Introduction
Osteoarthritis (OA) is a degenerative joint disease
that frequently affects middle-aged and elderly individuals
(1). OA is characterized by
mechanical abnormalities involving the degradation of joint
tissues, including the articular cartilage, subchondral bone and
the synovium (2). Although
numerous previous studies have focused on the cartilage, the
subchondral bone was recently identified to serve an important role
in the development of OA (3–6). The
mineral density of subchondral bone is reduced during the early
onset of OA, and bone mass is increased by the late stage, along
with the presence of subchondral sclerosis and osteophytes. In
addition, a vicious cycle develops between structural alterations
in the subchondral bone and cartilage injury, which are closely
associated with OA progression. Therefore, the subchondral bone is
notable target for the treatment of OA.
Structural alterations and abnormal bone remodeling
of the subchondral bone are frequently present in patients with OA.
The osteoprotegerin (OPG), receptor activator of nuclear factor-κB
(RANK) and RANK ligand (RANKL) system is one of the most important
molecular mechanisms that regulate bone remodeling (7). RANK is the receptor for RANKL, and
OPG is a decoy receptor for RANKL. The OPG/RANKL ratio is crucial
for the restoration of bone mass and repair of bone injury, due to
the fact that it maintains the homeostasis between bone resorption
and bone formation (8,9). Hence, delaying the pathological
progression of OA by adjusting the expression of OPG and RANKL, in
order to regulate the bone-remodeling rate, may lead to an
improvement in bone microstructure.
Rofecoxib (a cyclooxygenase 2 inhibitor), ibuprofen
and placebo are currently the standard treatment for OA (10), with the aim of reducing
inflammation, controlling pain and providing the cartilage with the
required nutrients. However, this approach involves the risk of
adverse reactions, for example gastrointestinal ulcers (10). The Tougu Xiaotong capsule (TGXTC),
characterized as a multi-target and multi-channel compound
(11), contains a proven recipe
for OA treatment, consisting of Morinda officinalis,
Paeonia lactiflora, Ligusticum chuanxiong and
Sarcandra glabra (12).
Previous studies have demonstrated that this compound may inhibit
chondrocyte apoptosis, promote chondrocyte proliferation (13–16),
suppress expression of matrix metalloproteinases and inflammatory
cytokines (17–19), improve the structure and function
of cartilage (20) and promote
osteoblast proliferation and activation (21). However, the regulatory effects of
TGXTC on subchondral bone remodeling remain largely unclear. In the
present study, the protective effects of TGXTC and glucosamine
hydrochloride on the regulation of subchondral bone remodeling were
compared, and the expression of OPG and RANKL were investigated in
a rabbit model of knee OA, in order to explore the underlying
mechanisms of TGXTC in OA treatment.
Materials and methods
Animals
A total of 72 female 6-month old New Zealand rabbits
weighing 2.0±0.3 kg, were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China) [license no. SCXK (Hu)
2012-0011]. These animals were raised in the Animal Center of
Fujian University of Traditional Chinese Medicine, Fujian, China
[license no. SYXK (Min) 2009-0001]. The care and use of the
laboratory animals complied with the Guidance Suggestions for the
Care and Use of Laboratory Animals, administered by the Ministry of
Science and Technology (Beijing, China) (22).
Experimental design
Subsequent to one week of acclimation, the 72
rabbits were randomly divided into six groups, including the normal
control, OA model, glucosamine hydrochloride (Bright Future
Pharmaceuticals Factory Hong Kong, Yuen Long, Hong Kong, SAR,
China), and low- (70 mg/kg/day), middle- (140 mg/kg/day) and high-
(280 mg/kg/day) dose TGXTC (The Second People's Hospital of Fujian
University of Traditional Chinese Medicine, Fuzhou, China; medical
license no. MIN ZIZHI Z20100006) groups, with 12 rabbits in each
group.
The rabbit model of OA was induced in all groups
except for the normal control group using a modified version of
Hulth's method (23). Rabbits were
anesthetized by ear vein injection of sodium pentobarbital (3%; 30
mg/kg; Shanghai Xitang Biotechnology, Co., Ltd., Shanghai, China)
and placed on an operating table in the supine position with 90°C
flexion of the right knee. The medial, collateral and anterior
cruciate ligaments were transected via the medial approach, and the
medial meniscus was removed. Successful transection was verified
with the drawer test, and then the joint capsule and skin were
sutured closed. Sodium penicillin (400,000 U; GE Healthcare Life
Sciences, Logan, UT, USA) was administered intramuscularly for 3
consecutive days postoperatively. One week later, all animals were
subjected to passive movement of the knee for 0.5 h daily for 4
weeks.
