Introduction
In acute coronary events, the establishment of early
and successful myocardial reperfusion is the most effective
strategy to limit infarct size (IS) and improve clinical outcomes.
However, reperfusion may induce further damage to the myocardium
itself (1). Remote ischemic
conditioning describes an innate cardioprotective mechanism, in
which brief periods of sublethal ischemia and reperfusion are
applied to a remote organ in order to protect the myocardium
against the detrimental effects of prolonged reperfusion injury
(2). This was first identified by
Przyklenk et al (3) in a
canine model; in which it was demonstrated that brief episodes of
ischemia in the circumflex branch protected remote virgin
myocardium from subsequent sustained left coronary artery ischemia.
Since then, the use of this procedure has been extended in a series
of experiments, demonstrating that intermittent ischemia of several
different remote organs induces protection against subsequent
myocardial ischemia/reperfusion (IR) injury (4,5). The
fact that remote ischemic conditioning can be performed
noninvasively using a blood pressure cuff on the upper/lower limb
made it more clinically feasible, compared with conventional local
ischemic conditioning (6). In
addition, unlike local ischemic conditioning, remote ischemic
conditioning can be applied during all three windows of IR,
including prior to (remote ischemic preconditioning; RIPC), during
(remote ischemic perconditioning; RIPerC) and following (remote
ischemic postconditioning; RIPostC) myocardial ischemia.
Considering the unpredictable nature of myocardial ischemic events,
RIPerC and RIPostC appear to be more practical than RIPC in
clinical settings, however, neither are as effective as local
ischemic preconditioning in terms of the ability to limit IS
(7). Our previous study
demonstrated that the combination of RIPerC and local ischemic
postconditioning (IPostC) produces synergistic effects and
reinforces the cardioprotective activities of local ischemic
preconditioning. However, RIPostC remains an invasive procedure and
has a limited time frame of use (8). Thus, it may be beneficial to
investigate the combination of two non-invasive procedures, RIPerC
and RIPostC, and determine whether these result in an additive
effect in the protection against myocardial IR injury. To
investigate this hypothesis, the present study analyzed the
protective efficacy of the combined use of RIPerC and RIPostC
against myocardial IR injury using an in vivo rat IR model,
and the results were compared with the use of either RIPerC or
RIPostC alone.
Materials and methods
Animals
A total of 90 male Sprague-Dawley rats (8-week-old),
weighing between 250 and 280 g (Experimental Animal Center, Fudan
University, Shanghai, China) were used in the present study. All
rats were housed at a controlled temperature (25°C) under a 12-h
light/dark cycle with ad libitum access to food and water.
The animal investigation protocol used was in compliance with the
Guide for the Care of Use of Laboratory Animals published by the
National Institutes of Health (NIH Publication no. 85-23, revised
1996) (9) and approved by the
Animal Care Committee of Shanghai Jiao Tong University Affiliated
Sixth People's Hospital, (Shanghai, China). All rats were housed
for 2 weeks to provide an acclimatization period prior to the
experiments.
Surgical preparation
The IR model was performed, as previously described
(10). In brief, the rats were
anesthetized by intraperitoneal injection with 1.2% pentobarbital
sodium (Sigma-Aldrich, St. Louis, MO, USA), at a dose of 50 mg/kg.
The left coronary artery (LCA) was ligated using a 6-0 Prolene
suture immediately distal to its first branch, and cardiac ischemia
was confirmed by the formation of a pale area below the suture,
which gradually became cyanotic. After 40 min, the suture was
released, and reperfusion was characterized by the rapid
disappearance of cyanosis, followed by vascular blush. Following
120 min of reperfusion, the rats were sacrificed with an overdose
