Introduction
Mast cells are major effector cells of allergic
inflammatory reactions with considerable influence on the
pathogenesis of a number of disorders, including contact asthma,
allergic rhinitis, tissue remodeling, rheumatoid arthritis and
anaphylaxis (1,2). Mast cells are activated by the
process of degranulation in response to antigen cross-linking of
immunoglobulin E (IgE) bound to the high-affinity IgE receptor (Fcε
RI), which results in phosphorylation of Syk tyrosine kinase,
mobilization of internal calcium, activation of protein kinase C,
mitogen-activated protein kinases (MAPKs), nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) and release of
inflammatory cytokines (3,4). Mast cell activation and degranulation
releases inflammatory mediators, such as histamine, and chemotactic
cytokines, including tumor necrosis factor (TNF)-α and interleukin
(IL)-6. Various acute and chronic allergic responses are induced by
these mediators (5,6).
Lignans from Sesamum indicum (sesame) seeds
are potent antioxidants, the most abundant of these lignans in
sesame seed oil is sesamin (7).
Sesamin inhibits lipopolysaccharide-induced IL-6 production by
suppressing p38 MAPK and NF-κB activation in murine microglia and
BV-2 cells (8). Sesame seed oil
accelerates recovery from colon inflammation in rats with induced
acute colitis by inhibiting inflammatory processes and sesamin
suppresses macrophage-derived chemokine expression in human
monocytes (9,10). However, the association between
sesamin and mast cell-mediated anaphylactic reactions is poorly
understood.
The aim of the present study was to assess the
effect of sesamin on inflammatory allergic reactions, and to
investigate the molecular mechanisms underlying the inhibitory
effect of sesamin on histamine release and pro-inflammatory
cytokine production in mast cells. In addition, the effect of
sesamin on systemic and local allergic reactions was examined to
assess its anti-allergic effect in vivo.
Materials and methods
Reagents and cell culture
Sesamin, anti-2,4-dinitrophenyl (DNP) IgE, DNP-human
serum albumin (HSA), phorbol 12-myristate 13-acetate (PMA), calcium
ionophore A23187, pyrrolidine dithiocarbamate (PDTC), azelastine,
anti-β actin antibodies, and HEPES were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Percoll solution was purchased
from Pharmacia Biotech (Uppsala, Sweden). Sesamin was dissolved in
a vehicle consisting of 0.5% (w/v) carboxy methylcellulose (Boster
Biological Technology Co., Ltd., Wuhan, China) and 0.025% Tween-20
(Sigma-Aldrich) in distilled water.
Animals
Male ICR mice (n=60; 6 weeks old; 25–30 g) and male
Sprague Dawley rats (n=10; 8 weeks old; 230–280 g) were obtained
from the in-house animal facility of Yanbian University Health
Science Center (Yanji, China). The animals were housed 3–5 per cage
in a laminar air-flow cabinet that was maintained at a temperature
of 22±1°C and relative humidity of 55±10%, under a 12-h light/dark
cycle for 1 week prior to the experiments. Water and a standard
diet were provided ad libitum throughout the study. The
experiments were performed in compliance with the guidelines
approved by the Institutional Animal Care and Use Committee of
Yanbian University School of Basic Medical Sciences (Yanji,
China).
Anti-DNP IgE-mediated passive cutaneous
anaphylaxis (PCA)
Anti-DNP IgE-mediated PCA was examined as reported
previously (11). The PCA reaction
was generated by sensitizing skin with an intradermal injection of
500 ng anti-DNP IgE in 50 ml phosphate-buffered saline (PBS). After
24 h, each mouse received an injection of 20 ml PBS containing 100
mg antigen, DNP-HSA, and 1% Evans blue (Sigma-Aldrich) via the tail
vein. Sesamin (50, 100 or 200 mg/kg) was admin istered orally 1 h
prior to the antigen challenge. Azelastine, an anti-histamine and
mast cell stabilizing agent, was orally administered at a dose of
10 mg/kg 1 h prior to the antigen challenge. The mice were
sacrificed by terminal anesthesia via intraperitoneal injection of
pentobarbital (50 mg/kg; Boster Biological Technology Co., Ltd.) 30
min subsequent to the antigen challenge and the dorsal skin around
the intradermal injection site was removed to weigh the pigmented
area, which was followed by extraction of the extravasated Evans
blue dye by incubation of the biopsies in 1 ml formamide at 55°C
for 24 h. The absorbance of the dye was measured at 620 nm using a
Spectra Max Plus spectrophotometer (Molecular Devices, LLC,
Sunnyvale, CA, USA). The concentrations of Evans blue dye were
quantified by interpolation on a standard curve of dye
concentrations in the range of 0.01–30 mg/ml.
Preparation of rat peritoneal mast cells
(RPMC)
RPMCs were isolated as described previously
(12). The rats were anesthetized
with 5 ml/L ether, 10 ml calcium-free HEPES-Tyrode buffer (137 mM
NaCl, 5.6 mM glucose, 12 mM NaHCO3, 2.7 mM KCl, 0.3 mM
NaH2PO4 and 0.1% gelatin; Sigma-Aldrich) was
then injected into the peritoneal cavity, and the abdomen was
gently massaged for ~90 sec. The peritoneal cavity was opened
carefully and the fluid that contained peritoneal cells was
collected by Pasteur pipette. RPMCs were purified using a Percoll
density gradient, as described previously (13). RPMC preparations were ~95% pure, as
assessed by toluidine blue staining (Sigma Aldrich), and >98% of
the cells were viable, as determined by Trypan blue uptake.
Purified mast cells (1×106 cells/ml) were resuspended in
HEPES-Tyrode buffer.