A total of five weeks postoperatively, the OA model
was successfully established in the rabbits. Intragastric
administration to the OA rabbits of glucosamine hydrochloride (75
mg/kg/day) and increasing doses of TGXTC (70, 140 and 280
mg/kg/day) was conducted either for 4 or 8 weeks, and an equivalent
volume of normal saline was administered to those in the normal
control or model groups.
Tissue collection
Following 4 or 8 weeks of the treatment, all animals
were sacrificed with 2% pentobarbital sodium (40 mg/kg.wt via ear
marginal vein injection; Merck Sharpe & Dohme, Shanghai, China)
and the tibia and femur were collected for further investigation.
The medial femoral condyle was prepared for histology and the tibia
for scanning electron microscopy, and the subchondral bone isolated
from the lateral femoral condyle was collected for the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
Histopathological examination
The femoral specimens were fixed with 4%
paraformaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) for 72 h, then decalcified with 10% EDTA
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) at room
temperature for 16 weeks. Subsequently, the medial two-thirds of
the medial femoral condyle was longitudinally cut into 1.2×1.2×0.5
cm sections and embedded in paraffin (Shanghai Guangkuo Chemical
Co., Ltd., Shanghai, China). Finally, 4-µm thick sagittal
sections were used for hematoxylin and eosin (H&E) staining and
were observed under a light microscope (DM4000 B; Leica
Microsystems GmbH, Wetzlar, Germany).
Scanning electron microscopy
Subsequent to fixation in 4% paraformaldehyde for 72
h, the medial two-thirds of the medial tibial condyle was sampled,
rinsed with 0.1 M phosphate-buffered saline (GE Healthcare Life
Sciences, Logan, UT, USA) in deionized water, dehydrated using
tertiary butanol (Sinopharm Chemical Reagent Co., Ltd.), dried in a
vacuum drier (Shanghai Jinghong Laboratory Instrument Co., Ltd.,
Shanghai, China), fixed onto the stage using conductive adhesive,
then observed with a tabletop scanning microscope (TM3030; Hitachi,
Ltd., Tokyo, Japan).
RT-qPCR
Total RNA was extracted from the subchondral bone of
the lateral femoral condyle using TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA) and quantified using a UV
spectrophotometer (ND-2000C; Thermo Fisher Scientific, Waltham, MA,
USA). cDNA (700 µg) was synthesized using the PrimeScript™
RT Reagent kit with gDNA Eraser (Takara Bio., Inc., Otsu, Japan).
The PCR system was prepared according to the manufacturer's
instructions, with 10 µl SYBR® Premix Ex
Taq II (Takara Bio., Inc.), 0.4 µl ROX Reference Dye
II (Takara Bio., Inc.), 0.8 µl upstream primer, 0.8
µl downstream primer, 2 µl cDNA and 6 µl
dH2O, with a total reaction volume of 20 µl. The
PCR amplification protocol was as follows: Initial denaturation at
95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for
3 sec and annealing at 60°C for 30 sec (S1000; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The fluorescence signal of
GAPDH acted as an internal reference for calculating the relative
gene expression levels. RT-qPCR was performed using an 7500 Fast
Real-Time PCR system (Applied Biosystems Life Technologies, Foster
City, CA, USA). Primers were designed and synthesized by Takara
Bio, Inc., and the sequences used are as follows: GAPDH, forward
5′-CCA CTT TGT GAA GCT CAT TTC T-3 and reverse 5′-TCG TCC TCC TCT
GGT GCT CT-3; OPG, forward 5′-ACT ACA CAG ACA CTT GGC ACA CC-3 and
reverse 5′-CTT CCT CGC ATT CAC ACA CAC -3; RANKL, forward 5′-GCT
AGG AGG GAG AGC AGC AA-3 and reverse 5′-TGA GAG AGG AAG ACG GCA
CA-3.