of pentobarbital sodium (150 mg/kg) and the hearts were harvested
for further assessment. For rats undergoing sham surgery, a suture
was placed in a corresponding location without ligation.
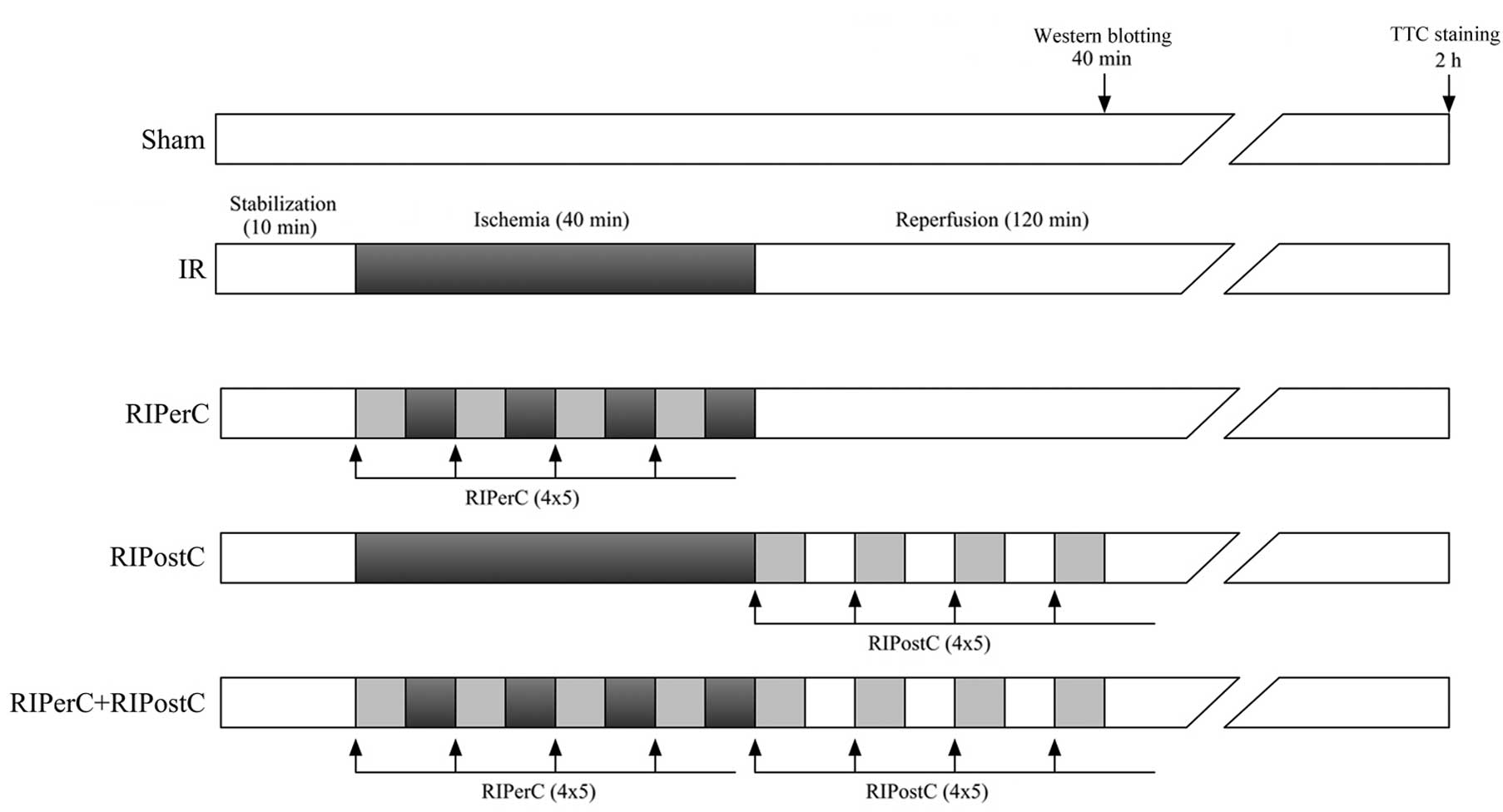
RIPerC and RIPostC were delivered via non-invasive
occlusion of both lower limbs using tourniquets, which was
validated by the disappearance of Doppler blood flow of the femoral
artery (5–10 MHz; M-Turbo System L38X; SonoSite, Inc., Bothell, WA,
USA). Both RIPerC and RIPostC consisted of four cycles of 5-min
limb ischemia/reperfusion, with RIPerC initiated at the onset of
coronary, while RIPostC initiated at the onset of coronary
reperfusion, as shown in Fig.
1.
Experimental protocols
The rats were randomly assigned to the following
experimental groups: i) Sham group (n=6; rats underwent sham
surgery); ii) IR group (n=6; rats underwent 40 min left anterior
descending artery occlusion followed by 2 h reperfusion) iii)
RIPerC group (n=6; as in the IR group, in addition to four cycles
of 5 min bilateral hindlimb occlusion followed by 5 min reperfusion
during myocardial ischemia); iv) RIPostC group (n=6; as in the IR
group, with four cycles of 5 min bilateral hindlimb occlusion
followed by 5 min reperfusion at the onset of coronary
reperfusion); v) RIPerC + RIPostC group (n=6; RIPerC combined with
RIPostC. Following 2 h of subsequent reperfusion, all rats were
sacrificed for IS quantification (Fig.
1).
An additional 30 Sprague-Dawley rats (six in each
group) underwent the procedures described above and were also
sacrificed 2 h following reperfusion. In these rats, a cross
section of the left ventricular (LV) myocardium (~5 mm thick) at
the papillary muscle level was obtained from each rat for terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) staining. The remaining myocardium from the area at risk
(AAR) was collected for quantification of the protein expression
levels of B cell lymphoma (Bcl)-2 and Bcl-2-associated X protein
(Bax).
In addition, a further six rats from each group
underwent the same procedure, were sacrificed following 40 min of
reperfusion, and the myocardium from the AAR was obtained for
western blot analysis. All tissues were snap-frozen in liquid
nitrogen (Shanghai Jiangnan Gas Co., Ltd., Shanghai, China) and
stored in a freezer at -80°C.
AAR and IS determination
Following 2 h of reperfusion, the LCA was
re-occluded, and 2% Evans blue dye (Sigma-Aldrich) was retrogradely
injected into the ascending aorta to delineate the AAR. The heart
was removed and sliced transversely from the base to the apex into
five sections (2-3 mm), which were incubated for 15 min at 37°C in
a phosphate-buffered 1% 2,3,5-triphenyltetrazolium chloride
solution (Sigma-Aldrich) to determine the infarcted area. All
slices were then fixed in 10% formalin (Goodbio, Shanghai, China),
and the extent of the area of necrosis was quantified by
computerized planimetry using ImagePro Plus software, version 6.0
(Media Cybernetics, Inc., Rockville, MD, USA) and corrected for the
weight of the tissue slices. IS is expressed as the percentage of
total weight of the LV AAR.
Determination of serum cardiac troponin I
(cTnI) and inflammatory cytokines
Subsequent to 2 h of reperfusion, blood samples were
collected into tubes containing microscopic silica particles and
rested for 30 min. Following centrifugation at 2,500 × g for 10 min
at 25°C, the supernatants were collected and stored at -80°C until
required for future analysis. The serum levels of cTnI, tumor
necrosis factor α (TNF-α) and interleukin 1β (IL-1β) were assessed
using Cardiac Troponin-I enzyme-linked immunosorbent assay (ELISA)
(cat. no. CTNI-HS), Rat TNF-alpha Platinum ELISA (cat. no. BMS622),
and Rat IL-1 beta Platinum ELISA (cat. no. BMS630) kits, according
to the manufacturer's instructions (cTnI, Life Diagnostics, Inc.,
West Chester, PA, USA; TNF-α and IL-1β, eBioscience, Inc., San
Diego, CA, USA). Levels of cTnI were expressed as ng/ml, whereas
levels of cytokines were expressed as pg/ml.
