Introduction
Pancreatic cancer is a major cause of mortality in
China, and its incidence has increased in the last decade, with
incidence approaching that of Western countries. At present,
pancreatic cancer remains the fourth leading cause of
cancer-associated mortality in the USA, and the 5-year relative
survival rate is approximately 7.2% (all stages included) (1,2).
If the tumor is resectable, surgical resection is
the only definitive option for treatment of pancreatic cancer
(3). Pancreatic cancer is
characterized by insidious onset, high local invasiveness and early
metastasis (4). Greater than 90%
of patients with pancreatic cancer present with local invasion and
overt metastasis at the time of the occurrence of clinical symptoms
and diagnosis (5). These are the
key factors for the failure of treatment aiming to prevent
recurrence and cancer-associated mortality (5). The 1-year survival rate of patients
who suffer from metastatic pancreatic cancer is lower than 20% and
the majority of patients do not live for longer than 2 years
following diagnosis (6). The
survival rate has not improved substantially during the last 40
years, despite the use of surgery, chemotherapy and radiation
therapy (7). Therefore,
understanding the metastatic mechanisms of pancreatic cancer and
targeted gene therapy for metastasis is important. Previous studies
have investigated the expression of KiSS-1 and its peptide,
metastin/kisspeptin, in pancreatic cancer. It has been observed
that KiSS-1 and kisspeptin are expressed in normal pancreatic
tissue, and the reduced expression levels of KiSS-1 and kisspeptin
are negatively correlated with TNM stage, invasion and metastasis
of pancreatic cancer (8–11). Studies have indicated that
kisspeptin binds to its receptor GPR54 (hOT7T175) and suppresses
the migration of PANC-1 pancreatic cancer cells, and activates ERK1
(9,11–14).
However, the mechanism of KiSS-1-mediated suppression of metastasis
in pancreatic cancer remains unclear. In previous studies,
eukaryotic expression plasmids of KiSS-1 were cloned and
constructed from human pancreatic tissue (15,16).
The present study aimed to investigate whether the metastatic
suppression of KiSS-1 on pancreatic cancer cells was dependent on
the GPR54 expression levels in pancreatic cancer cell lines.
Materials and methods
Materials and reagents
The pancreatic cancer cell lines, BxPC-3 and PANC-1,
were obtained from the American Type Culture Collection (Manassas,
VA, USA). Dulbecco's modified Eagle's medium (DMEM; cat. no.
41965-062), DMEM/F12 (cat. no. 11320-082), fetal bovine serum (FBS;
cat. no. 26140-079), TRIzol® Reagent (cat. no.
15596-018), SuperScript® II Reverse Transcriptase (cat.
no. 18064-014) and Lipofectamine® 2000 Transfection
Reagent (cat. no. 11668-019) were obtained from Invitrogen Life
Technologies (Beijing, China). The iQ SYBR® Green Super
mix (cat. no. 1708882) was purchased from Bio-Rad Laboratories,
Inc. (Shanghai, China), bicinchoninic acid (BCA) protein assay kit
(cat. no. 23227) was obtained from Thermo Fisher Scientific
(Shanghai, China); polyclonal goat anti-human GPR54 (N-14) (cat.
no. sc-48219) and goat anti-rabbit IgG (cat. no. sc-2004) and
donkey anti-goat IgG (cat. no. sc-2005) horseradish
peroxidase-labeled secondary antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Polyclonal rabbit anti
human metastin/kisspeptin (1–54) (cat. no. G-048-59) was purchased
from Phoenix Pharmaceuticals, Inc. (Burlingame, CA, USA).
Polyclonal rabbit anti-β-actin (cat. no. ab8227) was purchased from
Abcam (Pak Shek Kok, Hong Kong). Pierce Enhanced Chemiluminescence
(ECL) Western Blotting kit (cat. no. 32109) was obtained from GE
Healthcare Bio-Sciences (Pittsburgh, PA, USA). The
Vectastain® Elite ABC kit (cat. no. PK-6200) was
obtained from Vector Laboratories (Burlingame, CA, USA) and
BioCoat™ Matrigel™ invasion chambers in two 24-well plates (8
µm pore-size) were purchased from BD Biosciences (San Jose,
CA, USA). The pcDNA3 vector was obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA) and pcDNA3/KISS-1 was produced as
previously described (15).
Cell culture and transfection
The PxPC-3 and PANC-1 pancreatic cancer cell lines
were cultured in DMEM supplemented with 10% FBS, 1% penicillin and
1% streptomycin. Human trophoblast cells were obtained from legal
abortions (6–12 weeks of gestational age), with the approval of the
local ethical committee (in compliance with the Helsinki
Declaration) and the consent of the participating patients.
Trophoblast cells were isolated as described previously (17,18).
The primary trophoblast cells were cultured in DMEM/F12 medium
supplemented with 20% FBS, 1% penicillin and 1% streptomycin. Cells
were cultured at 37°C in a humidified incubator with 5%
CO2. The primary trophoblast cells were used as the
control for the relative mRNA expression of KiSS-1 and GPR54.
At 12 h prior to transfection, cells were seeded
into 6-well plates at a density of 5×105 cells/well.