Western blotting
The subchondral bone isolated from the lateral
femoral condyle was immersed 1:10 in lysis buffer containing 50mM
Tris (pH7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate,
0.1% SDS, 0.1% sodium orthovana-date and 2 mM EDTA (Beijing BLKW
Biotechnology Co., Ltd., Shanghai, China), homogenized using a
Tissuelyser-192 (Shanghai Jingxin Industrial Development Co., Ltd.,
Shanghai, China) on ice, then centrifuged at 4°C at 12,000 × g for
30 min. The protein samples were electrophoresed by 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis for 2 h
(Beyotime Institute of Biotechnology, Shanghai, China), transferred
onto polyvinylidene difluoride (Shanghai Jinghong Laboratory
Instrument Co., Ltd.) membranes, and blocked with 5% skimmed milk
for 2 h. Subsequently, the samples were incubated on a shaker at
4°C overnight with the following primary antibodies: Mouse
anti-β-actin (monoclonal; 1:5,000; cat. no. HC201; TransGen Biotech
Co., Ltd., Beijing, China), rabbit anti-OPG (polyclonal; 1:1,000;
cat. no. AV00033; Sigma-Aldrich, St. Louis, MO, USA) and
rabbit-anti RANKL (polyclonal; 1:200; cat. no. BA1323; Boster
Systems, Inc., Pleasanton, CA, USA). The samples were then rinsed
with Tris-buffered saline with Tween-20 (TBST; Shanghai Jinghong
Laboratory Instrument Co., Ltd.) and incubated with the following
corresponding secondary antibodies: Goat anti-mouse horseradish
peroxidase-conjugated IgG (monoclonal; 1:4,000; cat. no. HS201;
TransGen Biotech, Inc). and goat anti-rabbit horseradish
peroxidase-conjugated IgG (monoclonal, 1:4000; cat. no. HS101;
TransGen Biotech, Inc.). This was performed on a shaker at room
temperature for 1 h. Following incubation, the samples were rinsed
with TBST, and developed using an enhanced chemiluminescence
substrate (Beyotime Institute of Biotechnology). Image processing
was conducted using scanning densitometry (170–8070 Molecular
Imager ChemiDoc XRS System; Bio-Rad Laboratories, Inc.) to analyze
gray values and to determine the relative expression of the
proteins.
Statistical analysis
GraphPad Prism software, version 6.00 for Windows
was used for statistical analysis. All quantitative data are
expressed as the mean ± standard deviation. One-way analysis of
variance was used, and P<0.05 was considered to indicate a
statistically significant difference.
Results
TGXTC inhibits cartilage and subchondral
bone degradation
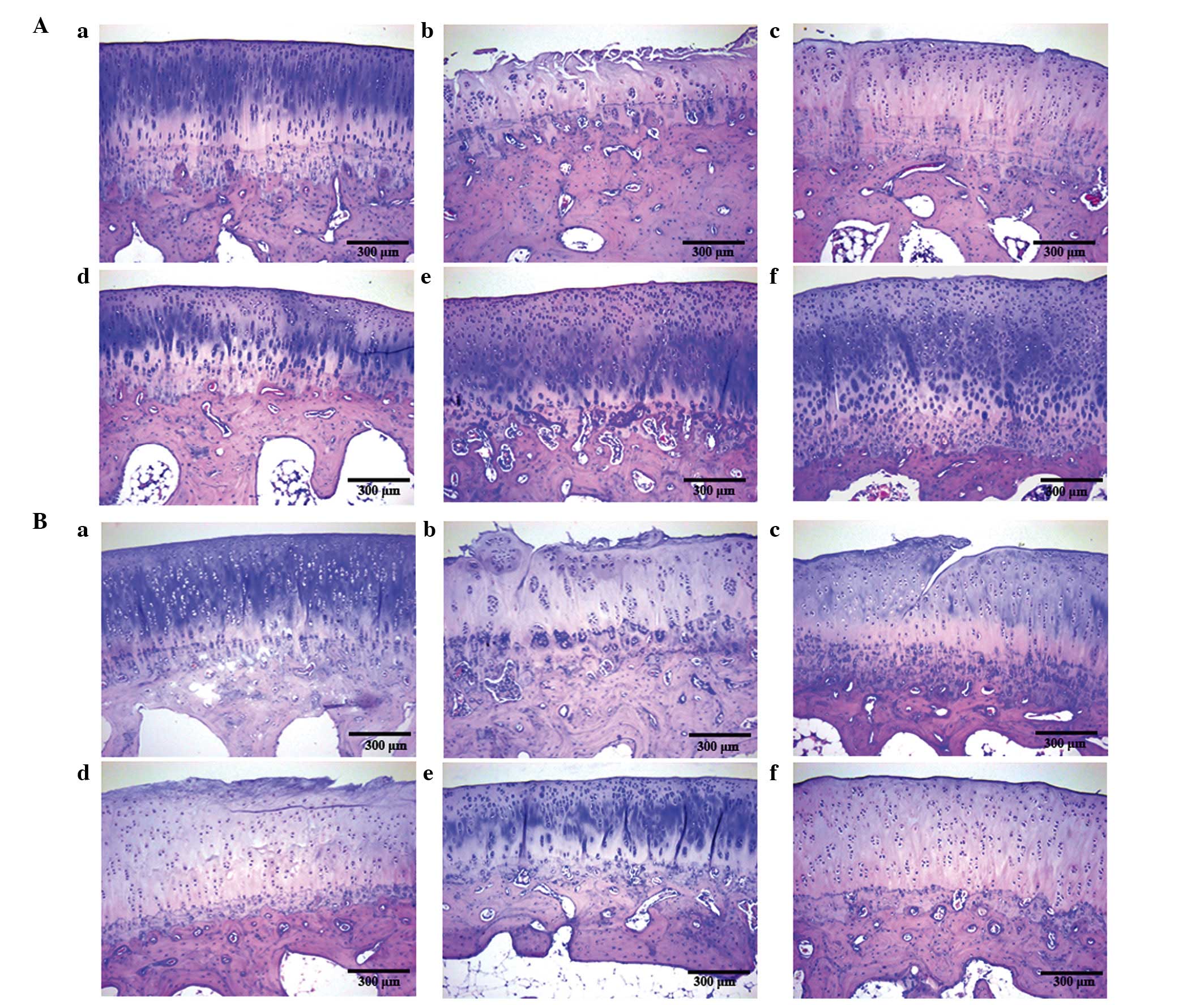
In order to determine the protective effects of
TGXTC on the morphology of cartilage and subchondral bone, the
sections were evaluated by H&E staining. There was no evidence
of degradation between cartilage and subchondral bone in the normal
control group (Fig. 1Aa and Ba).