Assessment of LV function
The right carotid artery was cannulated using a 1.6F
Pressure Catheter (Transonic Scisense, Inc., London, ON, Canada)
for measuring the hemodynamic parameters. The catheter was passed
retrogradely into the LV, and LV pressure tracings were digitized
using a PowerLab Physiological Recorder (ADInstruments Pty, Ltd.,
Bella Vista, Australia) and stored for later analysis. The LV
end-diastolic pressure (LVEDP), maximum/minimum first derivative of
LV pressure over time (± dP/dtmax) and mean arterial
pressure (MAP) were analyzed in a blinded-manner using LabChart
software, version 8 (ADInstruments Pty Ltd, Oxford, UK).
TUNEL staining
TUNEL staining was performed using a commercially
available kit (In Situ Cell Death Detection kit; Roche Diagnostics
GmbH, Mannheim, Germany), according to the manufacturer's protocol,
on heart tissue slices randomly selected from each group (n=6
tissue slices/group). A minimum of 100 cells from the peri-infarct
area were counted using a microscope (magnification, ×400; Q500MC;
Leica Microsystems GmbH, Wetzlar, Germany) in 10 fields for each
sample. The peri-infarct area was predetermined using hematoxylin
and eosin (Goodbio) staining, which was performed on the adjacent
tissue slide. The percentages of cells positive for TUNEL staining
were calculated as follows: Number of apoptotic cells / total
number of cells × 100%.
Western blotting
Western blotting was performed on the myocardium
from the AAR obtained following 40 min of reperfusion for
quantification of the levels of total and phosphorylated (p-)
STAT-3, Akt, extracellular signal-related kinase (ERK) 1/2 and
glycogen synthase kinase (GSK) 3β, and following 120 min of
reperfusion for Bcl-2 and Bax. Briefly, freshly frozen myocardial
tissue samples were ground into small pieces (~1×1×1 mm) in liquid
nitrogen. The samples were then transferred to microcentrifuge
tubes containing radioimmunoprecipitation lysis buffer (~150
μl per 10 mg tissue; Beyotime Institute of Biotechnology,
Shanghai, China) and 1 mM phenylmethylsulfonyl fluoride. The
samples were thoroughly homogenized and kept on ice for 1 h,
vortexing every 10 min. The samples were subsequently centrifuged
at 20,000 × g for 30 min at 4°C, and the supernatants were
transferred into fresh tubes and kept on ice. Following protein
quantification using a Bicinchoninic Acid assay (Beyotime Institute
of Biotechnology), equal quantities of protein (50 μg) were
separated on 10% Tris-glycine sodium dodecyl sulfate gels (Beyotime
Institute of Biotechnology), and transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). Subsequent
to blocking with 5% non-fat milk for 2 h and washing twice with
Tris-buffered saline containing 0.05% Tween (TBST; 5 min/wash;
Goodbio), the membranes were incubated overnight at 4°C with the
following primary antibodies: Rabbit monoclonal anti-Bcl-2
(1:1,000; cat. no. 2870; Cell Signaling Technology, Inc., Danvers,
MA, USA) and rabbit polyclonal anti-Bax (1:300; cat. no. sc-493;
Santa Cruz Biotechnology, Inc., Dallas TX, USA) to quantify
apoptotic signaling, rabbit monoclonal
anti-705Tyr-p-STAT-3 (1:1,000; cat. no. 9145), rabbit
polyclonal anti-total STAT-3 (1:1,000; cat. no. 9132), rabbit
monoclonal anti-473Ser-p-Akt (1:1,000; cat. no. 4060),
rabbit polyclonal anti-total Akt (1:800; cat. no. 9272), rabbit
monoclonal anti-202/204Tyr-p-ERK1/2 (1:1,000; cat. no.
4370), rabbit polyclonal anti-total ERK1/2 (1:1,000; cat. no.
9102), rabbit monoclonal anti-9Ser-p-GSK-3β (1:2,000;
cat. no. 5558) and rabbit monoclonal anti-total GSK-3β (1:1,000;
cat. no. 9315) (Cell Signaling Technology, Inc.) to quantify
salvage signaling pathways. Mouse monoclonal anti-β-actin (1:3,000;
cat. no. A1978; Sigma-Aldrich) was used as a loading control.
Following primary antibody incubation, the membranes were washed
five times with TBST (5 min/wash) and incubated with the
horseradish peroxidase-conjugated goat anti-rabbit (1:5,000; cat.
no. sc-2004) or goat anti-mouse (1:3,000; cat. no. sc-2005) IgG
secondary antibodies (Santa Cruz Biotechnology, Inc.).
Subsequently, the membranes were washed five times with TBST (5
min/wash), and the bands were detected using Western Blotting
Luminol reagent (cat. no. sc-2048; Santa Cruz Biotechnology, Inc.)
and quantified by densitometric analysis of digitized
autoradiograms using Quantity One version 4.6.2 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Each immunoblotting
experiment was repeated three times, and the averages of the
results were calculated.
Statistical analysis
All values are expressed as the mean ± standard
deviation. All data analyses were performed using SPSS statistical
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Differences
in the hemodynamic indexes were compared within groups using
repeated-measures analysis of variance, and between groups using
two-way analysis of variance followed by a least significant
difference (LSD) corrected multiple comparisons test. Differences
in other variables between groups were evaluated using one-way
analysis of variance, followed by Fisher's post-hoc LSD-corrected
multiple comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Myocardial IS
The AAR was similar among the groups (data not
shown). Compared with the IR group (49.45±6.59%), IS was
significantly reduced in the RIPerC (34.36±5.87%) group and RIPostC
(36.04±6.16%) group (P<0.05; Fig.