Mast cell viability assay
Mast cell viability was determined and the MTT
colorimetric assay was performed, as described previously (14). RPMCs were incubated with various
concentrations of sesamin (25–100 µg/ml) at 37°C for 2 h.
Following the addition of MTT (100 mg in 100 ml PBS), RPMCs were
incubated at 37°C for 1 h, and absorbance was measured at 570 nm
with the spectrophotometer.
Histamine release assay
The histamine content of RPMCs was evaluated
according to a radioenzymatic method, as previously described
(15). Purified RPMCs were
sensitized with 10 µg/ml anti-DNP IgE for 6 h and
preincubated with sesamin (25, 50 and 100 µM) or azelastine
(100 µM) at 37°C for 30 min prior to challenge with DNP-HSA
(100 ng/ml). Following centrifugation at 150 × g for 10 min at 4°C,
the supernatant was harvested for measurement of histamine content.
The percentage inhibition of histamine release was calculated
according to the following formula: Inhibition (%) =
[1−(T−B)/(C−N)]×100, where C is the control (IgE without sesamin),
N is the normal group (no IgE and no sesamin), T is the test group
(IgE and sesamin) and B is the blank group (sesamin without
IgE).
Measurement of 45Ca
uptake
The calcium uptake of the mast cells was measured
using the procedure of Choi et al (16). RPMCs were resuspended in
HEPES-Tyrode buffer containing 45Ca (1.5 mCi/ml; 1 Ci =
3.7×1010 Bq; Perkin-Elmer Inc., Waltham, MA, USA) at 4°C
for 10 min. Mast cell suspensions were sensitized with 10
µg/ml anti-DNP IgE for 6 h and preincubated with prewarmed
buffer containing sesamin. The reaction proceeded for 30 min at
37°C prior to the challenge with DNP-HSA (100 ng/ml) and was
terminated by the addition of 1 mM lanthanum chloride
(Sigma-Aldrich). The samples were centrifuged three times at 150 ×
g for 10 min, and mast cells were lysed with 10% Triton X-100
(Sigma-Aldrich) with vigorous agitation. Sample radioactivity was
determined with a scintillation β-counter (B1600/1600 Tri-Carb
Liquid Scintillation Analyzer; Canberra Industries, Inc., Meriden,
CT, USA).
Assay of TNF-α and IL-6 secretion
The HMC-1 human mast cell line (American Type
Culture Collection, Manassas, VA, USA) was used. The cells were
treated with 20 nM PMA and 1 µM A23187 for 4 h in the
absence or presence of various concentrations of sesamin (25, 50
and 100 µM). TNF-α and IL-6 concentrations in the
supernatant were determined using Biosource ELISA kits according to
the manufacturer's instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA).
Western blot analysis
HMC-1 cells were treated with 20 nM PMA and 1
µM A23187 for 4 h in the absence or presence of sesamin (25,
50 or 100 µM). Cell extracts were prepared by using a
detergent lysis procedure, as described previously (17). Samples of protein (30 µg)
were loaded per lane and subject to 12% SDS-PAGE (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 120 V for 90 min.
Separated proteins were transferred to polyvinylidene difluoride
(PVDF) membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
p38 MAPK, extracellular signal-regulated kinases (ERK) and c-Jun
N-terminal kinase (JNK) activation were determined using rabbit
monoclonal anti-p38 MAPK (D13E1; cat. no. 8690s; 1:1000),
phosphorylated (p)-p38 MAPK (Thr180/Tyr182; 3D7; cat. no. 4092s;
1:1,000), p-p44/42 MAPK (Erk1/2; Thr202/Tyr204; 197G2; cat. no.
14227s; 1.1000), p44/42 MAPK (Erk1/2; 137F5; cat. no. 4348s;
1:1,000), P-SAPK/JNK (Thr183/Tyr185; 81E11; cat. no. 4668s;
1:1,000) and SAPK/JNK (cat, no, 9252s; 1:1,000) antibodies (Cell
Signaling Technology, Inc., Beverly, MA, USA). TNF-α and IL-6 were
measured using rabbit polyclonal anti-TNF-α (h-156; cat. no.
sc-8301; 1:1,000) and anti-IL-6 (H-183; cat. no. sc-7920; 1:1,000)
antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
incubated at 4°C for 2 h. Immunodetection was performed using an
enhanced chemiluminescence detection kit (Amersham ECL Select
Detection System; Amersham Pharmacia Biotech).
Nuclear protein extraction for analysis
of NF-κB
Nuclear protein was extracted according to the
procedure of Choi and Yan (18).