However, the cartilage surface of the OA model group was partially
damaged, with the disruption of the four-layer structure,
disordered chondrocyte clusters and tidemark replication, in
addition to the reduced staining intensity of the cartilage matrix
(Fig. 1Ab and Bb). Subchondral
sclerosis, involving increases in trabecular number and thickness
and narrowing of the intertrabecular space was observed (Fig. 2A), suggesting the middle or late
stages of OA.
Following 4 weeks of treatment, increased staining
intensity of the cartilage matrix, reduced trabecular number and an
increased intertrabecular space were observed in the glucosamine
hydrochloride and TGXTC groups when compared with the OA model
group, suggesting that glucosamine hydrochloride and TGXTC improve
the morphology of cartilage and subchondral bone of the OA.
Compared with the glucosamine hydrochloride group, increased the
number of chondrocytes (Fig.
1Ac–f) and smooth or straight trabecular bone (Fig. 2B and C) were observed in the TGXTC
groups, indicating that TGXTC may be more suitable for treating the
OA model induced by a modified version of Hulth's method.
Subsequent to 8 weeks of treatment, more pronounced
degradation of the cartilage and subchondral bone was observed in
the OA model group. Although the cartilage surface appeared to
contain fissures, extending deep into the radial layer in the
glucosamine hydrochloride group, the structure of the cartilage and
subchondral bone in this group appeared clearer than that of the OA
model (Fig. 1Bc). Increased
regular chondrocyte clusters and reduced tidemarks were observed in
the TGXTC groups compared with the OA model and glucosamine
hydrochloride groups, which is consistent with the results of the
treatment for 4 weeks. Notably, the middle-dose TGXTC group
exhibited greater improvement than the other doses, suggesting that
the protective role of TGXTC was not dose-dependent.
TGXTC inhibits OPG expression and
promotes RANKL expression
In order to further investigate the mechanism of
TGXTC on subchondral bone remodeling, the expression levels of OPG
and RANKL were analyzed using RT-qPCR and western blotting. A total
of nine weeks subsequent to the induction of OA (4-week treatment
group), the OPG mRNA and protein expression levels were
significantly increased in the OA model group compared with the
normal control group (P<0.05). A total of 13 weeks subsequent to
the induction of OA (8-week treatment group), the mRNA and protein
expression levels of OPG were not significantly different between
the OA model and normal control groups, suggesting that increased
OPG expression is only observed during the middle stage of OA
progression.
Following 4 weeks of treatment, reduced OPG mRNA and
protein expression levels were observed in the TGXTC and
glucosamine hydrochloride groups, compared with the OA model group
(P<0.05; Figs. 3A, 4A and C), suggesting that the excessive
bone formation induced by OPG was inhibited by TGXTC and
glucosamine hydrochloride.