Cells were transfected when plate confluence had reached 80–90%.
The cells were transfected with 3.3 µg/well PcDNA3.1-KISS-1
vector using 45 µl Lipofectamine® 2000. Following
6 h of incubation at 37°C, the plasmid containing medium was
replaced with normal cell culture medium. Cells were transfected
with the empty pcDNA3 vector as a negative control. All
transfections were performed in triplicate for each time point.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was conducted as previously described with
minor modifications (18).
Briefly, total RNA was extracted from untransfected and transfected
BxPC-3, PANC-1 and trophoblast cells using TRIzol®
Reagent. RNA (500 ng) was converted to cDNA using
SuperScript® II Reverse Transcriptase. RT-qPCR analysis
was performed using the ABI PRISM® 7700 Sequence
Detector (Applied Biosystems Life Technologies, Foster City, CA,
USA). RT-qPCR was conducted using the reagents and instructions of
the iQ SYBR Green Super mix. The PCR primers for human genes KiSS-1
(NM_002256), GPR54 (NM_032551) and the internal control β-actin
(NM_001101) are as previously described (18): KiSS-1, forward ACT CAC TGG TTT CTT
GGC AGC T and reverse CAG AGG CCA CCT TTT CTA ATG G; GPR54, forward
CGA CTT CAT GTG CAA GTT CGTC and reverse CAC ACT CAT GGC GGT CAG
AG; β-actin, forward ACC AAC TGG GAC GAC ATG GAG AAAA and reverse
TAC GGC CAG AGG CGT ACA GGG ATA G. The PCR was performed for 60 sec
at 95°C followed by 40 cycles of 15 sec denaturation at 95°C and 60
sec annealing at 60°C. The RT-qPCR reaction was conducted in
triplicate in a final volume of 25 µl with 100 ng cDNA. The
quantity of cDNA for each experimental gene was normalised to the
quantity of β-actin cDNA in each sample. Relative expression was
determined using the ΔΔCt (threshold cycle) method according to the
manufacturer's instructions.
Western blot analysis
Western blotting was conducted as described
previously (18). Briefly, protein
extracts were prepared from cells by adding modified RIPA lysis
buffer supplemented with protease inhibitor cocktail tablets (Roche
Diagnostics, Basel, Switzerland). Protein concentrations were
quantified using the BCA protein assay. Protein samples (30
µg) were migrated on a 15% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene difluoride membrane (GE Healthcare Bio-Sciences).
The membrane was blocked with blocking buffer (Tris-buffered saline
with Tween 20 with 5% non-fat milk) and incubated with the primary
antibody. The following primary antibodies were used: Rabbit
anti-human metastin/kisspeptin (1–54) (1:800), goat anti-human
GPR54 (N-14) (1:500) and polyclonal rabbit anti-β-actin (1:1,000).
Endogenous β-actin expression served as an internal control.
Primary antibody binding was detected using the following secondary
antibodies: Anti-rabbit IgG and anti-goat IgG antibody conjugated
to horseradish perioxidase (1:10,000; Santa Cruz Biotechnology).
Detection was achieved using the ECL Western Blotting kit (GE
Healthcare Bio-Sciences) and X-ray film (Kodak, Shanghai,
China).
Cell proliferation assay
This method provides a quantitative measurement of
the number of cells with metabolically active mitochondria and is
based on the mitochondrial reduction of a tetrazolium bromide salt
[MTT assay; 3-(4,5-dimethylthiazol-2)-2, 5-diphenyltetrazolium
bromide; cat. no. M5655, Sigma-Aldrich, St. Louis, MO, USA]. Cells
were seeded at a density of 3×103 cells/well in a
96-well plate and cultured in the presence of 10% FBS for 0, 1, 2,
3, 4, 5 and 6 days. The cells were pulsed with MTT 20
µl/well (5 mg/ml in phosphate-buffered saline for 4 h. The
purple-blue MTT formazan precipitate was dissolved in 100 µl
of dimethyl sulfoxide and agitated for 10 min. Absorbance was
measured at 490 nm with a Beckman-DU 640 Spectrophotometer (Beckman
Coulter, Inc., Brea, CA, USA). Experiments were repeated six
times.
Invasion assays
For the invasion assays, BioCoat™
Matrigel® invasion chambers in two 24-well plates with
polycarbonate filters (8 µm) were used. Cells
(5×104 cells/ml) in 500 µl complete medium were
seeded into the upper chamber. Next, 600 µl complete medium
was added to the lower chamber, and the plate was incubated at 37°C
in a 5% CO2 incubator for 48 h. Cells on the lower
surface of the filter were stained with hexamethylpararosaniline
and counted under a light microscope (Nikon Eclipse Ci-S; Nikon
Instruments Inc., Shanghai, China). Each group of cells, which
included BxPC-3 (blank control), BxPC-3/vector (negative control),
BxPC-3/pcDNA3/KiSS-1, PANC-1 (blank control), PANC-1/vector
(negative control) and PANC-1/pcDNA3/KiSS-1, was seeded in three
wells and the experiment was performed in triplicate for these two
different cell lines. Invaded cells were counted in five randomly
selected fields for each filter under a light microscope with 100x
magnification (Nikon Eclipse Ci-S). The invasion index was defined
as follows: (Number of cells that migrated through the 8 µm
pores of the filter in the experimental group/number of cells that
migrated through the filter in the blank control group) ×100.