Cells were harvested, washed three times and lysed in 2 volumes of
lysis buffer A [50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 10% glycerol,
0.5 mM dithiothreitol, 5 mM MgCl2, 1 mM
phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor
cocktail; Sigma-Aldrich] for 5 min at 4°C. Subsequently, the cell
suspension was centrifuged at 1,000 × g for 15 min at 4°C. The
supernatant fraction was incubated on ice for 10 min and
centrifuged at 100,000 × g for 1 h at 4°C to obtain cytosolic
protein extracts. The pelleted nuclei were resuspended in buffer B
(1.3 M sucrose, 1.0 mM MgCl2 and 10 mM potassium
phosphate buffer; pH 6.8; Sigma-Aldrich) and centrifuged at 1,000 ×
g for 15 min. The pellets were suspended in buffer B with a final
sucrose concentration of 2.2 M and centrifuged at 100,000 × g for 1
h. The resulting nuclear pellets were washed once with a solution
containing 0.25 M sucrose, 0.5 mM MgCl2 and 20 mM
Tris-HCl (pH 7.2) and centrifuged at 1,000 × g for 10 min. The
pellets were solubilized with a solution containing 50 mM Tris-HCl
(pH 7.2), 0.3 M sucrose, 150 mM NaCl, 2 mM EDTA, 20% glycerol, 2%
Triton X-100, 2 mM PMSF and protease inhibitor cocktail. The
mixture was maintained on ice for 1 h with gentle stirring and
centrifuged at 12,000 × g for 30 min. The resulting supernatant
served as the soluble nuclear protein sample. For western blot
analysis, samples (30 µg protein per lane) were subjected to
10% SDS-PAGE at 120 V for 90 min, and separated proteins were
transferred to PVDF membranes using the wet transfer method (250
mA; 90 min). Nonspecific sites were blocked with 5% non-fat dry
milk in PBS for 1 h, and the blots were incubated with antibodies
against NF-κB (Upstate Biotechnology, Inc., Lake Placid, NY, USA)
overnight at 4°C. Horseradish peroxidase-conjugated goat
anti-rabbit IgG (cat. no. sc-2004; 1:1,000) was used to detect
bound antibodies. Protein bands were visualized by exposing the
membranes to photographic film (X-OMAT BT photographic film; Kodak,
Rochester, NY, USA) following treatment with the enhanced
chemiluminescence system reagents.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. Statistical evaluation of the results was performed
using one-way analysis of variance followed by Duncan's multiple
range test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sesamin inhibits PCA induced by anti-DNP
IgE
To confirm the anti-allergic effects of sesamin
in vivo, extravasation was induced by a local injection of
anti-DNP IgE into the dorsal skin followed by an intravenous
antigenic challenge in PCA model rats. After 24 h, the animals were
injected intravenously with DNP-HSA and Evans blue dye. Sesamin was
administered orally 1 h prior to the antigen challenge. Sesamin