2). However, no further reductions in IS were observed in the
RIPerC + RIPostC group, compared with either the group exposed to
RIPerC alone or the group exposed to RIPostC alone (31.43±5.43%;
Fig. 2).
 | Figure 2Myocardial infarct size 2 h
post-reperfusion. (A) Representative sequential LV slices from each
group, indicating the AAR, delineated with Evans blue staining, in
the normal heart tissue and the area of necrosis, determined using
2,3,5-triphenyltetrazolium chloride staining (pale area = infarcted
tissue). (B) Percentages of LV weights in the AN/AAR. Data are
expressed as the mean ± standard deviation. *P<0.05,
vs. IR group. LV, left ventricular; AN, area of necrosis; AAR, area
at risk; IR, ischemia/reperfusion; RIPerC, remote ischemic
perconditioning; RIPostC, remote ischemic postconditioning. |
Myocardial apoptosis
As shown in Fig. 3A and
B, the results of the TUNEL staining demonstrated that the
percentages of positively-stained cells were lower in the RIPerC
(22.35±4.22%) and RIPostC (24.63±4.44%) groups, compared with the
IR group (35.81±5.27%; P<0.05). The combination of RIPerC and
RIPostC did not further reduce the percentage of apoptotic cells,
compared with the percentages of apoptosis in either of the groups
exposed to RIPerC or RIPostC alone (20.33±3.67%). Similarly, the
protein expression ratio of Bcl-2/Bax was found to be higher in all
the conditioning groups, compared with the IR group (P<0.05),
with no significant differences identified among the groups
(Fig. 3C and D).
Serum levels of cTnI
Following 2 h of reperfusion, the serum levels of
cTnI were significantly increased in the IR group, compared with
the sham group. However, the increase in the levels of cTnI were
significantly attenuated in the RIPerC, RIPostC and RIPerC +
RIPostC groups (P<0.05, vs. IR group), with no significant
differences observed among the three groups (Table I).
 | Table ISerum levels of CTnI following 2 h of
reperfusion. |
Table I
Serum levels of CTnI following 2 h of
reperfusion.
| Group | CTnI (ng/ml) |
|---|
| Sham | 3.31±0.75a |
| IR | 139.85±21.18 |
| RIPerC | 54.02±9.60a |
| RIPostC | 50.90±10.95a |
| RIPerC +
RIPostC | 46.96±8.81a |
Levels of serum inflammatory
cytokines
Compared with the sham group, the serum levels of
TNF-α and IL-1β following 2 h of reperfusion were significantly
increased in the IR group (P<0.05, vs. sham group), and were
significantly attenuated by RIPerC, RIPostC and RIPerC + RIPostC
(P<0.05, vs. IR group). However, no differences were observed
between the groups (Table
II).
 | Table IISerum levels of TNF-α and IL-1β 2 h
post-reperfusion. |
Table II
Serum levels of TNF-α and IL-1β 2 h
post-reperfusion.
| Group | TNF-α (pg/ml) | IL-1β (pg/ml) |
|---|
| Sham | 10.50±2.74a | 10.17±2.32a |
| IR | 215.67±41.80 | 148.67±20.16 |
| RIPerC |
173.33±32.72a |
128.83±17.99a |
| RIPostC |
180.17±30.30a |
126.00±16.99a |
| RIPerC +
RIPostC |
167.33±25.44a |
120.17±14.35a |
LV functions
The heart rates were observed to be consistent among
the groups at all time points (data not shown). During the periods
of ischemia and reperfusion, there were increases in LVEDP, and a
significant reduction in MAP, +dP/dtmax and
−dP/dtmax in each group, compared with the data at
baseline (P<0.05, vs. baseline). However, compared with the IR
group, none of the conditioning methods had a significant effect on
MAP, LVEDP, +dP/dtmax or −dP/dtmax at any
given time point. Individual group data are presented in Table III.
 | Table IIILeft ventricular functions at
different stages of IR. |
Table III
Left ventricular functions at
different stages of IR.