Statistical analysis
All experiments were conducted a minimum of three
times. Within each experiment, a minimum of three replicates were
used. Data are presented as the mean ± standard deviation. Data
were analyzed using SPSS software, version 16.0 (SPSS, Inc.,
Chicago IL, USA). Analysis of variance was conducted followed by
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of KiSS-1 and its receptor
GPR54 in human pancreatic cancer cell lines
The human pancreatic ductal adenocarcinoma cell
lines BxPc-3 (CRL-1687) and PANC-1 (CRL-1469) were obtained from
the American Type Culture Collection and were grown as recommended.
BxPc-3 cells were originally derived from a 61-year-old Caucasian
female with a well differentiated pancreatic carcinoma without any
evidence of metastasis. PANC-1 cells were derived from a primary
pancreatic ductal adenocarcinoma in a 56-year-old Caucasian male
that extended to involve the duodenal wall and had metastasized to
one peripancreatic lymph node.
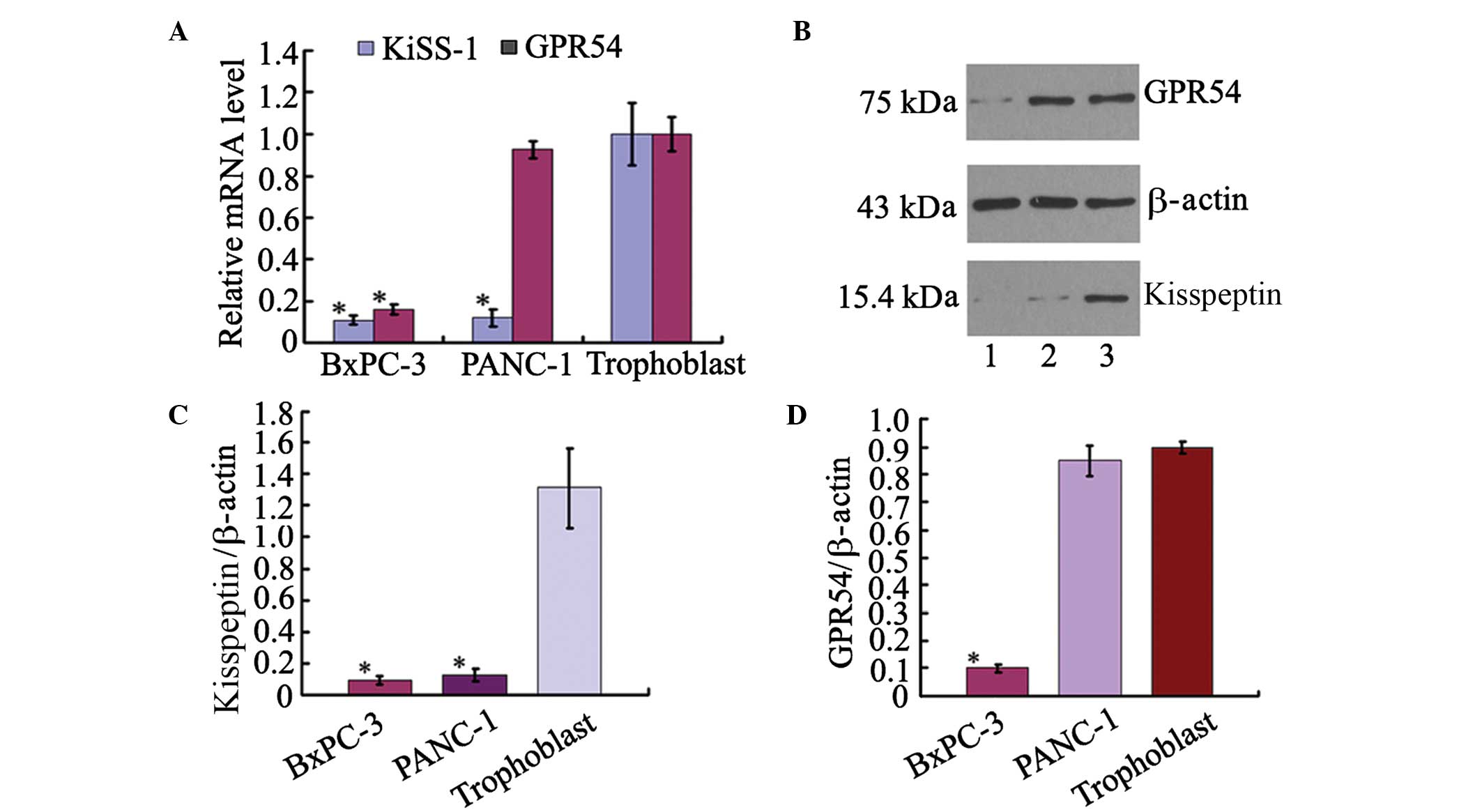
To determine the expression levels of KiSS-1 and its
receptor GPR54 in pancreatic ductal adenocarcinoma, the levels of
KiSS-1 and GPR54 mRNA were measured using RT-qPCR in cultured
pancreatic ductal adenocarcinoma cells. Compared with the primary
cultured human trophoblast cells, KiSS-1 mRNA was expressed at
reduced levels in BxPc-3 and PANC-1 cells, and the relative
expression of KiSS-1 mRNA was 10% of that in human trophoblast
cells (Fig. 1A). The protein
levels of kisspeptin and GPR54 were measured using western
blotting. The expression level of kisspeptin was observed to be low
in BxPc-3 and PANC-1 cells compared with trophoblast cells
(Fig. 1B and C). GPR54 mRNA
expression was reduced in BxPc-3 cells, with levels 15.9% of that
in human trophoblasts. However, the expression of GPR54 mRNA in
PANC-1 cells was 92.7% of that in human trophoblast cells, 5.83
fold higher than in BxPc-3 cells (Fig.