(50–200 mg/kg) inhibited dye extravasation in a dose-dependent
manner (Table I). Similarly,
azelastine significantly inhibited the PCA reaction at a dose of 10
mg/kg compared with the control (P<0.05).
 | Table IInhibitory effect of sesamin on
anti-DNP IgE-mediated passive cutaneous anaphylaxis in rats. |
Table I
Inhibitory effect of sesamin on
anti-DNP IgE-mediated passive cutaneous anaphylaxis in rats.
| Treatment
(mg/kg) | Anti-DNP IgE | Evans blue
(µg/g) | Inhibition (%) |
|---|
| Sesamin | | | |
| Group N |
| 0 | – | 46.51±4.21 | – |
| Group B |
| 50 | – | 47.23±3.42 | – |
| 100 | – | 48.34±5.25 | – |
| 200 | – | 45.75±4.34 | – |
| Azelastine |
| 10 | – | 44.92±3.89 | – |
| Sesamin |
| Group C |
| 0 | + | 263.82±13.64 | – |
| Group T |
| 50 | + | 235.49±11.28 | 13.37 |
| 100 | + |
186.54±10.81a | 36.40 |
| 200 | + |
162.56±13.26a | 46.25 |
| Azelastine |
| 10 | + | 150.63±9.83a | 51.36 |
Effect of sesamin on mast cell
viability
The viability of RPMCs exposed to sesamin was
determined by MTT assay. RPMC viability was ~100% following
exposure to 100 µM sesamin for 2 h (data not shown). Thus,
sesamin was not cytotoxic in RPMCs.
Sesamin inhibits the release of histamine
induced by IgE in RPMCs
RPMCs were preincubated with sesamin at
concentrations of 25–100 µM. As demonstrated in Fig. 1, sesamin inhibited anti-DNP
IgE-mediated histamine release from RPMCs in a
concentration-dependent manner (13, 43 and 62% inhibition at 25, 50
and 100 µM, respectively). Similarly, azelastine
significantly inhibited histamine release at a dose of 100
µM (75% inhibition; P<0.05). These results suggest that
sesamin inhibits IgE-induced anaphylactic reactions by blocking
histamine release from mast cells.
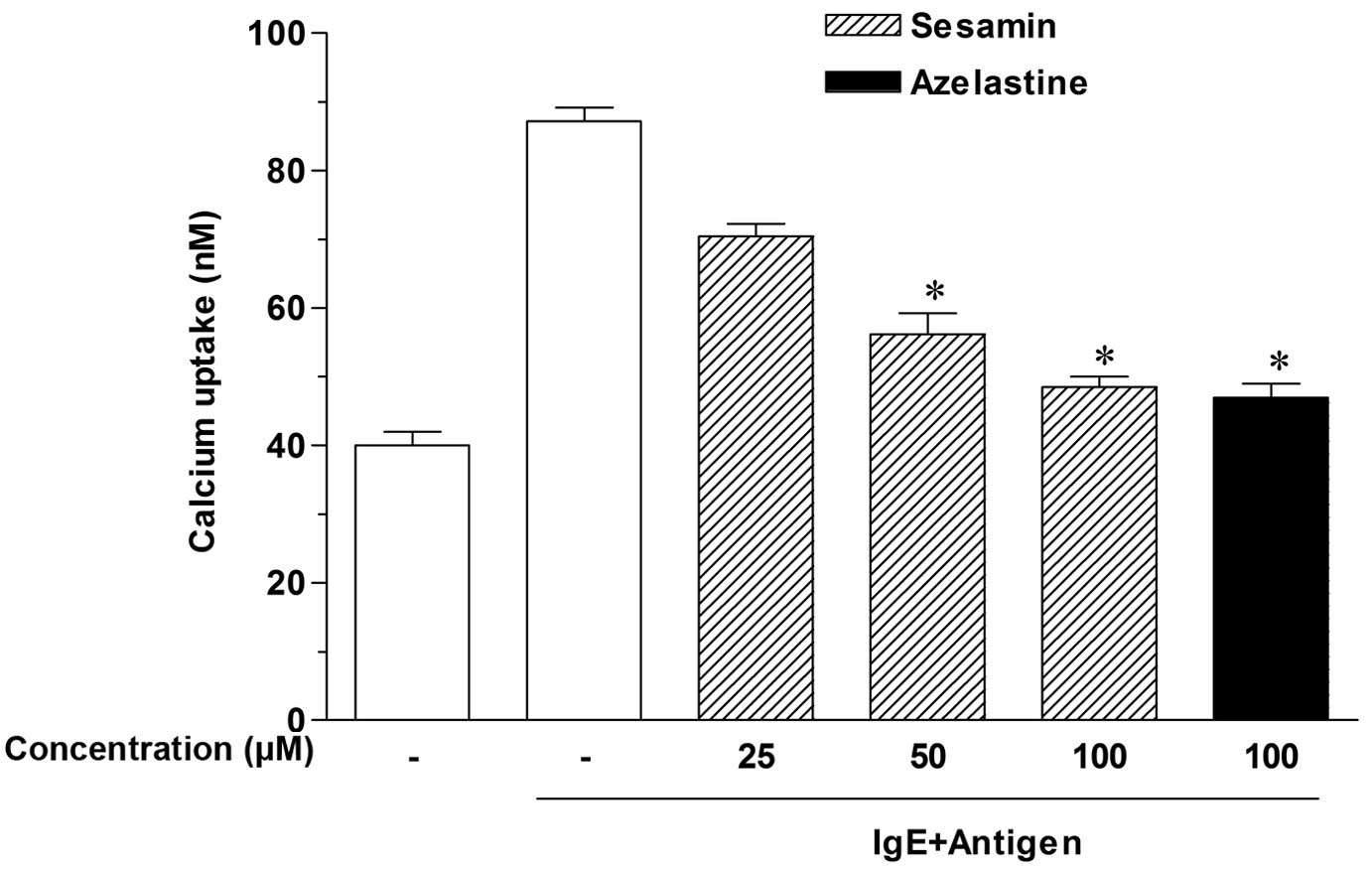
Sesamin inhibits calcium uptake into
RPMCs
Calcium movement across mast cell membranes is
critical to histamine release (19). Thus, to investigate the mechanisms
by which sesamin inhibits histamine release, calcium uptake was
measured. As presented in Fig. 2,
antigen-elicited calcium uptake was inhibited in a
concentration-dependent manner by sesamin, and significant effects
were produced at concentrations of 50–100 µM (P<0.05).
These results suggest that sesamin downregulates IgE-mediated
histamine release by reducing calcium uptake by RPMCs.
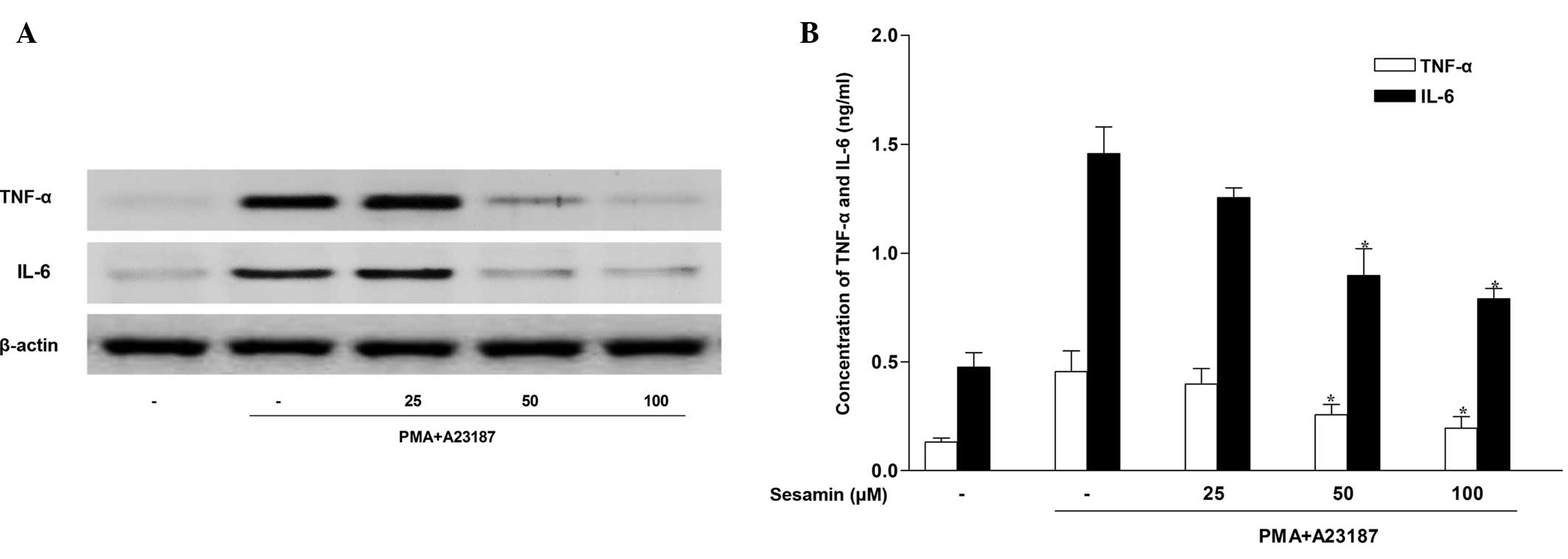
Sesamin inhibits pro-inflammatory
cytokine expression in human mast cells
The HMC-1 cell line has been established as a useful
cell type in which to study signaling events leading to cytokine
activation (20). As mast cell
activation stimulates cytokine release, the present study
investigated whether sesamin exposure regulated the release of
pro-inflammatory cytokines, such as TNF-α and IL-6 in HMC-1 cells.
