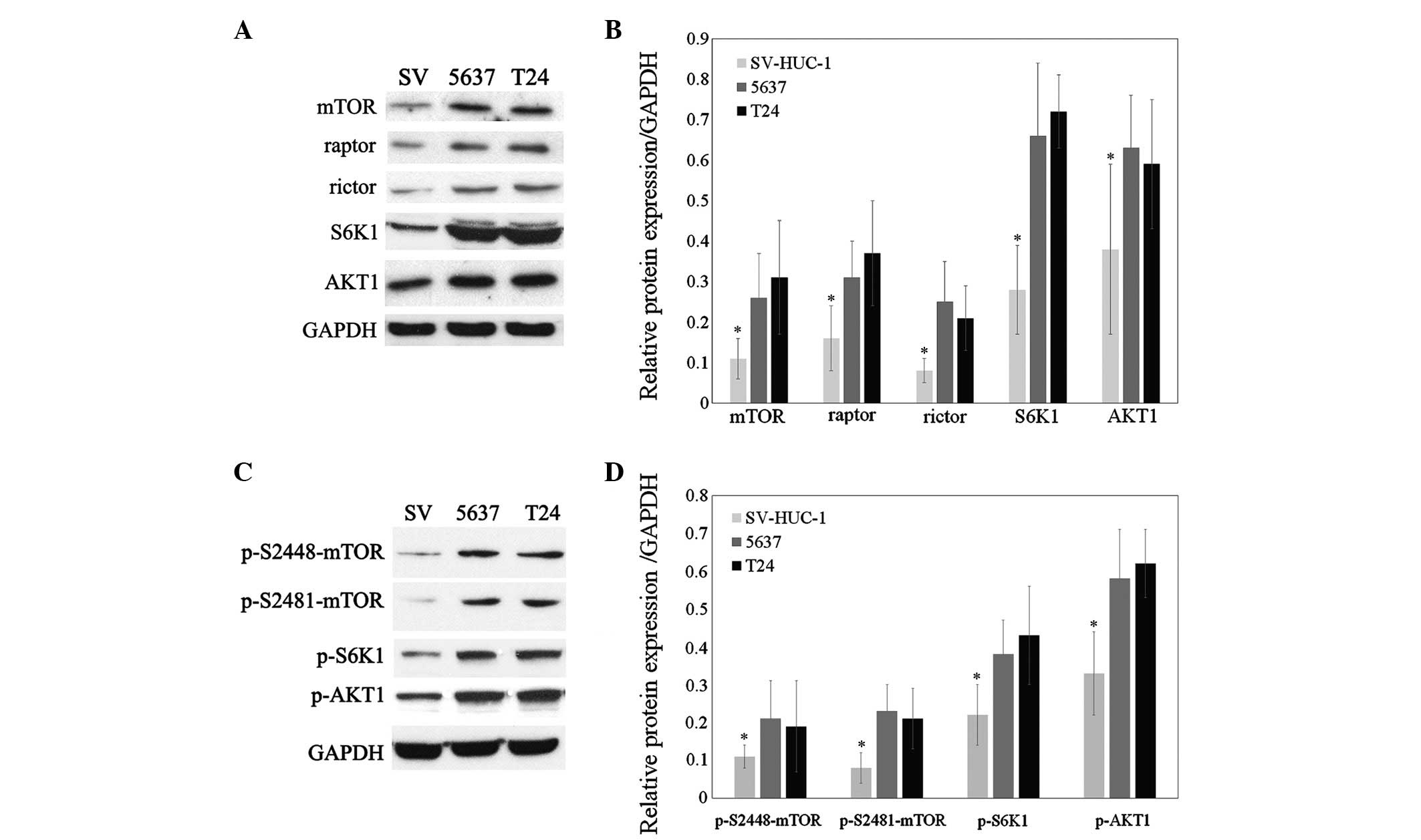 | Figure 3Expression and activation of signaling
proteins of the mTOR pathway in 5637 and T24 cells. (A) Western
blot analysis of mTOR, raptor, rictor, AKT1 and S6K1 in SV-HUC-1,
5637 and T24 cells. (B) Densitometric quantification of band
intensities in A relative to GAPDH. (C) Western blot analysis of
phosphorylated mTOR, AKT1 and S6K1 in SV-HUC-1, 5637 and T24 cells.