1A). The protein expression level of GPR54 was significantly
higher in PANC-1 cells than that in BxPc-3 cells, as detected by
western blotting (Fig. 1D).
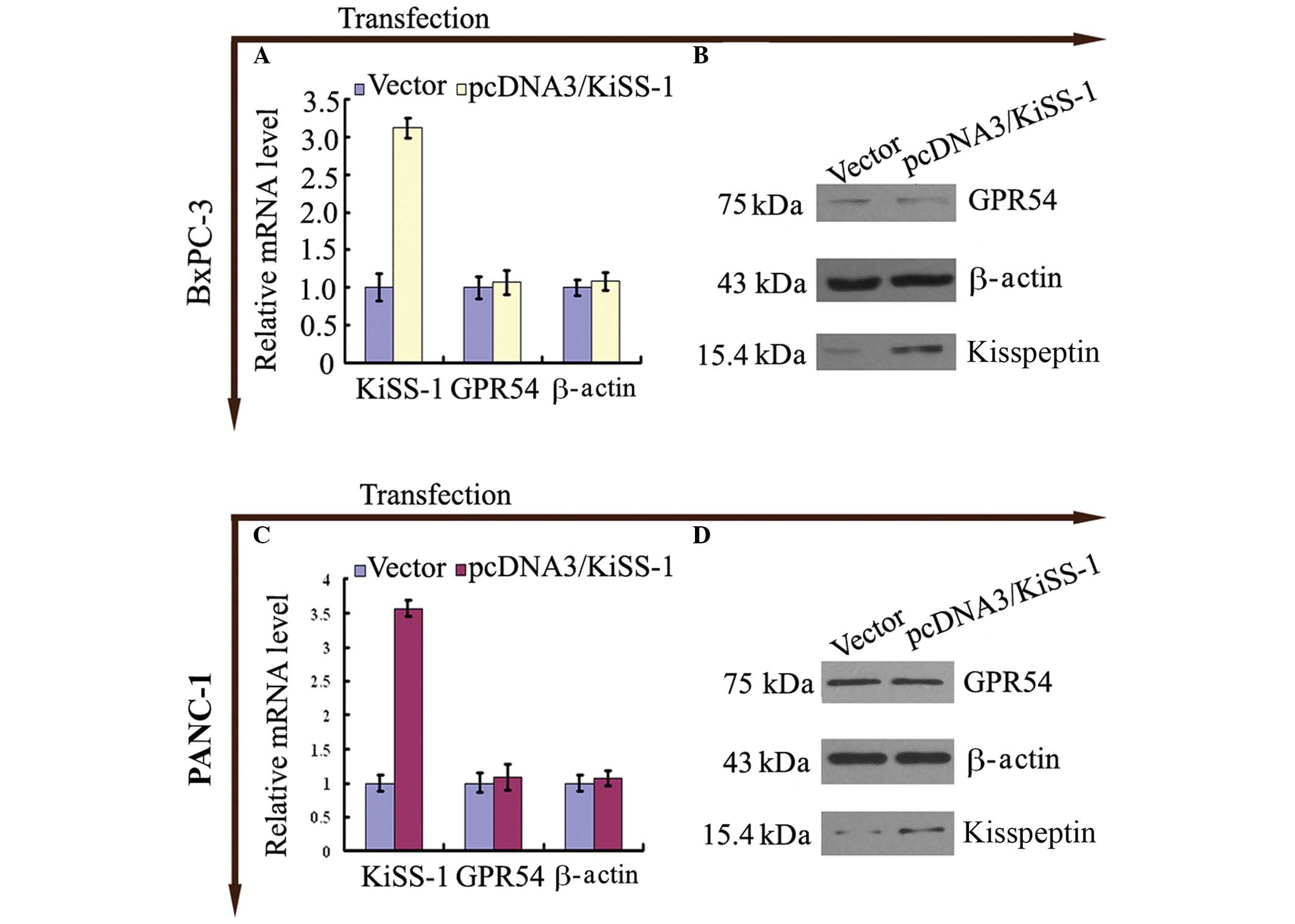
Transfection of KiSS-1 increases
expression of KiSS-1 with no significant alterations in GPR54
expression
Following trans-fection of BxPc-3 and PANC-1 cells
with pcDNA3-KiSS-1, an increase in the expression levels of KiSS-1
mRNA (Fig. 2A and C) and
kisspeptin (Fig 2B and D) was
observed (P<0.05; Fig. 2).