Western blotting indicated that stimulation of HMC-1 cells with 20
nM PMA and 1 µM A23187 for 4 h induced TNF-α and IL-6
production. Sesamin inhibited protein expression of TNF-α and IL-6
in stimulated HMC-1 cells in a concentration-dependent manner
(Fig. 3A). The ability of sesamin
to suppress the secretion of TNF-α and IL-6 was investigated by
evaluating their abundance in the HMC-1 cell medium. Consistent
with the western blot analysis, ELISA analysis demonstrated that
treatment with sesamin inhibited TNF-α and IL-6 secretion induced
by PMA and A23187 in stimulated HMC-1 cells (Fig. 3B).
Sesamin inhibits p38 MAPK activation
The MAPK signaling cascade is an important pathway
in the regulation of pro-inflammatory molecules that mediate
cellular responses (21). Sesamin
inhibited the activation of p38 MAPK in HMC-1 cells (Fig. 4A), however, it had no effect on
phosphorylation of ERK and JNK (data not shown). Furthermore,
western blot analysis and ELISA (Fig.
4B and C) demonstrated that exposure to the selective p38 MAPK
inhibitor, SB 203580 (5 µM) decreased TNF-α and IL-6
production that had been induced by PMA and A23187 stimulation in
HMC-1 cells.
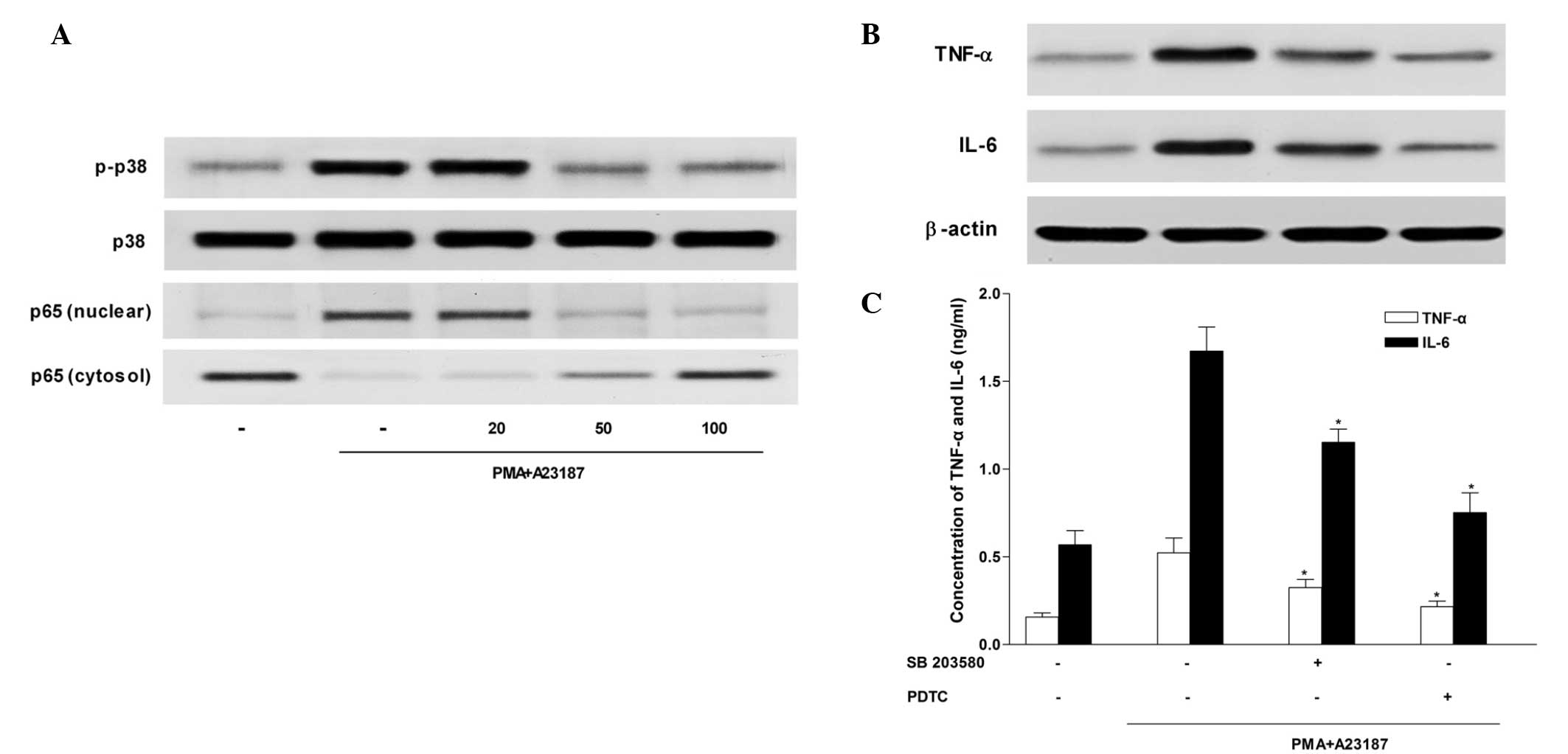 | Figure 4Inhibitory effect of sesamin on p38
MAPK and NF-κB p65 activation in HMC-1 cells. HMC-1 cells were
stimulated with 20 nM PMA and 1 µM calcium ionophore A23187
for 4 h in the absence or presence of sesamin (25, 50 or 100
µM). Western blot analysis was used to determine (A) p38
MAPK, extracellular signal-regulated kinases (data not shown), and
c-Jun N-terminal kinase activation (data not shown). HMC-1 cells
were pretreated with inhibitors of p38 MAPK and NF-κB (SB 203580
and PDTC, respectively) for 30 min prior to stimulation with PMA
(20 nM) and A23187 (1 µM), and production and secretion of
TNF-α and IL-6 were evaluated by (B) western blotting and (C)
ELISA. *P<0.05. p38 MAPK, p38 mitogen-activated
protein kinase; NF-κB, nuclear factor κ-light-chain-enhancer of
activated B cells TNF, tumour necrosis factor; IL-6, interleukin-6;
PMA, phorbol 12-myristate 13-acetate; PDTC, pyrrolidine
dithiocarbamate. |
Sesamin inhibits NF-κB activation
Previous studies have demonstrated that NF-κB is
critical in immune and inflammatory responses, and identified that
it is an important transcriptional regulator of inflammatory
cytokines (22–24). To investigate the intracellular
mechanism responsible for the inhibitory effect of sesamin on TNF-α
and IL-6 expression levels, the effect of sesamin on NF-κB activity
was examined using western blotting. Stimulation of HMC-1 cells
with PMA and A23187 induced nuclear translocation of NF-κB p65.