| Time point | MAP (mmHg) | LVEDP (mmHg) |
+dP/dtmax (mmHg/sec) |
−dP/dtmax (mmHg/sec) |
|---|
| Baseline |
| Sham | 113.2±5.4 | 4.0±0.5 | 9,985.2±957.7 | 5,927.7±519.2 |
| IR | 114.9±6.1 | 4.1±0.5 |
10,128.9±1,044.2 | 5,987.4±488.5 |
| RIPerC | 116.4±6.3 | 4.2±0.5 |
10,207.3±1,068.6 | 6,036.2±594.1 |
| RIPostC | 113.7±5.9 | 4.1±0.6 | 10,041.1±914.5 | 5,960.1±566.8 |
| RIPerC +
RIPostC | 115.4±6.2 | 4.2±0.5 |
10,157.3±1,054.1 | 6,020.6±598.3 |
| 20 min
post-ischemia |
| Sham | 110.3±6.1b | 4.0±0.6a |
9,897.3±952.1a | 5,892.5±590.4 |
| IR | 103.3±10.7b | 5.1±0.6b |
8,308.5±885.2b |
5,257.3±620.9b |
| RIPerC | 106.8±12.4b | 5.1±0.8b |
8,392.3±876.8b |
5,327.0±721.1b |
| RIPostC | 104.1±9.8b | 5.1±0.8b |
8,283.7±951.4b |
5,239.2±619.2b |
| RIPerC +
RIPostC | 105.6±11.3b | 5.1±0.8b |
8,377.4±1,087.2b |
5,324.1±545.8b |
| 40 min
post-ischemia |
| Sham | 107.7±6.2a,b | 4.1±0.6a |
9,798.6±941.8a |
5,854.4±507.6a |
| IR | 95.3±8.5b | 5.7±0.8b |
7,006.2±815.2b |
4,860.7±544.5b |
| RIPerC | 98.7±9.7b | 5.6±0.9b |
7,114.5±875.1b |
4,972.3±749.3b |
| RIPostC | 95.5±7.2b | 5.7±0.9b |
6,982.9±703.4b |
4,830.3±668.9b |
| RIPerC +
RIPostC | 98.4±9.0b | 5.6±0.9b |
7,103.7±858.7b |
4,968.9±596.1b |
| 30 min
post-reperfusion |
| Sham | 104.9±5.3a,b | 4.1±0.6a |
9,607.5±936.7a,b |
5,756.3±547.1a,b |
| IR | 86.8±9.2b | 6.6±0.9b |
5,276.2±679.4b |
4,255.1±486.6b |
| RIPerC | 89.1±8.8b | 6.5±0.9b |
5,414.1±751.2b |
4,401.2±623.4b |
| RIPostC | 88.7±8.3b | 6.5±0.9b |
5,417.8±610.8b |
4,384.7±556.5b |
| RIPerC +
RIPostC | 90.6±8.4b | 6.4±0.9b |
5,422.4±660.3b |
4,404.9±538.2b |
| 1 h
post-reperfusion |
| Sham | 104.3±5.7a,b | 4.1±0.6a |
9,401.9±965.5a,b |
5,650.2±491.7a,b |
| IR | 84.4±8.1b | 6.3±0.8b |
5,286.2±606.3b |
4,278.8±514.2b |
| RIPerC | 85.9±8.3b | 6.1±0.8b |
5,452.3±694.7b |
4,473.1±616.1b |
| RIPostC | 85.2±6.9b | 6.2±0.8b |
5,432.8±609.1b |
4,450.5±576.8b |
| RIPerC +
RIPostC | 87.6±8.3b | 6.1±0.8b |
5,474.6±723.4b |
4,480.4±518.4b |
| 2 h
post-reperfusion |
| Sham | 103.2±6.1a,b | 4.1±0.6a |
9,238.7±928.5a,b |
5,507.3±517.4a,b |
| IR | 85.5±8.2b | 5.9±0.8b |
5,307.3±655.1b |
4,320.1±535.6b |
| RIPerC | 88.8±7.8b | 5.7±0.7b |
5,603.3±731.4b |
4,561.5±633.4b |
| RIPostC | 87.4±7.9b | 5.8±0.8b |
5,587.6±704.3b |
4,538.6±591.7b |
| RIPerC +
RIPostC | 90.1±8.4b | 5.7±0.8b |
5,634.1±727.0b |
4,575.3±540.8b |
Reperfusion injury salvage kinase (RISK)
and survivor activating factor enhancement (SAFE) pathways
The protein levels of total Akt, ERK1/2, GSK-3β
(RISK pathway) and signal transducer and activator of transcription
(STAT) 3 (SAFE pathway) were found to be similar among the groups.
The levels of p-Akt, p-ERK1/2, p-GSK-3β and p-STAT-3 are expressed
as densitometric levels, normalized by levels of total protein.
In the IR group, the phosphorylation levels of Akt,
ERK1/2, GSK-3β and STAT-3 were significantly increased following 40
min of reperfusion, compared with the sham group (P<0.05, vs.
sham group; Fig. 4). In the
RIPerC, RIPostC and RIPerC + RIPostC groups, further increases in
the phosphorylation levels of STAT-3, Akt, ERK1/2 and GSK-3β were
detected (P<0.05, vs. IR group; Fig. 4), however, no significant
differences were observed among the three groups.
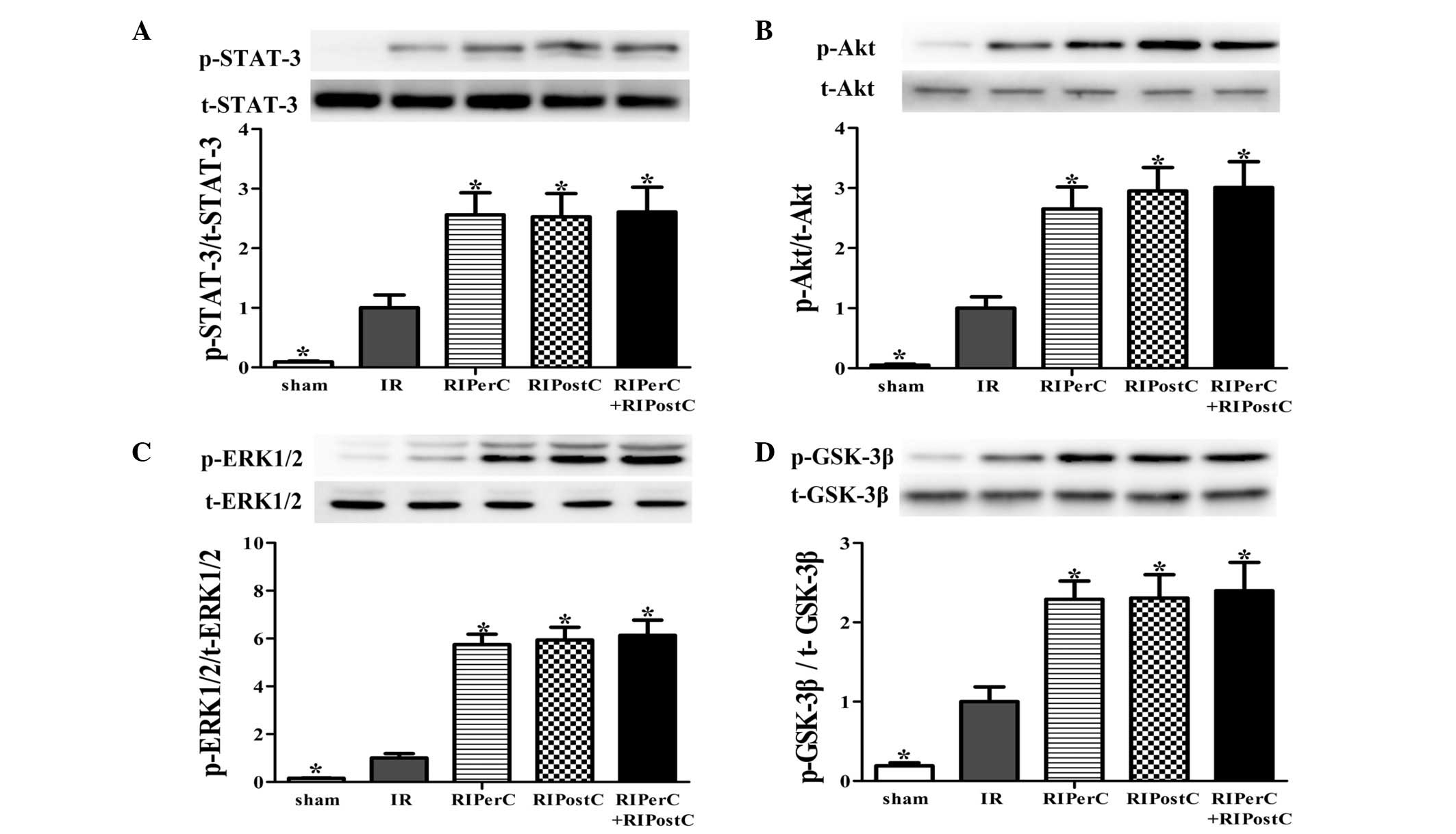 | Figure 4Reperfusion injury salvage kinase
(Akt, ERK1/2 and GSK-3β) and survivor activating factor enhancement
(STAT-3) pathway activation following 40 min reperfusion. Western
blot analysis of total and phosphorylated (A) STAT-3, (B) Akt, (C)
ERK1/2 and (D) GSK-3β proteins in rat hearts in left ventricular
homogenates of heart tissues subjected to IR, and the expression
ratios of phosphorylated/total protein are shown. All expression