(D) Densitometric quantification of band intensities in C relative
to GAPDH. mTOR, mammalian target of rapamycin. Blots are
representative of three individual experiments and values are
expressed as the mean ± standard deviation; *P<0.05
vs. 5637 and T24. p, phosphorylated; mTOR, mammalian target of
rapamycin; S6K1, S6 kinase 1; SV, normal bladder epithelial cell
line SV-HUC-1. |
PP242 downregulates mTORC2/AKT1 activity
but not mTORC1/S6K1 activity in bladder cancer cells
In order to elucidate the potential molecular
mechanism via which PP242 inhibits cell proliferation, the effects
of PP242 on the activation of mTORC1 and mTORC2 activation in 5637
cells were assessed. Western blot analysis revealed that following
treatment with PP242 for 48 h, the amount of mTOR phosphorylated on
ser2448 and S6K1 phosphorylated on threonine 389 was not obviously
different from those in the control and DMSO-treated groups.
However, the amount of mTOR phosphorylated on ser2481 and AKT1
phosphorylated on ser473 was significantly decreased following
treatment with PP242 (Fig. 4A and
B; P<0.05). These results demonstrated that PP242 suppressed
cell proliferation, at least in part, by deactivating the
mTORC2/AKT1 signaling pathway.
Discussion
As the fourth most common type of cancer, bladder
cancer occurs three times more frequently in men than in women in
the USA. An estimated 74,690 of novel cases of urinary bladder
cancer were diagnosed in the USA in 2014 (19), of which 70% were of the
non-muscle-invasive type, which has a tendency to recur in the same
or another region of the bladder at either the same stage as that
of the initial tumor or a more advanced stage (20). The ability to migrate is important
for the formation of metastasis/recurrence of non-muscle invasive
bladder cancer. Therefore, the treatment of bladder cancer is
mostly directed at reducing recurrences and preventing progression.
Although combined chemotherapy initially produces high response
rates, most patients experience recurrences, and the majority of
them succumb to the disease shortly thereafter (21). Aberrant expression of mTORC1/mTORC2
has been observed in numerous types of cancer (8–13)
and their inhibitor, PP242, has been demonstrated to reduce the
activity of mTORC2/AKT1, thus decreasing cell survival,
proliferation and migration (14).
Accordingly, the development of more efficient anti-cancer agents
is urgently required to improve the outcome of bladder cancer. The
present study showed that the mTORC1/2 inhibitor PP242 inhibited
the growth, proliferation and migration of bladder cancer
cells.
Previous studies have shown that PP242 can restrain
the growth of gastric cancer cell lines (22) and clearly demonstrated its enhanced
efficacy compared with that of rapamycin in endometrial tumor
models (23). PP242 is a selective
ATP-competitive mTOR inhibitor and inhibits mTORC1 as well as
mTORC2 simultaneously, thereby preventing the feedback activation
loop of AKT and therefore exerting a greater anti-tumor activity
than rapamycin and its analogues (24). The present study demonstrated that
PP242 is a potent inhibitor of mTORC2 in bladder cancer cells and
suppresses their proliferation in a dose-dependent manner. These
inhibitory effects of PP242 were demonstrated to be mediated by its
targeting of TORC2 but not TORC1. Mechanistic studies indicated
that PP242 selectively inhibited the phosphorylation of AKT1 at
Ser473, the downstream substrate of mTORC2, while it did not affect
the phosphorylation of S6K1, the downstream substrate of mTORC1
(25). The results of the current
study demonstrate that the role of PP242 in bladder cancer cells is
more important for mTORC2, however further research is required to
confirm the exact mechanism. Furthermore, numerous previous studies
demonstrated that PP242 inhibited cell proliferation and induced
apoptosis by its targeting of TORC2 (26–30).