Sesamin inhibited nuclear translocation of NF-κB p65 induced by PMA
and A23187, and increased the expression levels of NF-κB p65 in the
cytosol preparations (Fig. 4A).
These results indicate that sesamin inhibits NF-κB activity by
preventing its translocation into the nucleus. In addition, western
blotting (Fig. 4B) and ELISA
(Fig. 4C) demonstrated that TNF-α
and IL-6 production induced by PMA and A23187 stimulation were
decreased by treatment with PDTC, a potent NF-κB inhibitor.
Discussion
Mast cell activation leads to the release of
inflammatory mediators including, histamine, heparin and various
cytokines, and is significant in allergic inflammation (25–27).
Previous studies have demonstrated that stimulation of mast cells
with IgE initiates the activation of the cell signaling, which
leads to histamine release and in turn produces local effects,
including increased cutaneous blood flow and enhanced vascular
permeability, resulting in tissue swelling and itching due to
stimulation of cutaneous sensory nerves (28). In the present study, sesamin
effectively suppressed histamine release from mast cells. In
addition, the experiments conducted in the present study using the
PCA animal model [a well-established model for evaluating localized
mast cell-mediated allergic reactions in vivo (29)], were consistent with the in
vitro experiments, and indicated that sesamin significantly
inhibited mast cell membrane perturbation in PCA rats. This finding
suggests that sesamin may inhibit IgE-mediated anaphylaxis by
inhibiting mast cell activation.
The intracellular calcium response induced by Fcε RI
cross-linking on mast cells is critical to mast cell degranulation
(30,31). Agents that decrease intracellular
calcium levels reduce mast cell degranulation, and histamine
release is inhibited by decreases in intracellular calcium content
due to the activation of mast cells (32). Changes in calcium homeostasis occur
as a result of calcium release from internal stores or calcium
uptake from the external environment; calcium uptake is essential
for Fcε RI-induced degranulation (33). Therefore, in the present study,
calcium uptake was measured using a radiotracer assay. The
intracellular calcium content of RPMCs was increased by incubation
with IgE in comparison with that of the basal cells. However, as
expected based upon the inhibition of histamine production by
sesamin, sesamin markedly inhibited antigen-induced calcium influx
into RPMCs. These results indicate that the effects of sesamin on
allergic reactions may be associated with a decrease in mast cell
intracellular calcium content. Furthermore, these findings
demonstrate that sesamin inhibits histamine release by suppressing
calcium uptake into mast cells.
The HMC-1 cell line is suitable for the study of
cytokine activation pathways (20,34).
The variety of cytokines produced by HMC-1 cells following
stimulation with PMA and A23187 supports the well-established role
of mast cells in immediate-type hypersensitivity. TNF-α is a key
mediator in a number of cytokine-dependent inflammatory events,
which induce chemotaxis of neutrophils and T cells, enhance
macrophage cytotoxicity, and stimulate the expression of adhesion
molecules in mast cells (35).
IL-6 is one of the most important mediators of fever and acute
phase responses, and it is predominantly secreted by T cells,
macrophages and mast cells (36).
Triggering and sustaining allergic inflammation in mast cells are
significant functions of TNF-α and IL-6, and local accumulation of
these two cytokines is associated with the PCA reaction (37). In the present study, sesamin
inhibited the PMA- and A23187-induced secretion of TNF-α and IL-6,
indicating that sesamin may suppress acute allergic inflammation by
decreasing pro-inflammatory cytokine production.
The MAPK cascade is one of the important signaling
pathways in the immune response (38). Although expression of TNF-α and
IL-6 is regulated by MAPKs, the precise signaling pathways involved
and the contributions of the three primary types of MAPKs (p38
MAPK, ERKs and JNKs) remain unclear; however, p38 MAPK is
considered to be an important regulator of inflammatory responses.
Activation of p38 MAPK is essential for the expression of
pro-inflammatory cytokines (39,40).
In the current study, stimulation with PMA and A23187 activated p38
MAPK in HMC-1 cells, and sesamin specifically inhibited p38 MAPK
activation. Furthermore, sesamin and SB 203580, a specific
inhibitor of p38 MAPK, reduced TNF-α and IL-6 production. These
data suggest that sesamin inhibits p38 activation and downstream
TNF-α and IL-6 production.