levels were normalized to that of β-actin. Data are expressed as
the mean ± standard deviation. *P<0.05, vs. IR group.
ERK1/2, extracellular signal-related kinase 1/2; GSK-3β, glycogen
synthase kinase 3; STAT-3, signal transducer and activator of
transcription 3; IR, ischemia/reperfusion; RIPerC, remote ischemic
perconditioning; RIPostC, remote ischemic postconditioning; p-,
phosphorylated; t-, total. |
Discussion
In the present study, RIPerC was combined with
RIPostC in an in vivo rat IR model, and its protective
efficacy was compared with RIPerC and RIPostC alone. The results
demonstrated that RIPerC and RIPostC were equally effective in
providing protection against myocardial IR injury, however, the
combination of RIPerC and RIPostC did not produce additive
protective effects, compared with either treatment alone.
Additionally, the protective activities were found to be associated
with activation of the RISK and SAFE pathways.
Remote ischemic conditioning confers potent
protective effects against myocardial IR injury by conducting brief
periods of IR to a remote organ (2), and RIPerC and RIPostC have been
reported to be beneficial in animal investigations (11,12)
and randomized clinical trials (13,14).
However, not all results are consistent (15,16).
In the present study, the efficacy of RIperC and RIPostC was
investigated by performing four cycles of 5 min occlusion and 5 min
reperfusion to hindlimbs, either during or subsequent to myocardial
ischemia. The results suggested that RIPerC and RIPostC were
equally effective in protecting against myocardial IR injury in
terms of myocardial IS, cell apoptosis, serum troponin I levels and
inflammatory responses, which is consistent with previous studies
demonstrating all three remote conditioning strategies (RIPC,
RIPerC and RIPostC) have similar therapeutic potential for cardiac
IR injury (17).
The mechanisms responsible for the cardioprotective
effects of remote ischemic conditioning remain to be fully
elucidated. Current evidence suggests that the majority of
mechanisms identified for conventional local ischemic conditioning
are also applicable to remote ischemic conditioning. The
well-described RISK and SAFE pathways have been reported to be
involved in remote ischemic conditioning (18,19).
The present study demonstrated that RIPerC and RIPostC increased
the phosphorylation of Akt, Erk1/2, GSK-3β and STAT-3, with no
significant differences between the two procedures, suggesting that
the protective effects of RIPerC and RIPostC are associated with
activation of the RISK and SAFE pathways, is consistent with
previous studies (10,19).
Ischemic conditioning was originally described to be
an 'all or nothing' event (20,21).
Subsequent studies have reported that the protective effect of
ischemic conditioning varies with the strength of the stimulus,
optimized by the number of cycles and duration, suggesting a
dose-dependent response (22,23).
A previous study combined different doses of RIPerC with IPostC,
and the results demonstrated that the combination of the optimized
dose of RIPerC and IPostC offered higher protective potential
against myocardial IR injury, compared with either treatment alone
(10). The present study aimed to
investigate whether the combination of RIPerC and RIPostC, which
can be induced non-invasively using standard blood pressure cuffs,
provide additive protection against myocardial IR injury. However,
the results demonstrated that the combination RIPerC and RIPostC
failed to produce further protective effects against myocardial IR
injury, compared with either alone, as indicated by similar
myocardial IS values, levels of cell apoptosis, serum levels of
troponin I and LV function.