In order to confirm that PP242 targets mTORC2, the present study
also examined the phosphorylation of mTOR. After treatment with
PP242, phosphorylation of mTOR on ser2448, which is an activation
site of mTORC1 (31) was not
affected, while phosphorylation of mTOR on ser2481, an activation
site of mTORC2 (32), was
obviously decreased. These results indicated that mTORC2 was a
target of PP242 in bladder cancer cells.
Furthermore, the present study identified that the
migratory ability of bladder cancer cells was decreased by PP242
treatment, suggesting that the mTORC2 pathway also participates in
the process of tumor progression. Recent studies showed that PP242
inhibited cancer-cell migration by blocking the mTOR pathway in
certain tumor types (22,33,34).
Further study is required to determine the complex regulatory role
of TORC2 signaling in the migration of bladder cancer cells.
In conclusion, the present study supported the
concept that PP242 exerts its anti-tumor effects through inhibiting
cell proliferation and migration by suppressing mTORC2, but not the
mTORC1, in bladder cancer cells. Indeed, pre-clinical studies have
indicated that PP242 is a potent anti-cancer drug.
Acknowledgments
The present study was supported by the Liaoning
Province Science and Technology Plan Project (no. 2012225016), the
Liaoning Provincial Natural Science Foundation (no. 2013021066) and
the Shenyang City Project of Key Laboratory (no. F13-293-1-00).
References
|
1
|
Zuo W, Wang ZZ and Xue J: Artesunate
induces apoptosis of bladder cancer cells by miR-16 regulation of
COX-2 expression. Int J Mol Sci. 15:14298–14312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang H, Peng X, Bai Q, Zhou Y, Yu X,
Zhang Q, Zhu J and Mi M: Ampelopsin suppresses breast
carcinogenesis by inhibiting the mTOR signaling pathway.
Carcinogenesis. 35:1847–1854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rivas DA, Yaspelkis BB III, Hawley JA and
Lessard SJ: Lipid-induced mTOR activation in rat skeletal muscle
reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-beta
-D-ribofuranoside. J Endocrinol. 202:441–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wahane SD, Hellbach N, Prentzell MT, Weise
SC, Vezzali R, Kreutz C, Timmer J, Krieglstein K, Thedieck K and
Vogel T: PI3K-p110-alpha-subtype signalling mediates survival,
proliferation and neurogenesis of cortical progenitor cells via
activation of mTORC2. J Neurochem. 130:255–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Z, Zhang G, Xu X, Su W and Yu B:
MTOR-rictor is the Ser473 kinase for AKT1 in mouse one-cell stage
embryos. Mol Cell Biochem. 361:249–257. 2012. View Article : Google Scholar
|
|
6
|
Jash S, Dhar G, Ghosh U and Adhya S: Role
of the mTORC1 complex in satellite cell activation by RNA-induced
mitochondrial restoration: Dual control of cyclin D1 through
microRNAs. Mol Cell Biol. 34:3594–3606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goodman CA: The role of mTORC1 in
regulating protein synthesis and skeletal muscle mass in response
to various mechanical stimuli. Rev Physiol Biochem Pharmacol.