Previous reports have indicated that NF-κB is
critical in immune and inflammatory responses (22). Activation of NF-κB has also been
observed following IgE-induced TNF-α and IL-6 production in mast
cells (41). NF-κB is present in
the cytoplasm, where it is associated with nuclear factor of κ
light polypeptide gene enhancer in B-cells inhibitor α (IκBα), an
NF-κB inhibitory protein. Upon stimulation, IκBα is phosphorylated
and undergoes proteolytic degradation, which results in the release
of NF-κB from IκBα and its translocation into the nucleus, where it
promotes the transcription of genes, including TNF-α and IL-6
(42). In the present study,
sesamin reduced NF-κB p65 levels in the nucleus and increased
levels of NF-κB p65 protein in cytosolic extracts from mast cells
stimulated with PMA and A23187. Furthermore, sesamin and PDTC, a
potent NF-κB inhibitor, downregulated TNF-α and IL-6 production.
These results indicate that sesamin prevents translocation of NF-κB
into the nucleus and, thus, inhibits its transcriptional activity,
as well as its downstream TNF-α and IL-6 production.
In conclusion, sesamin inhibits IgE-mediated
anaphylactic reactions in vivo and in vitro.
Inhibition of pro-inflammatory mediator release by sesamin appears
to be involved in the inhibition of p38 MAPK and NF-κB activity, in
addition to the suppression of calcium uptake. The present study
provides insight into the underlying mechanisms by which sesamin
produces anti-allergic effects, and indicates that the
administration of sesamin may contribute to the prevention and
treatment of mast cell-mediated allergic diseases.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81260665 and
81260016), the Project of Research and Innovation of Jilin Youth
Leader and Team (grant no. 20140519013JH), the Natural Science
Research Foundation of Jilin Province for Sciences and Technology
(grant no. 20130101121JC) and the Natural Science Research
Foundation of the Education Department of Jilin Province (grant no.
2014 21).
References
|
1
|
Galli SJ, Borregaard N and Wynn TA:
Phenotypic and functional plasticity of cells of innate immunity:
Macrophages, mast cells and neutrophils. Nat Immunol. 12:1035–1044.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar V and Sharma A: Mast cells: Emerging
sentinel innate immune cells with diverse role in immunity. Mol
Immunol. 48:14–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalesnikoff J and Galli SJ: New
developments in mast cell biology. Nat Immunol. 9:1215–1223. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beaven MA, Rogers J, Moore JP, Hesketh TR,
Smith GA and Metcalfe JC: The mechanism of the calcium signal and
correlation with histamine release in 2H3 cells. J Biol Chem.
259:7129–7136. 1984.PubMed/NCBI
|
|
5
|
Beaven MA and Metzger H: Signal
transduction by Fc receptors: The Fc epsilon RI case. Immunol
Today. 14:222–226. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Plaut M, Pierce JH, Watson CJ, Hanley-Hyde
J, Nordan RP and Paul WE: Mast cell lines produce lymphokines in
response to cross-linkage of Fc epsilon RI or to calcium
ionophores. Nature. 339:64–67. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nasirullah and Latha RB: Storage stability
of sunflower oil with added natural antioxidant concentrate from
sesame seed oil. J Oleo Sci. 58:453–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeng KC, Hou RC, Wang JC and Ping LI:
Sesamin inhibits lipopolysaccharide-induced cytokine production by
suppression of p38 mitogen-activated protein kinase and nuclear
factor-kappaB. Immunol Lett. 97:101–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Periasamy S, Hsu DZ, Chandrasekaran VR and
Liu MY: Sesame oil accelerates healing of
2,4,6-trinitrobenzenesulfonic acid-induced acute colitis by
attenuating inflammation and fibrosis. J Parenter and Enteral Nutr.
37:674–682. 2013. View Article : Google Scholar
|
|
10
|
Hsieh CC, Kuo CH, Kuo HF, Chen YS, Wang
SL, Chao D, Lee MS and Hung CH: Sesamin suppresses
macrophage-derived chemokine expression in human monocytes via
epigenetic regulation. Food Funct. 5:2494–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi YH and Yan GH: Ellagic Acid
attenuates immunoglobulin E-mediated allergic response in mast
cells. Biol Pharm Bull. 32:1118–1121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi YH and Yan GH: Pycnogenol inhibits
immunoglobulin E-mediated allergic response in mast cells.
Phytother Res. 23:1691–1695. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hachisuka H, Nomura H, Sakamoto F, Mori O,
Okubo K and Sasai Y: Effect of antianaphylactic agents on
substance-P induced histamine release from rat peritoneal mast
cells. Arch Dermatol Res. 280:158–162. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshimura T, Hamaguchi E, Usami E,
Nakashima K, Kawaguchi M, Suzuki N, Okamoto Y, Nakao T and Yamazaki
F: Increased in vitro release of interferon-gamma from
ampicillin-stimulated peripheral blood mononuclear cells in
Stevens-Johnson syndrome. Biol Pharm Bull. 27:929–931. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harvima RJ, Harvima IT and Fraki JE:
Optimization of histamine radio enzyme assay with purified
histamine-N-methyltransferase. Clin Chim Acta. 171:247–256. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi YH, Yan GH, Chai OH, Lim JM, Sung SY,
Zhang X, Kim JH, Choi SH, Lee MS, Han EH, et al: Inhibition of
anaphylaxis like reaction and mast cell activation by water extract
from the fruiting body of Phellinus linteus. Biol Pharm Bull.
29:1360–1365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nunomura S, Kitanaka S and Ra C:
3-O-(2,3-dimethylbutanoyl)-13-O-decanoylingenol from Euphorbia
kansui suppresses IgE-mediated mast cell activation. Biol Pharm
Bull. 29:286–290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi YH and Yan GH: Anti-allergic effects
of scoparone on mast cell-mediated allergy model. Phytomedicine.