The expression of GPR54 in BxPc-3 and PANC-1 cells
transfected with pcDNA3-KiSS-1 was not altered compared with
control cells (P>0.05; Fig.
2).
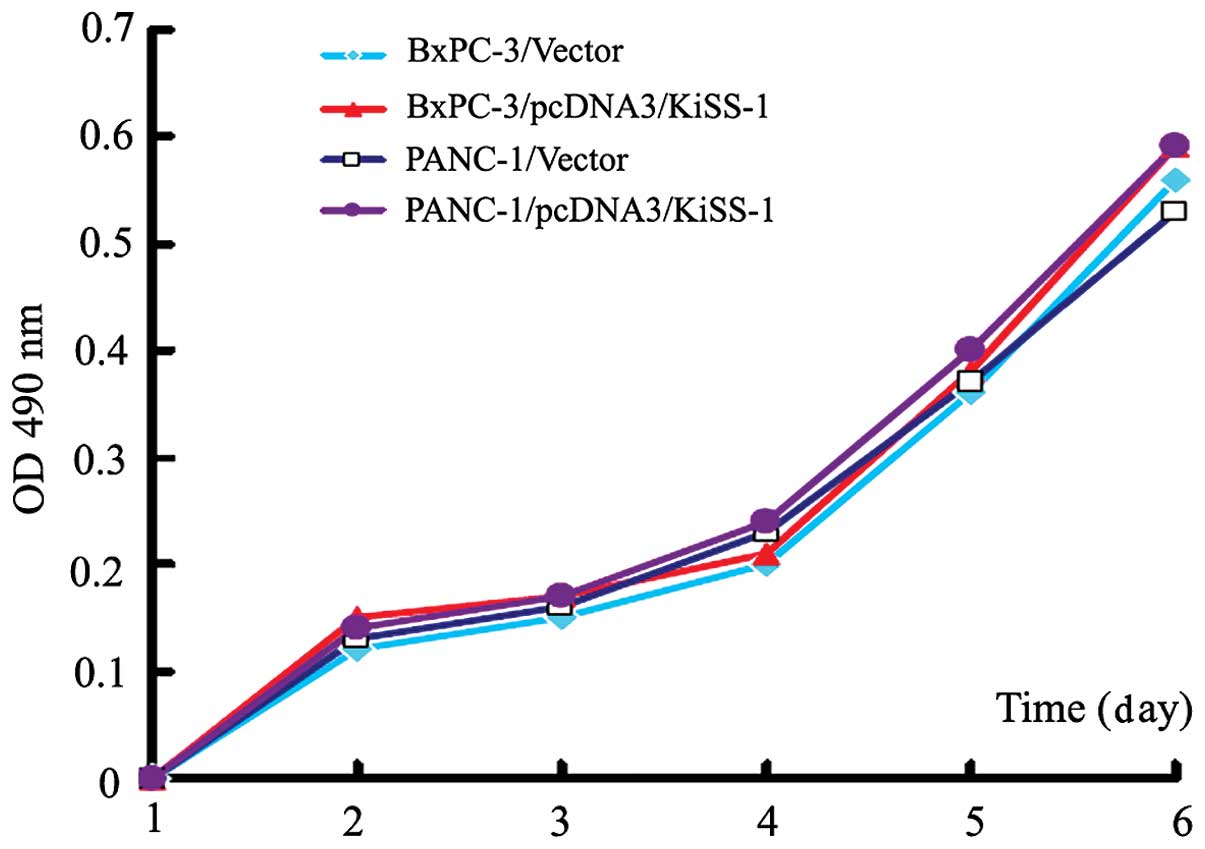
Overexpression of KiSS-1 does not alter
cellular proliferation in BxPc-3 and PANC-1 cells
The effect of KiSS-1 overexpression on the
proliferation of pancreatic cancer cells was investigated.
Following transfection, the absorbance values (490 nm) of
pcDNA3-KiSS-1-transfected BxPc-3 and PANC-1 cells did not differ
from those of control cells (P>0.05). These results indicate
that KiSS-1 overexpression had no effect on the proliferation of
BxPc-3 and PANC-1 cells (Fig.
3).
Overexpression of KiSS-1 suppresses cell
invasion
The invasive ability of BxPc-3 and PANC-1 cells was
measured by counting the number of cells that digested Matrigel and
migrated through the 8 µm pores in the filter. The invasion
of BxPc-3 and PANC-1 cells was observed to be significantly reduced
by overexpression of KiSS-1, with invasion indices of 50.8±4.1% and
48.3±4.3%, respectively (P<0.05; Fig. 4). These data indicate that
overexpression of KiSS-1 is capable of inhibiting the invasion of
BxPc-3 and PANC-1 cells (Fig.
4).
Discussion
The onset of local invasion and lymphatic metastasis
of pancreatic cancer limits the survival rate following surgical
intervention and other therapies (19,20).