A previous study suggested that the additive
protection induced by the combination of RIPerC and IPostC was
associated with the additional phosphorylation of Akt and ERK1/2
(10), whereas Tamareille et
al (19) reported that the
enhanced protective effects observed with RIPerC + IPostC were
accompanied by increased levels of p-STAT-3. However, in the
present study, none of the above-mentioned kinases were found to be
further activated by the combination of RIPerC and RIPostC. Taken
together, the presents study hypothesized that there is a certain
mechanistic aspect of IPostC, which is not shared by RIPostC, and
that the difference may lie upstream of the RISK and SAFE pathways,
and that the 'passive' effects of IPostC may be involved (24). The immediate full-flow reperfusion
of the coronary artery following lethal ischemia has been found to
lead to sudden changes in the extracellular environment, including
altered osmolarity, ion concentrations and pH, which may lead to
intracellular edema, opening of the mitochondrial permeability
transition pore and myocardial cell death (25). Local IPostC at the onset of
reperfusion may function as a type of gradual reperfusion, to
reduce the severity of these changes, thereby protecting the
myocardium against the potentially lethal consequences (26,27).
This hypothesis is supported by the fact that IPostC is only
effective in the first ~3 mins of reperfusion (26,28),
whereas RIPostC has a wider time frame. However, further
investigations are required to in order to clarify the mechanistic
differences between local and remote ischemic conditioning.
It has been suggested that the inflammatory response
is also important in the mechanism of myocardial IR injury, and
local and remote ischemic conditioning have been identified to
exhibit anti-inflammatory effects (29,30).
The present study demonstrated that RIPerC, RIPostC and the two in
combination significantly alleviated the systemic inflammatory
response induced by myocardial IR injury, as indicated by reduced
serum levels of TNF-α and IL-1β, with no significant difference
among the groups, consistent with previous studies (31,32).
In the present study, an optimized dose (number of
cycles and duration) of the RIPerC stimulus was determined
according to a previous study (10), and the same algorithm was used for
RIPostC for comparison purposes. However, whether additional cycles
or a longer duration of RIPostC stimulation enhances the
cardioprotective effects of optimized RIPerC remain to be
elucidated. Of note, the lack of further protection by combining
RIPerC and RIPostC can only be interpreted in light of the ischemic
duration and animal model selected in the present study.
In conclusion, the present study demonstrated that
RIPerC and RIPostC were equally effective in protecting against
myocardial IR injury, and that the combination of RIPerC and
RIPostC failed to provide additional protection, compared with
either alone. These cardioprotective effects were found to be
associated with increased activation of the RISK and SAFE
pathways.
Abbreviations:
|
RIPerC
|
remote ischemic perconditioning
|
|
RIPostC
|
remote ischemic postconditioning
|
|
LV
|
left ventricular
|
|
LCA
|
left coronary artery
|
|
IS
|
infarct size
|
|
AAR
|
area at risk
|
|
AN
|
area of necrosis
|
|
SAFE
|
survivor activating factor
enhancement
|
|
RISK
|
reperfusion injury salvage kinase
|
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81100099).
References
|
1
|
Moens AL, Claeys MJ, Timmermans JP and
Vrints CJ: Myocardial ischemia/reperfusion injury, a clinical view
on a complex pathophysiological process. Int J Cardiol.
100:179–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmidt MR, Sloth AD, Johnsen J and Bøtker
HE: Remote ischemic conditioning: The cardiologist's perspective. J
Cardiovasc Med (Hagerstown). 13:667–674. 2012. View Article : Google Scholar
|
|
3
|
Przyklenk K, Bauer B, Ovize M, Kloner RA
and Whittaker P: Regional ischemic 'preconditioning' protects
remote virgin myocardium from subsequent sustained coronary
occlusion. Circulation. 87:893–899. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gho BC, Schoemaker RG, van den Doel MA,
Duncker DJ and Verdouw PD: Myocardial protection by brief ischemia
in noncardiac tissue. Circulation. 94:2193–2200. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lang SC, Elsässer A, Scheler C, Vetter S,
Tiefenbacher CP, Kübler W, Katus HA and Vogt AM: Myocardial
preconditioning and remote renal preconditioning-identifying a
protective factor using proteomic methods? Basic Res Cardiol.
101:149–158. 2006. View Article : Google Scholar
|
|
6
|
Kharbanda RK, Mortensen UM, White PA,
Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K,
Redington AN and MacAllister R: Transient limb ischemia induces
remote ischemic preconditioning in vivo. Circulation.
106:2881–2883. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Candilio L, Hausenloy DJ and Yellon DM:
Remote ischemic conditioning: A clinical trial's update. J
Cardiovasc Pharmacol Ther. 16:304–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skyschally A, van Caster P, Iliodromitis
EK, Schulz R, Kremastinos DT and Heusch G: Ischemic
postconditioning: Experimental models and protocol algorithms.
Basic Res Cardiol. 104:469–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 7th edition. National Academy
Press; Washington DC: 1996
|
|
10
|
Xin P, Zhu W, Li J, Ma S, Wang L, Liu M,
Li J, Wei M and Redington AN: Combined local ischemic
postconditioning and remote perconditioning recapitulate
cardioprotective effects of local ischemic preconditioning. Am J
Physiol Heart Circ Physiol. 298:H1819–H1831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt MR, Smerup M, Konstantinov IE,
Shimizu M, Li J, Cheung M, White PA, Kristiansen SB, Sorensen K,
Dzavik V, et al: Intermittent peripheral tissue ischemia during
coronary ischemia reduces myocardial infarction through a
KATP-dependent mechanism: First demonstration of remote ischemic
perconditioning. Am J Physiol Heart Circ Physiol. 292:H1883–H1890.