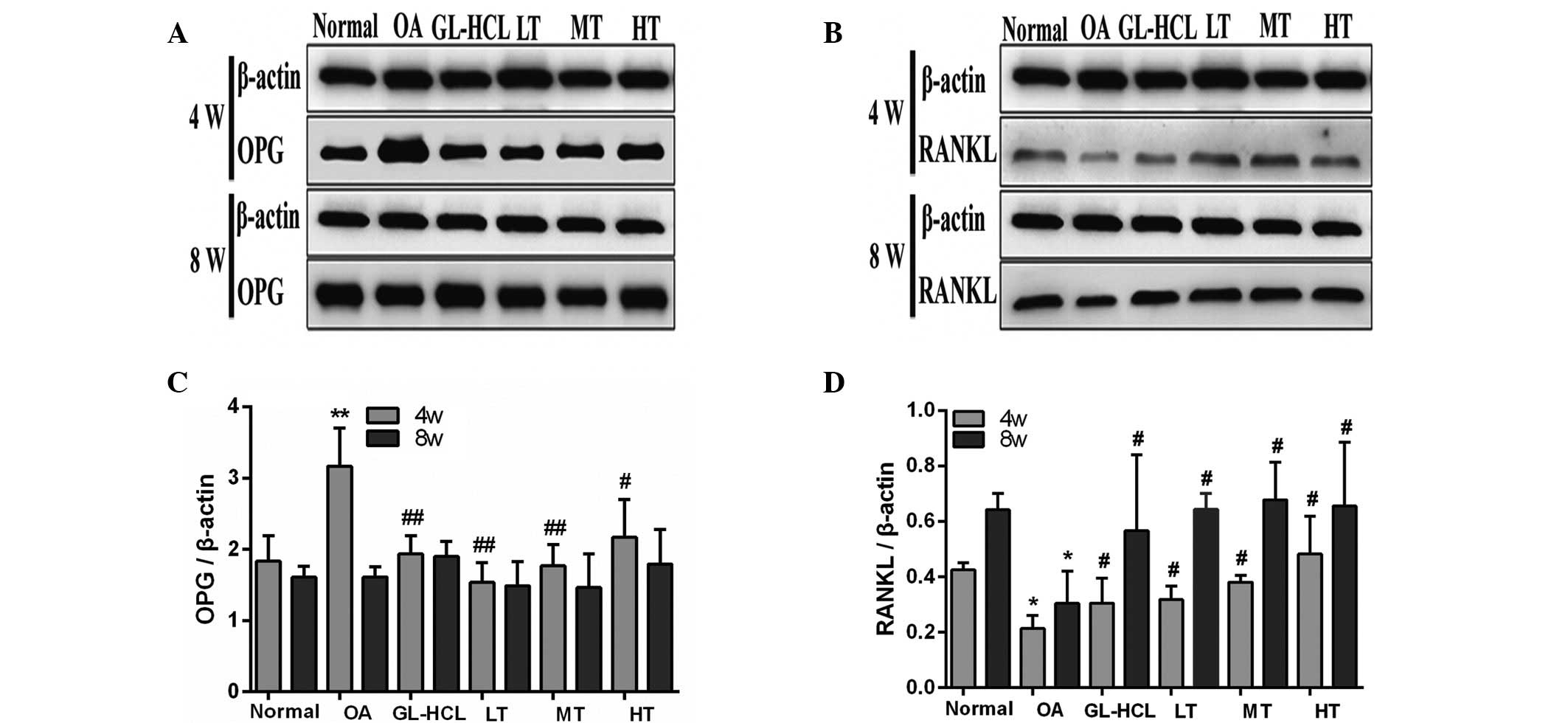 | Figure 4The protein expression of OPG and
RANKL. The protein expression of OPG and RANKL were determined by
the western blot assay. Chemiluminescent imaging for (A) OPG
protein and (B) RANKL. Relative expression of (C) OPG and (D)
RANKL. *P<0.05, **P<0.01 vs. normal
control group. #P<0.05, ##P<0.01 vs. OA
model group. OPG, osteoprotegerin; RANKL, receptor activator of
nuclear factor-κB ligand; OA, osteoarthritis; GL-HCL, glucosamine
hydrochloride; TGXTC, Tougu Xiaotong capsule; LT, low-dose (70
mg/kg/day) TGXTC group; MT, middle-dose (140 mg/kg/day) TGXTC
group; HT, high-dose (280 mg/kg/day) TGXTC group. |
The mRNA and the protein expression levels of RANKL
in the OA model group were significantly lower (P<0.05) than
those in the normal control group subsequent to 9 weeks (4-week
treatment group) or 13 weeks (8-week treatment group) of inducing
OA, which indicates that insufficient bone resorption had occurred.
Compared with the OA model group, a significant increase
(P<0.05) in the RANKL mRNA and protein expression levels was
observed between the glucosamine hydrochloride group and the TGXTC
groups at 4 weeks. No significant difference in RANKL mRNA
expression was observed between the glucosamine hydrochoride group
and the OA group, however, the expression was increased in the
TGXTC group at 8 weeks, compared with the OA model group. In
addition, a significant increase (P<0.05) in RANKL protein
expression levels was observed in the glucosamine hydrochloride
group and the TGXTC groups at 8 weeks, compared with the OA model
group (Figs. 3B, 4B and D). This suggests that TGXTC and
glucosamine hydrochloride may selectively promote bone resorption
through inducing the expression of RANKL.