The majority of cases of pancreatic cancer-associated mortality are
due to complications resulting from tumor metastasis rather than as
a consequence of the original tumor growth. There is increasing
interest in understanding the metastatic mechanisms of pancreatic
cancer in order to identify possible ways to inhibit local invasion
and metastatic cancer progression.
Previous studies have demonstrated that the
expression levels of KiSS-1 mRNA in pancreatic cancer are lower
compared with normal pancreatic tissue (8,10).
Additionally, there are correlations between KiSS-1 mRNA expression
levels and clinical stage, metastasis and nerve invasion, without
any correlation with histological classification (10). The pancreatic cancer cell line
BxPC-3 is highly differentiated without any metastasis whilst
PANC-1 is poorly differentiated with local and lymph node
metastasis. Based on their different differentiation degrees and
metastatic potential, BxPC-3 and PANC-1 cells were selected for the
current study.
The mRNA and protein expression levels of KiSS-1 and
its receptor GPR54 were measured in BxPc-3 and PANC-1 cells.
Numerous previous studies have indicated that KiSS1 and its
receptor hOT7T175/GPR54 are expressed in human placental and
pancreatic tissues (21–23). Previously, studies have
investigated the mRNA and protein expression of KiSS-1 in
trophoblasts and the placentas of normal and preeclamptic
pregnancies, and observed that KiSS-1 is highly expressed (17,18).
Therefore, primary cultured trophoblasts were selected as the
control for the present study. In the present study, the expression
level of KiSS-1 mRNA was observed to be low in BxPc-3 and PANC-1
cells. However, the expression of GPR54 mRNA was higher in PANC-1,
with levels comparable to those of human primary cultured
trophoblasts, while the expression levels of GPR54 mRNA were low in
BxPC-3 cells. Consistent with these results, Masui et al
(12) observed low levels of
KiSS-1 mRNA expression in BxPC-3, Capan-2, CFPAC-1 and PANC-1
cells, whilst PANC-1 cells demonstrated high expression levels of
hOT7T175/GPR54 mRNA
In the present study, the protein levels of KiSS-1
and GPR54 in the BxPC-3 and PANC-1 pancreatic cancer cell lines
were investigated using western blotting. This demonstrated that
kisspeptin expression was minimal in BxPC-3 and PANC-1 cells.
However, the protein level of GPR54 was increased in PANC-1 cells
and reduced in BxPC-3 cells. Nagai et al (24) analyzed 53 cases of pancreatic
ductal carcinoma and observed that kisspeptin and GPR54 expression
was significantly correlated with tumor size, recurrence and
survival of patients, however was not correlated with the degree of
tissue differentiation. This indicated that kisspeptin may serve a
role in the metastasis of pancreatic cancer. In the current study,
BxPC-3 cells were selected as a representative cell line with low
expression of KiSS-1 and GPR54, whilst PANC-1 cells were a
representative cell line with low expression of KiSS-1 and high
expression of GPR54. In the current study, overexpression of KiSS-1
had no effect on cellular proliferation in BxPC-3 and PANC-1 cells,
which is consistent with previous studies in melanoma and breast
cancer (25,26). As Masui et al (12) reported, the addition of kisspeptin
had no effect on the proliferation of BxPC-3 and PANC-1 cells.
Together, this suggests that KiSS-1 and kisspeptin do not affect
the proliferative capacity of human pancreatic cancer cell
lines.
In the current study, it was demonstrated that the
invasive ability of pancreatic cancer cells was significantly
reduced following the overexpression of KiSS-1, whilst the empty
vector had no effect. It was observed that overexpression of KiSS-1
did not have differential effects upon the two pancreatic cancer
cell lines, suggesting that the inhibitory effect on invasion of
pancreatic cancer is not dependent on the degree of
differentiation. Shirasaki et al (26) observed that the expression of
KiSS-1 was lost during the progression of melanocytic tumors in
vivo. Jiang et al (27)
reported that restoring KiSS-1 expression was able to significantly
suppress the metastasis of ovarian cancer and melanoma. The
MDA-MB-435 ductal breast carcinoma cell line is metastatic and does
not express KiSS-1, however when full-length KiSS-1 cDNA was
transfected into MDA-MB-435 cells and injected into the mammary fat
pads of athymic nude mice, lung metastasis was significantly
suppressed and the incidence of regional lymph node metastasis was
reduced (25). Masui et al
(12) examined the effect of
exogenous kisspeptin on pancreatic cancer cell proliferation in
AsPC-1 and PANC-1 cells, and observed that the migration of AsPC-1
cells was not altered by kisspeptin, while PANC-1 cells were
significantly inhibited by kisspeptin. Furthermore, the effect of
exogenous kisspeptin on the invasion of these cell lines was
associated with the expression level of GPR54/hOT7T175 (12). In a model of melanoma, secretion of
KiSS-1 was required for metastasis suppressor activity (28). When KiSS-1 was transfected into a
metastatic subclone of the SUIT-2 pancreatic adenocarcinoma cell
line, S2VP10, and injected into the tail of the pancreas of severe
combined immunodeficiency mice, mice with KiSS-1 expression
developed fewer liver and lung metastases than the controls
(29). Furthermore, it was
observed that the re-expression of KiSS-1 in S2VP10 without
expression of GPR54 resulted in suppression of invasion. These
observations indicate the existence of an intracrine signaling loop
for KiSS-1. Consistent with the results reported by McNally et
al (29), the current study
also demonstrated that the expression of KiSS-1 mRNA and kisspeptin
in BxPC-3 and PANC-1 cells increased following transfection with
KiSS-1, whilst the expression level of GPR54 mRNA and protein was
unaltered. In the current study, GPR54 was observed exhibit greater
expression in PANC-1 cells and lower expression in BxPC-3 cells.