166:43–95. 2014.PubMed/NCBI
|
|
8
|
Faller WJ, Jackson TJ, Knight JR, Ridgway
RA, Jamieson T, Karim SA, Jones C, Radulescu S, Huels DJ, Myant KB,
et al: MTORC1-mediated translational elongation limits intestinal
tumour initiation and growth. Nature. 517:497–500. 2015. View Article : Google Scholar :
|
|
9
|
Kaibori M, Shikata N, Sakaguchi T,
Ishizaki M, Matsui K, Iida H, Tanaka Y, Miki H, Nakatake R, Okumura
T, et al: Influence of rictor and raptor expression of mtor
signaling on long-term outcomes of patients with hepatocellular
carcinoma. Dig Dis Sci. 60:919–928. 2015. View Article : Google Scholar
|
|
10
|
Randall JM, Millard F and Kurzrock R:
Molecular aberrations, targeted therapy and renal cell carcinoma:
Current state-of-the-art. Cancer Metastasis Rev. 33:1109–1124.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Treilleux I, Arnedos M, Cropet C, Wang Q,
Ferrero JM, Abadie-Lacourtoisie S, Levy C, Legouffe E, Lortholary
A, Pujade-Lauraine E, et al: Translational studies within the
TAMRAD randomized GINECO trial: Evidence for mTORC1 activation
marker as a predictive factor for everolimus efficacy in advanced
breast cancer. Ann Oncol. 26:120–125. 2015. View Article : Google Scholar
|
|
12
|
Sandhöfer N, Metzeler KH, Rothenberg M,
Herold T, Tiedt S, Groiß V, Carlet M, Walter G, Hinrichsen T,
Wachter O, et al: Dual PI3 K/mTOR inhibition shows antileukemic
activity in MLL rearranged acute myeloid leukemia. Leukemia.
29:828–838. 2015. View Article : Google Scholar
|
|
13
|
Yue H, Li W, Liu P, Gao J, Miao J and Zhao
J: Inhibition of autophagy promoted sphingosylphosphorylcholine
induced cell death in non-small cell lung cancer cells. Biochem
Biophys Res Commun. 453:502–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joha S, Nugues AL, Hétuin D, Berthon C,
Dezitter X, Dauphin V, Mahon FX, Roche-Lestienne C, Preudhomme C,
Quesnel B and Idziorek T: GILZ inhibits the mTORC2/AKT pathway in
BCR-ABL(+) cells. Oncogene. 31:1419–1430. 2012. View Article : Google Scholar :
|
|
15
|
Zheng X, Liang Y, He Q, Yao R, Bao W, Bao
L, Wang Y and Wang Z: Current models of mammalian target of
rapamycin complex 1 (mTORC1) activation by growth factors and amino
acids. Int J Mol Sci. 15:20753–20769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y, Xi Q, Chen Y, Wang J, Peng P, Xia
S and Yu S: A dual mTORC1 and mTORC2 inhibitor shows antitumor
activity in esophageal squamous cell carcinoma cells and sensitizes
them to cisplatin. Anticancer Drugs. 24:889–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Z, Zhang G and Kong C: High
expression of Cdc25B and low expression of 14-3-3σ is associated
with the development and poor prognosis in urothelial carcinoma of
bladder. Tumour Biol. 35:2503–2512. 2014. View Article : Google Scholar
|
|
18
|
Fan W, Cheng K, Qin X, Narsinh KH, Wang S,
Hu S, Wang Y, Chen Y, Wu JC, Xiong L and Cao F: MTORC1 and mTORC2
play different roles in the functional survival of transplanted
adipose-derived stromal cells in hind limb ischemic mice via
regulating inflammation in vivo. Stem Cells. 31:203–214. 2013.
View Article : Google Scholar
|
|
19
|
Baack Kukreja JE, Scosynev E, Brasachio
RA, Toy EP, Messing EM and Wu G: Bladder cancer incidence and
mortality in patients treated with radiation for uterine cancer.
BJU Int. 114:844–851. 2014. View Article : Google Scholar
|
|
20
|
Kamat AM, Vlahou A, Taylor JA, Hudson ML,
Pesch B, Ingersoll MA, Todenhöfer T, van Rhijn B, Kassouf W, Barton
Grossman H, et al: Considerations on the use of urine markers in
the management of patients with high-grade non-muscle-invasive
bladder cancer. Urol Oncol. 32:1069–1077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petrelli F, Coinu A, Cabiddu M, Ghilardi
M, Vavassori I and Barni S: Correlation of pathologic complete
response with survival after neoadjuvant chemotherapy in bladder
cancer treated with cystectomy: A meta-analysis. Eur Urol.