TGXTC inhibits the OPG/RANKL ratio
To further determine the regulation of TGXTC on the
homeostasis between bone resorption and bone formation, the
OPG/RANKL ratio was analyzed for mRNA and protein expression. The
OPG/RANKL ratio for the mRNA and the protein expression levels in
the OA model group were significantly higher than those in the
normal control group following 9 (P<0.05) or 13 (P<0.05)
weeks of OA induction. This indicated that an imbalance of bone
metabolism was involved in OA progression. Following 4 weeks of
treatment, the OPG/RANKL ratio was significantly inhibited by the
addition of glucosamine hydrochloride and TGXTC (P<0.05),
compared with the OA model group (Fig.
5A). Following 8 weeks of treatment, similar results to the 4
weeks treatment group were observed in the mRNA (P<0.05) and
protein expression levels of the low or medium TGXTC dose groups
(P<0.05), however no reduction was observed in the protein level
of the glucosamine hydrochloride group and in the high TGXTC dose
group. This suggests that TGXTC may be more suitable for regulating
the homeostasis between bone resorption and bone formation induced
by the OPG/RANKL pathway in the late stage of OA, and that this
protective effect is not dose-dependent.
 | Figure 5OPG/RANKL ratio (A) 4 and (B) 8 weeks
of treatment. The OPG/RANKL ratio was used to determine the
protective effect of TGXTC on subchondral bone remodeling.
*P<0.05, **P<0.01 vs. normal control
group. #P<0.05, ##P<0.01 vs. OA model
group. OPG, osteoprotegerin; RANKL, receptor activator of nuclear
factor-κB ligand; OA, osteoarthritis; GL-HCL, glucosamine
hydrochloride; TGXTC, Tougu Xiaotong capsule; LT, low-dose (70
mg/kg/day) TGXTC group; MT, middle-dose (140 mg/kg/day) TGXTC
group; HT, high-dose (280 mg/kg/day) TGXTC group; PCR, polymerase
chain reaction; WB, western blotting. |
Discussion
TGXTC, a traditional Chinese medicine, has been
demonstrated to be clinically effective in the treatment of OA,
which has been indicated by in vitro and in vivo
studies where TGXTC was observed to reverse cartilage degeneration
in OA (11–20). However, whether TGXTC has a
protective effect on subchondral bone remodeling remains unclear.
In the current study, TGXTC was demonstrated to be able to
efficiently inhibit the imbalance of subchondral bone remodeling of
OA, via the OPG/RANKL pathway.
OA progression involves various pathological
alterations, including those involving cartilage, subchondral bone
and the synovial membrane, while the degeneration of cartilage is
the most typical characteristic of this disease (24). In the present study, multiple
pathological alterations of the cartilage, containing those of the
four-layer structure, cell number and arrangement, tidemarks, in
addition to matrix staining were clearly observed in the OA model
group, which indicates the incidence of OA. Following treatment
with glucosamine hydrochloride and TGXTC, all of these pathological
alterations were observed to be alleviated. In addition, the
therapeutic effects of TGXTC are suggested to be preferable to
those of glucosamine hydrochloride, indicating the benefits of
treatment with TGXTC.
In addition to those in the cartilage, structural
alterations in the subchondral bone can further aggravate the
progression of OA. Thus, regulating subchondral bone remodeling may
improve the subchondral bone structure, which would be beneficial
by delaying the OA progression (25,26).
The OPG/RANKL/RANK system is one of the most critical molecular
mechanisms underlying the regulation of bone remodeling, and
additionally serves an important role in maintaining the OPG/RANKL
balance in bone remodeling (7).
RANKL is secreted by osteoblasts and acts as a strong regulator of
bone resorption. RANKL binds to its receptor, RANK, on osteoclast
precursor cell, which induces osteoclast maturation, thereby
mediating bone resorption (27).
OPG, which is secreted by osteoblasts and bone marrow stromal
cells, is essential for preventing bone resorption, and is a decoy
receptor for RANKL (7). By binding
to RANKL, OPG inhibits osteoclast proliferation and
differentiation, reduces the production of mature osteoclasts and
reduces bone resorption (28).
Additionally, bone remodeling is controlled by the balance of the
OPG/RANKL ratio (7,29), with a higher OPG/RANKL ratio
mediating bone resorption (30)
and a lower OPG/RANKL ratio mediating bone formation (31).
Abnormal bone remodeling during OA results in an
imbalance between bone resorption and bone formation, leading to
structural alterations in the subchondral bone. A total of 9 weeks
subsequent to the induction of OA, OPG production was increased,
RANKL production was reduced and the OPG/RANKL production ratio was
significantly increased in the OA model group. This suggested that
OPG expression reached a peak during the middle stage of OA, and
the compensatory remodeling of the subchondral bone was faster.
Although the increase in OPG levels was inhibited by compensatory
remodeling following 13 weeks of OA induction, levels of RANKL were
inhibited during the progression of OA. In addition, a higher
OPG/RANKL ratio was observed in the OA model group, suggesting that
there was continuous bone formation, which may be due to the
subchondral sclerosis occurring during the late stages of OA.