Following transfection with KiSS-1, the invasive ability of these
cell lines was suppressed, however the proliferative ability was
not altered. This suggests that the suppression of invasion
mediated by KiSS-1 overexpression was not dependent on the receptor
expression levels.
In the intracrine signaling loop, certain cytokines
exert their biological function by binding to their receptor in the
cytoplasm without the requirement to be secreted out of the cell
(30). In this way, certain
chemokines and peptide hormones serve an important role in cell
apoptosis, migration, invasion and metastasis (30,31).
Previous studies have indicated that intracrine signaling occurs in
pancreatic adenocarcinoma (29,32,33).
Alternatively, there is a possibility that other peptide fragments
of KiSS-1, which do not bind GPR54, are released to the
extracellular space, however further studies investigating this are
required. In an electrophoretic mobility shift assay, KiSS-1 was
demonstrated to inhibit the binding of nuclear factor-κB and the
matrix metal-loproteinase-9 (MMP-9) promoter, thereby reducing
MMP-9 gene transcription and the level of MMP-9, inhibiting the
mobility, chemotaxis and invasion of tumor cells (34). It was additionally identified that
KiSS-1 gene transfection significantly inhibited MMP-9 gene
transcription, and reduced the expression of MMP-9 protein. It is
suggested that KiSS-1 suppression of the pancreatic cancer
metastasis mechanism is predominantly associated with the
inhibition of MMP-9 transcription and the intracellular autocrine
loop (34).
In conclusion, the current study demonstrated that
increases in KiSS-1 expression suppressed the invasion of
pancreatic cancer cells without impacting cellular proliferation.
Therefore, targeted gene therapy to increase the expression levels
of KiSS-1 and its peptides may be a potential therapeutic strategy
for the treatment of pancreatic cancer metastasis.
Acknowledgments
The current study was supported by grants from the
People's Liberation Army Medical Science and Technique Foundation
during the 11th Five-Year Plan for Science Technology (Young
Scholar Program; grant no. 06Q014), the National Natural Science
Foundation of China (General Program; grant no. 81370735) and the
Liaoning Provincial Natural Science Foundation (Doctor Startup Fund
Program; grant no. 20071038).
References
|
1
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Institute: Cancer
statistics: SEER stat fact sheets: Pancreas cancer. http://seer.cancer.gov/statfacts/html/pancreas.html.
Accessed July 1, 2014.
|
|
3
|
Shaib Y, Davila J, Naumann C, et al: The
impact of curative intent surgery on the survival of pancreatic
cancer patients: a U.S. population-based study. Am J Gastroenterol.
102:1377–1382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwon D, McFarland K, Velanovich V and
Martin RC II: Borderline and locally advanced pancreatic
adenocarcinoma margin accentuation with intraoperative irreversible
electroporation. Surgery. 156:910–920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuveson DA and Neoptolemos JP:
Understanding metastasis in pancreatic cancer: A call for new
clinical approaches. Cell. 148:21–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zakharova OP, Karmazanovsky GG and Egorov
VI: Pancreatic adenocarcinoma: Outstanding problems. World J
Gastrointest Surg. 4:104–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang C, Qiao C, Ma S, Zhou W and Dai X:
Expression of KiSS-1 in human pancreatic cancer and relationship
with their invasion and metastasis. China J Mod Med. 15:1620–1623.
16312005.
|
|
9
|
Makri A, Pissimissis N, Lembessis P,
Polychronakos C and Koutsilieris M: The kisspeptin (KiSS-1)/GPR54
system in cancer biology. Cancer Treat Rev. 34:682–692. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Qiao C and Dai X: Expression of
KiSS-1 in human pancreatic cancer and its clinical significance.
Chin J Cancer Prev Treat. 13:207–215. 2006.
|
|
11
|
Ji K, Ye L, Mason MD and Jiang WG: The
Kiss-1/Kiss-1R complex as a negative regulator of cell motility and
cancer metastasis (Review). Int J Mol Med. 32:747–754.