65:350–357. 2014. View Article : Google Scholar
|
|
22
|
Xing X, Zhang L, Wen X, Wang X, Cheng X,
Du H, Hu Y, Li L, Dong B, Li Z and Ji J: PP242 suppresses cell
proliferation, metastasis and angiogenesis of gastric cancer
through inhibition of the PI3K/AKT/mTOR pathway. Anticancer Drugs.
25:1129–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korets SB, Musa F, Curtin J, Blank SV and
Schneider RJ: Dual mTORC1/2 inhibition in a preclinical xenograft
tumor model of endometrial cancer. Gynecol Oncol. 132:468–473.
2014. View Article : Google Scholar
|
|
24
|
Zhou HY and Huang SL: Current development
of the second generation of mTOR inhibitors as anticancer agents.
Chin J Cancer. 31:8–18. 2012.
|
|
25
|
Chen L, Xu B, Liu L, Liu C, Luo Y, Chen X,
Barzegar M, Chung J and Huang S: Both mTORC1 and mTORC2 are
involved in the regulation of cell adhesion. Oncotarget.
30:7136–7150. 2015. View Article : Google Scholar
|
|
26
|
Ravichandran K, Zafar I, Ozkok A and
Edelstein CL: An mTOR kinase inhibitor slows disease progression in
a rat model of poly-cystic kidney disease. Nephrol Dial Transplant.
30:45–53. 2015. View Article : Google Scholar
|
|
27
|
Qin Y, Zhao X and Fang Y: PP242 synergizes
with suberoylanilide hydroxamic acid to inhibit growth of ovarian
cancer cells. Int J Gynecol Cancer. 24:1373–1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goncharov DA, Kudryashova TV, Ziai H,
Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM and
Goncharova EA: Mammalian target of rapamycin complex 2 (mTORC2)
coordinates pulmonary artery smooth muscle cell metabolism,
proliferation and survival in pulmonary arterial hypertension.
Circulation. 129:864–874. 2014. View Article : Google Scholar :
|
|
29
|
Becker MN, Wu KJ, Marlow LA, Kreinest PA,
Vonroemeling CA, Copland JA and Williams CR: The combination of an
mTORc1/TORc2 inhibitor with lapatinib is synergistic in bladder
cancer in vitro. Urol Oncol. 32:317–326. 2014. View Article : Google Scholar
|
|
30
|
Bogani C, Bartalucci N, Martinelli S,
Tozzi L, Guglielmelli P, Bosi A and Vannucchi AM; Associazione
Italiana per la Ricerca sul Cancro AGIMM Gruppo Italiano Malattie
Mieloproliferative: MTOR inhibitors alone and in combination with
JAK2 inhibitors effectively inhibit cells of myeloproliferative
neoplasms. PLoS One. 8:e548262013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Müller J, Ehlers A, Burkhardt L, Sirma H,
Steuber T, Graefen M, Sauter G, Minner S, Simon R, Schlomm T and
Michl U: Loss of pSer2448-mTOR expression is linked to adverse
prognosis and tumor progression in ERG-fusion-positive cancers. Int
J Cancer. 132:1333–1340. 2013. View Article : Google Scholar
|
|
32
|
Vazquez-Martin A, Oliveras-Ferraros C,
Bernadó L, López-Bonet E and Menendez JA: The serine
2481-autophos-phorylated form of mammalian Target of Rapamycin
(mTOR) is localized to midzone and midbody in dividing cancer
cells. Biochem Biophys Res Commun. 380:638–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Lai P, Zhang Z, Huang M, Wang L,
Yin M, Jin D, Zhou R and Bai X: Targeted inhibition of mTORC2
prevents osteosarcoma cell migration and promotes apoptosis. Oncol
Rep. 32:382–388. 2014.PubMed/NCBI
|
|
34
|
Li H, Lin J, Wang X, Yao G, Wang L, Zheng
H, Yang C, Jia C, Liu A and Bai X: Targeting of mTORC2 prevents
cell migration and promotes apoptosis in breast cancer. Breast
Cancer Res Treat. 134:1057–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|