Subsequent to 4 weeks of TGXTC treatment, OPG
expression was reduced, RANKL expression was increased and the
OPG/RANKL ratio was significantly reduced to levels similar to
those of the normal control group. However, in addition to those
treated with glucosamine hydrochloride or high doses of TGXTC,
these effects were also observed in the low and medium TGXTC dose
groups during following 8 weeks of treatment. This indicates that
TGXTC may reduce the remodeling rate and stabilize bone remodeling
to delay subchondral sclerosis with the appropriate dose, thus may
be preferable for use in the regulation of subchondral bone
remodeling in OA.
Taken together, these results suggest that TGXTC may
alleviate damage to the cartilage and subchondral bone, and balance
subchondral bone remodeling, delaying subchondral sclerosis via the
regulation of OPG and RANKL expression. This may provide a novel
therapeutic strategy for use in the treatment of OA.
Acknowledgments
The current study was supported by the Key Project
of Fujian Province Department of Science and Technology (grant no.
2012Y4006), the Natural Science Foundation of Fujian Province
(grant nos. 2014J01356 and 2015J01690), the Developmental Fund of
CHEN Keji Integrative Medicine (grant no. CKJ2011004) and the
National Natural Science Foundation of China (grant no.
81202712).
References
|
1
|
Englund M, Guermazi A, Gale D, Hunter DJ,
Aliabadi P, Clancy M and Felson DT: Incidental meniscal findings on
knee MRI in middle-aged and elderly persons. N Engl J Med.
359:1108–1115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hunter DJ: Osteoarthritis. Best Pract Res
Clin Rheumatol. 25:801–814. 2011. View Article : Google Scholar
|
|
3
|
Radin EL and Rose RM: Role of subchondral
bone in the initiation and progression of cartilage damage. Clin
Orthop Relat Res. 213:34–40. 1986.PubMed/NCBI
|
|
4
|
Orth P, Cucchiarini M, Kaul G, Ong MF,
Gräber S, Kohn DM and Madry H: Temporal and spatial migration
pattern of the subchondral bone plate in a rabbit osteochondral
defect model. Osteoarthritis Cartilage. 20:1161–1169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bünger MH, Birkbak M, Pedersen JS, et al:
Effect of bisphosphonate treatment on subchondral bone
nanostructure in the dunkin hartley guinea pig model of
osteoarthritis studied by scanning small-angle X-ray scattering.
Bone. 50(S1): S1172012. View Article : Google Scholar
|
|
6
|
Hudelmaier M, Wirth W, Nevitt M, et al:
Longitudinal rates of change in subchondral bone size in healthy
knees and knees with radiographic osteoarthritis. Osteoarthritis
Cartilage. 21:S2422013. View Article : Google Scholar
|
|
7
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trouvin AP and Goëb V: Receptor activator
of nuclear factor-κB ligand and osteoprotegerin: Maintaining the
balance to prevent bone loss. Clin Interv Aging. 5:345–354.
2010.
|
|
9
|
Tanaka H, Mine T, Ogasa H, Taguchi T and
Liang CT: Expression of RANKL/OPG during bone remodeling in vivo.
Biochem Biophys Res Commun. 411:690–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hawkey C, Laine L, Simon T, Beaulieu A,
Maldonado-Cocco J, Acevedo E, Shahane A, Quan H, Bolognese J and
Mortensen E: Comparison of the effect of rofecoxib (a
cyclooxygenase 2 inhibitor), ibuprofen, and placebo on the
gastroduodenal mucosa of patients with osteoarthritis: a
randomized, double-blind, placebo-controlled trial. The Rofecoxib
Osteoarthritis Endoscopy Multinational Study Group. Arthritis
Rheum. 43:370–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng C, Ye H, Xu X and Liu XX:
Computational pharmacology study of tougu xiaotong granule in
preventing and treating knee osteoarthritis. Chin J Integr Med.
15:371–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin MN and Liu XX: Tougu Xiaotong
decoction for treating 30 cases of osteoarthritis of the knee.
Fujian J Tradit Chin Med. 36:15–16. 2005.In Chinese.
|
|
13
|
Li XH, Wu MX, Ye HZ, Chen WL, Lin JM,
Zheng LP and Liu XX: Experimental study on the suppression of
sodium nitroprussiate-induced chondrocyte apoptosis by Tougu
Xiaotong Capsule (透骨消痛胶囊)-containing serum. Chin J Integr Med.