2013.PubMed/NCBI
|
|
12
|
Masui T, Doi R, Mori T, Toyoda E, Koizumi
M, Kami K, Ito D, Peiper SC, Broach JR, Oishi S, et al: Metastin
and its variant forms suppress migration of pancreatic cancer
cells. Biochem Biophys Res Commun. 315:85–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szereszewski JM, Pampillo M, Ahow MR,
Offermanns S, Bhattacharya M and Babwah AV: GPR54 regulates ERK1/2
activity and hypothalamic gene expression in a Gα(q/11) and
β-arrestin-dependent manner. PLoS One. 5:e129642010. View Article : Google Scholar
|
|
14
|
Francis VA, Abera AB, Matjila M, Millar RP
and Katz AA: Kisspeptin regulation of genes involved in cell
invasion and angiogenesis in first trimester human trophoblast
cells. PLoS One. 9:e996802014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang C, Qiao C and Dai X: Cloning of human
KiSS-1 gene and construction of its eukaryotic expression vector. J
Clin Med Univ. 34:218–219. 2005.In Chinese.
|
|
16
|
Li N, Wang HX, Zhang J, Ye YP and He GY:
KISS-1 inhibits the proliferation and invasion of gastric carcinoma
cells. World J Gastroenterol. 18:1827–1833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiao C, Wang CH, Shang T and Lin QD:
Clinical significance of KiSS-1 and matrix metalloproteinase-9
expression in trophoblasts of women with preeclampsia and their
relation to perinatal outcome of neonates. Zhonghua Fu Chan Ke Za
Zhi. 40:585–590. 2005.In Chinese. PubMed/NCBI
|
|
18
|
Qiao C, Wang C, Zhao J, Liu C and Shang T:
Elevated expression of KiSS-1 in placenta of Chinese women with
early-onset preeclampsia. PLoS One. 7:e489372012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boyle J, Czito B, Willett C and Palta M:
Adjuvant radiation therapy for pancreatic cancer: A review of the
old and the new. J Gastrointest Oncol. 6:436–444. 2015.PubMed/NCBI
|
|
20
|
Sinn M, Striefler JK, Sinn BV, Sallmon D,
Bischoff S, Stieler JM, Pelzer U, Bahra M, Neuhaus P, Dörken B, et
al: Does long-term survival in patients with pancreatic cancer
really exist? Results from the CONKO-001 study. J Surg Oncol.
108:398–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muir AI, Chamberlain L, Elshourbagy NA,
Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM,
Chambers JK, Murdock P, et al: AXOR12, a novel human G
protein-coupled receptor, activated by the peptide KiSS-1. J Biol
Chem. 276:28969–28975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohtaki T, Shintani Y, Honda S, Matsumoto
H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, et
al: Metastasis suppressor gene KiSS-1 encodes peptide ligand of a
G-protein-coupled receptor. Nature. 411:613–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JH, Miele ME, Hicks DJ, Phillips KK,
Trent JM, Weissman BE and Welch DR: KiSS-1, a novel human malignant
melanoma metastasis-suppressor gene. J Natl Cancer Inst.
88:1731–1737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagai K, Doi R, Katagiri F, Ito T, Kida A,
Koizumi M, Masui T, Kawaguchi Y, Tomita K, Oishi S, et al:
Prognostic value of metastin expression in human pancreatic cancer.
J Exp Clin Cancer Res. 28:92009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JH and Welch DR: Suppression of
metastasis in human breast carcinoma MDA-MB-435 cells after
transfection with the metastasis suppressor gene, KiSS-1. Cancer
Res. 57:2384–2387. 1997.PubMed/NCBI
|
|
26
|
Shirasaki F, Takata M, Hatta N and
Takehara K: Loss of expression of the metastasis suppressor gene
KiSS1 during melanoma progression and its association with LOH of
chromosome 6q16.3-q23. Cancer Res. 61:7422–7425. 2001.PubMed/NCBI
|
|
27
|
Jiang Y, Berk M, Singh LS, Tan H, Yin L,
Powell CT and Xu Y: KiSS1 suppresses metastasis in human ovarian
cancer via inhibition of protein kinase C alpha. Clin Exp
Metastasis. 22:369–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nash KT, Phadke PA, Navenot JM, Hurst DR,
Accavitti-Loper MA, Sztul E, Vaidya KS, Frost AR, Kappes JC, Peiper
SC, et al: Requirement of KISS1 secretion for multiple organ
metastasis suppression and maintenance of tumor dormancy. J Natl
Cancer Inst. 99:309–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McNally LR, Welch DR, Beck BH, Stafford
LJ, Long JW, Sellers JC, Huang ZQ, Grizzle WE, Stockard CR, Nash
KT, et al: KISS1 over-expression suppresses metastasis of
pancreatic adenocarcinoma in a xenograft mouse model. Clin Exp
Metastasis. 27:591–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Re RN: The origins of intracrine hormone
action. Am J Med Sci. 323:43–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gortz A, Nibbs RJ, McLean P, Jarmin D,
Lambie W, Baird JW and Graham GJ: The chemokine ESkine/CCL27
displays novel modes of intracrine and paracrine function. J
Immunol. 169:1387–1394. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leung PS: The physiology of a local
renin-angiotensin system in the pancreas. J Physiol. 580:31–37.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lau ST and Leung PS: Role of the RAS in
pancreatic cancer. Curr Cancer Drug Targets. 11:412–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan C, Wang H and Boyd DD: KiSS-1
represses 92-kDa type IV collagenase expression by down-regulating
NF-kappaB binding to the promoter as a consequence of Ikappa Balpha
-induced block of p65/p50 nuclear translocation. J Biol Chem.
276:1164–1172. 2001. View Article : Google Scholar
|