16:1089–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCloskey MA and Zhang L: Potentiation of
Fcepsilon receptor I-activated Ca(2+) current (I(CRAC))
by cholera toxin: Possible mediation by ADP ribosylation factor. J
Cell Biol. 148:137–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sillaber C, Bevec D, Butterfield JH,
Heppner C, Valenta R, Scheiner O, Kraft D, Lechner K, Bettelheim P
and Valent P: Tumor necrosis factor alpha and interleukin-1 beta
mRNA expression in HMC-1 cells: Differential regulation of gene
product expression by recombinant interleukin-4. Exp Hematol.
21:1271–1275. 1993.PubMed/NCBI
|
|
21
|
Takeishi Y, Huang Q, Abe J, Che W, Lee JD,
Kawakatsu H, Hoit BD, Berk BC and Walsh RA: Activation of
mitogen-activated protein kinases and p90 ribosomal S6 kinase in
failing human hearts with dilated cardiomyopathy. Cardiovasc Res.
53:131–137. 2002. View Article : Google Scholar
|
|
22
|
Siebenlist U, Franzoso G and Brown K:
Structure, regulation and function of NF kappa B. Annu Rev Cell
Biol. 10:405–455. 1994. View Article : Google Scholar
|
|
23
|
Liu SF and Malik AB: NF-kappa B activation
as a pathological mechanism of septic shock and inflammation. Am J
Physiol Lung Cell Mol Physiol. 290:L622–L645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doggrell SA: NF-kappa B–a target in the
inflammation of bone destruction. Expert Opin Ther Targets.
9:191–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kemp SF and Lockey RF: Anaphylaxis: A
review of causes and mechanisms. J Allergy Clin Immunol.
110:341–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dvorak AM: Mast cell-derived mediators of
enhanced microvascular permeability, vascular permeability
factor/vascular endothelial growth factor, histamine, and
serotonin, cause leakage of macromolecules through a new
endothelial cell permeability organelle, the vesiculo-vacuolar
organelle. Chem Immunol Allergy. 85:185–204. 2005. View Article : Google Scholar
|
|
27
|
Rattmann YD, Pereira CR, Cury Y, Gremski
W, Marques MC and da Silva-Santos JE: Vascular permeability and
vasodilation induced by the Loxosceles intermedia venom in rats:
involvement of mast cell degranulation, histamine and 5-HT
receptors. Toxicon. 51:363–372. 2008. View Article : Google Scholar
|
|
28
|
Neel NF, Creasy BM, Rankin JN, Pierce EM,
McCoy ME, Daner RH, Fowler JA, Daniel JC and Lantz CS: Absence of
interleukin-3 does not affect the severity of local and systemic
anaphylaxis but does enhance eosinophil infiltration in a mouse
model of allergic peritonitis. Immunol Lett. 95:37–44. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kabu K, Yamasaki S, Kamimura D, Ito Y,
Hasegawa A, Sato E, Kitamura H, Nishida K and Hirano T: Zinc is
required for Fc epsilon RI-mediated mast cell activation. J
Immunol. 177:1296–1305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishida K, Yamasaki S, Ito Y, Kabu K,
Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS,
et al: Fc{epsilon} RI-mediated mast cell degranulation requires
calcium-independent microtubule-dependent translocation of granules
to the plasma membrane. J Cell Biol. 170:115–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tshori S and Razin E: Editorial: Mast cell
degranulation and calcium entry–the Fyn-calcium store connection. J
Leukoc Biol. 88:837–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chakravarty N and Yu WJ: Role of
intracellular calcium in histamine release from rat mast cells.
Agents and Actions. 14:386–391. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shumilina E, Lam RS, Wolbing F, Matzner N,
Zemtsova IM, Sobiesiak M, Mahmud H, Sausbier U, Biedermann T, Ruth
P, et al: Blunted IgE-mediated activation of mast cells in mice
lacking the Ca2+-activated K+ channel KCa3.1.
J Immunol. 180:8040–8047. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Möller A, Henz BM, Grutzkau A, Lippert U,
Aragane Y, Schwarz T and Kruger-Krasagakes S: Comparative cytokine
gene expression: Regulation and release by human mast cells.
Immunology. 93:289–295. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thomas PS: Tumour necrosis factor-alpha:
The role of this multifunctional cytokine in asthma. Immunol Cell
Biol. 79:132–140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu ZQ, Zhao WH and Shimamura T: Regulation
of mast cell development by inflammatory factors. Curr Med Chem.
14:3044–3050. 2007. View Article : Google Scholar
|
|
37
|
Mican JA, Arora N, Burd PR and Metcalfe
DD: Passive cutaneous anaphylaxis in mouse skin is associated with
local accumulation of interleukin-6 mRNA and immunoreactive
interleukin-6 protein. J Allergy Clin Immunol. 90:815–824. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arbabi S and Maier RV: Mitogen-activated
protein kinases. Crit Care Med. 30(Suppl): S74–S79. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ashwell JD: The many paths to p38
mitogen-activated protein kinase activation in the immune system.
Nat Rev Immunol. 6:532–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Manthey CL, Wang SW, Kinney SD and Yao Z:
SB202190, a selective inhibitor of p38 mitogen-activated protein
kinase, is a powerful regulator of LPS-induced mRNAs in monocytes.
J Leukoc Biol. 64:409–417. 1998.PubMed/NCBI
|
|
41
|
Jeong HJ, Koo HN, Na HJ, Kim MS, Hong SH,
Eom JW, Kim KS, Shin TY and Kim HM: Inhibition of TNF-alpha and
IL-6 production by Aucubin through blockade of NF-kappaB activation
RBL-2H3 mast cells. Cytokine. 18:252–259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Klemm S and Ruland J: Inflammatory signal
transduction from the Fc epsilon RI to NF-kappa B. Immunobiology.
211:815–820. 2006. View Article : Google Scholar : PubMed/NCBI
|