17:436–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu ZL, Li XH, Wu GW, Ye HZ, WU MX and Liu
XX: Effect of drug-containing serum of Tougu Xiaotong Capsule to
apoptotic pathway of chondrocyte mitochondria. Chin J Tradit Chin
Med. 26:343–346. 2011.
|
|
15
|
Li X, Lang W, Ye H, Yu F, Li H, Chen J,
Cai L, Chen W, Lin R, Huang Y, et al: Tougu Xiaotong capsule
inhibits the tidemark replication and cartilage degradation of
papain-induced osteoarthritis by the regulation of chondrocyte
autophagy. Int J Mol Med. 31:1349–1356. 2013.PubMed/NCBI
|
|
16
|
Ye HZ, Li XH, Chen JS, Zheng CS, Yang JP,
Wong XP, Zeng ZP, Zheng Z and Liu XX: Study on the effect of Tougu
Xiaotong capsule medicated serum on the expression of cyclin D1
mRNA in chondrocytes. J Tradit Chin Orthopedic Traumatol. 24:3–7.
2012.In Chinese.
|
|
17
|
Huang YM, Chen WL, Liu XX, Huang MY, Lin
RH, Li Min, Xiao CY and Wu GW: Histochemical study of
osteoarthritis treated by Tougu Xiaotong granule. Chin J Tradit Med
Traumatol Orthop. 19:1–3. 2011.In Chinese.
|
|
18
|
Liu BL, Zou JL, Liang GQ, Liu XX, Li XH
and Wu GW: Regulatory effects of Tougu Xiaotong granule on
wnt/β-catenin signal pathway of articular chondrocytes. Chin J
Tissue Eng Res. 15:8574–8578. 2011.In Chinese.
|
|
19
|
Liu BL, Liang GQ, Liu XX, Wu GW and Li XH:
Expression of cyclooxygenase-2 and inducible nitric oxide synthase
in primary knee osteoarthritis interfered by Tougu Xiaotong
granules. Chin J Tissue Eng Res Clinic Rehabilit. 15:2034–2037.
2011.In Chinese.
|
|
20
|
Chen Y, Xiao XJ, Bao XP, et al: Analgesia
and anti-inflammatory effects of Tougu Xiaotong prescription. China
J Chin Med. 28:1675–1676. 2013.In Chinese.
|
|
21
|
Huang YM, Chen WL, Lin RH, Huang MY, Li
ZF, Liao NS and Liu XX: Tougu Xiaotong capsule promotes the
proliferation and differentiation of osteoblasts. Chinese J Tissue
Eng Res. 17:5923–5928. 2013.In Chinese.
|
|
22
|
Guidance Suggestions for the Care and Use
of Laboratory Animals. The Ministry of Science and Technology of
the People's Republic of China; Beijing, China: pp. 1–5. 2006, In
Chinese.
|
|
23
|
Liu XX, Li XH and Zhou JT: Experimental
study on replicating knee osteoarthritis by modified Hulth's
modeling method. Zhongguo Zhong Xi Yi Jie He Za Zhi. 25:1004–1008.
2005.In Chinese.
|
|
24
|
Kuettner KE and Cole AA: Cartilage
degeneration in different human joints. Osteoarthritis Cartilage.
13:93–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cox LG, van Donkelaar CC, van Rietbergen
B, Emans PJ and Ito K: Decreased bone tissue mineralization can
partly explain subchondral sclerosis observed in osteoarthritis.
Bone. 50:1152–1161. 2013. View Article : Google Scholar
|
|
26
|
Zhu S, Chen K, Lan Y, Zhang N, Jiang R and
Hu J: Alendronate protects against articular cartilage erosion by
inhibiting subchondral bone loss in ovariectomized rats. Bone.
53:340–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kearns AE, Khosla S and Kostenuik PJ:
Receptor activator of nuclear factor kappaB ligand and
osteoprotegerin regulation of bone remodeling in health and
disease. Endocr Rev. 29:155–192. 2008. View Article : Google Scholar
|
|
28
|
Whyte MP, Obrecht SE, Finnegan PM, Jones
JL, Podgornik MN, McAlister WH and Mumm S: Osteoprotegerin
deficiency and Juvenile Paget's disease. N Engl Med. 347:175–184.
2002. View Article : Google Scholar
|
|
29
|
Trouvin AP and Goëb V: Receptor activator
of nuclear factor-κB ligand and osteoprotegerin: Maintaining the
balance to prevent bone loss. Clin Interv Aging. 5:345–354.
2010.
|
|
30
|
Brzóska MM and Rogalska J: Protective
effect of zinc supplementation against cadmium-induced oxidative
stress and the RANK/RANKL/OPG system imbalance in the bone tissue
of rats. Toxicol Appl Pharmacol. 272:208–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maddi A, Hai H, Ong ST, Sharp L, Harris M
and Meghji S: Long wave ultrasound may enhance bone regeneration by
altering OPG/RANKL ratio in human osteoblast-like Cells. Bone.
39:283–288. 2006. View Article : Google Scholar : PubMed/NCBI
|