2007. View Article : Google Scholar
|
|
12
|
Andreka G, Vertesaljai M, Szantho G, Font
G, Piroth Z, Fontos G, Juhasz ED, Szekely L, Szelid Z, Turner MS,
et al: Remote ischaemic postconditioning protects the heart during
acute myocardial infarction in pigs. Heart. 93:749–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Botker HE, Kharbanda R, Schmidt MR,
Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen
TM, Trautner S, et al: Remote ischaemic conditioning before
hospital admission, as a complement to angioplasty and effect on
myocardial salvage in patients with acute myocardial infarction: A
randomised trial. Lancet. 375:727–734. 2010. View Article : Google Scholar
|
|
14
|
Sloth AD, Schmidt MR, Munk K, Kharbanda
RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT and Bøtker HE;
CONDI Investigators: Improved long-term clinical outcomes in
patients with ST-elevation myocardial infarction undergoing remote
ischaemic conditioning as an adjunct to primary percutaneous
coronary intervention. Eur Heart J. 35:168–175. 2014. View Article : Google Scholar
|
|
15
|
Carrasco-Chinchilla F, Muñoz-García AJ,
Domínguez-Franco A, Millán-Vázquez G, Guerrero-Molina A,
Ortiz-García C, Enguix-Armada A, Alonso-Briales JH,
Hernández-García JM, de Teresa-Galván E and Jiménez-Navarro MF:
Remote ischaemic postconditioning: Does it protect against
ischaemic damage in percutaneous coronary revascularisation?
Randomised placebo-controlled clinical trial. Heart. 99:1431–1437.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sachdeva J, Dai W, Gerczuk PZ and Kloner
RA: Combined remote perconditioning and postconditioning failed to
attenuate infarct size and contractile dysfunction in a rat model
of coronary artery occlusion. J Cardiovasc Pharmacol Ther.
19:567–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu SB, Liu Y, Zhu Y, Yin GL, Wang RP,
Zhang Y, Zhu J and Jiang W: Remote preconditioning, perconditioning
and post-conditioning: A comparative study of their
cardio-protective properties in rat models. Clinics (Sao Paulo).
68:263–268. 2013. View Article : Google Scholar
|
|
18
|
Breivik L, Helgeland E, Aarnes EK, Mrdalj
J and Jonassen AK: Remote postconditioning by humoral factors in
effluent from ischemic preconditioned rat hearts is mediated via
PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic
Res Cardiol. 106:135–145. 2011. View Article : Google Scholar :
|
|
19
|
Tamareille S, Mateus V, Ghaboura N,
Jeanneteau J, Croué A, Henrion D, Furber A and Prunier F: RISK and
SAFE signaling pathway interactions in remote limb ischemic
perconditioning in combination with local ischemic
postconditioning. Basic Res Cardiol. 106:1329–1339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li GC, Vasquez JA, Gallagher KP and
Lucchesi BR: Myocardial protection with preconditioning.
Circulation. 82:609–619. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morris SD and Yellon DM:
Angiotensin-converting enzyme inhibitors potentiate preconditioning
through bradykinin B2 receptor activation in human heart. J Am Coll
Cardiol. 29:1599–1606. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barbosa V, Sievers RE, Zaugg CE and Wolfe
CL: Preconditioning ischemia time determines the degree of glycogen
depletion and infarct size reduction in rat hearts. Am Heart J.
131:224–230. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schulz R, Post H, Vahlhaus C and Heusch G:
Ischemic preconditioning in pigs: a graded phenomenon: its relation
to adenosine and bradykinin. Circulation. 98:1022–1029. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsang A, Hausenloy DJ and Yellon DM:
Myocardial postcon-ditioning: reperfusion injury revisited. Am J
Physiol Heart Circ Physiol. 289:H2–H7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cohen MV and Downey JM: Ischemic
postconditioning: From receptor to end-effector. Antioxid Redox
Signal. 14:821–831. 2011. View Article : Google Scholar
|
|
26
|
Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera
JS, Halkos ME, Kerendi F, Guyton RA and Vinten-Johansen J:
Postconditioning attenuates myocardial ischemia-reperfusion injury
by inhibiting events in the early minutes of reperfusion.
Cardiovasc Res. 62:74–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cohen MV, Yang XM and Downey JM: The pH
hypothesis of postconditioning: staccato reperfusion reintroduces
oxygen and perpetuates myocardial acidosis. Circulation.
115:1895–1903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang XM, Proctor JB, Cui L, Krieg T,
Downey JM and Cohen MV: Multiple, brief coronary occlusions during
early reperfusion protect rabbit hearts by targeting cell signaling
pathways. J Am Coll Cardiol. 44:1103–1110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheung MM, Kharbanda RK, Konstantinov IE,
Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van
Arsdell GS and Redington AN: Randomized controlled trial of the
effects of remote ischemic preconditioning on children undergoing
cardiac surgery: first clinical application in humans. J Am Coll
Cardiol. 47:2277–2282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimizu M, Saxena P, Konstantinov IE,
Cherepanov V, Cheung MM, Wearden P, Zhangdong H, Schmidt M, Downey
GP and Redington AN: Remote ischemic preconditioning decreases
adhesion and selectively modifies functional responses of human
neutrophils. J Surg Res. 158:155–161. 2010. View Article : Google Scholar
|
|
31
|
Albrecht M, Zitta K, Bein B, Wennemuth G,
Broch O, Renner J, Schuett T, Lauer F, Maahs D, Hummitzsch L, et
al: Remote ischemic preconditioning regulates HIF-1α levels,
apoptosis and inflammation in heart tissue of cardiosurgical
patients: A pilot experimental study. Basic Res Cardiol.
108:3142013. View Article : Google Scholar
|
|
32
|
Wang NP, Pang XF, Zhang LH, Tootle S,
Harmouche S and Zhao ZQ: Attenuation of inflammatory response and
reduction in infarct size by postconditioning are associated with
down-regulation of early growth response 1 during reperfusion in
rat heart. Shock. 41:346–354. 2014. View Article : Google Scholar
|