Introduction
Family with sequence similarity 172, member A
(FAM172A), also termed C5orf21, was first cloned from human aortic
tissues and its existence was confirmed in our previous study in
2009 (1,2), however, its biological function and
role in disease states remains to be fully elucidated. In our
previous studies, the protein expression of FAM172A was observed in
human endothelial cells, vascular smooth muscle cells and
macrophages, and was upregulated by high glucose in a
concentration- and time-dependent manner (2,3),
which indicated that FAM172A may be involved in the pathogenesis of
diabetic macroangiopathy.
Previously, Harding et al (4) reported that type 1 and type 2
diabetes are associated with increased incidence and mortality
rates from cancer. In addition, Ayturk et al (5) indicated that insulin resistance is an
independent risk factor for thyroid nodule formation, and several
studies reported that insulin resistance was markedly associated
with the occurrence of papillary thyroid carcinoma (PTC) (6–8),
accounting for ~80–85% of all cases of thyroid cancer (9,10).
Whether the FAM172A protein also has positive
effects on PTC remains to be fully elucidated. Therefore, the
present study aimed to first investigate the protein expression
levels of FAM172A in human PTC, followed by the examination of the
effect of the FAM172A protein on the proliferation of IHH-4 human
PTC cells and its potential molecular mechanisms.
Materials and methods
Human thyroid tissues
A total of 12 thyroid specimens wre collected from
nine patients (age, 34–67 years; 7 female patients, 2 male
patients) with thyroid diseases, including three normal thyroid,
three thyroid adenoma, three PTC and three PTC corresponding
pericarcinous tissue samples were collected from patients
undergoing thyroidectomy in the Department of Surgery, Shanghai
Jiao Tong University Affiliated Sixth People's Hospital (Shanghai,
China) between July 2012 and January 2013, and were stored at
−80°C. The present study was approved by the human research ethics
committee of Shanghai Jiao Tong University Affiliated Sixth
People's Hospital, and written informed consent was obtained from
all participants prior to commencement.
Immunohistochemical staining
The thyroid specimens were fixed with 4%
paraformaldehyde (Shanghai Ling Feng Chemical Reagent Co., Ltd.,
Shanghai, China) in ice-cold phosphate-buffered saline (PBS) for 10
min, and rinsed three times in PBS, followed by incubation with 20%
sucrose overnight. Subsequently, the frozen tissue samples were
sectioned (4 µm) and mounted on polylysine pre-coated glass
slides with paraformaldehyde solution with 3%
H2O2 for 30 min at room temperature, prior to
being washed three times with PBS. After being blocked with 0.05%
Tween-20 (Shanghai Ling Feng Chemical Reagent Co., Ltd.) and 5%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in PBS for 30 min at room temperature, the slides
were incubated with rabbit anti-FAM172A polyclonal antibody (cat.
no. ab121364; Abcam, Cambridge, MA, USA) at a dilution of 1:200 at
4°C overnight. The slides were then washed with PBS and incubated
with goat anti-rabbit IgG peroxidase-conjugated antibody (1:1,000;
cat. no. bs10350; Bioword Technology, Inc., St. Louis Park, MN,
USA) for 1 h at 37°C. Following further washing with PBS, the
slides were stained with DAB and were visualized using fluorescence
microscopy (Axio Scope.A1; Zeiss, Oberkochen, Germany).
Cell culture and treatment
The IHH-4 human PTC cell line was provided by
Professor Haixia Guan (First Hospital of China Medical University,
Shenyang, China) and cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) and Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 0.1 mg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) in a 37°C, 5% CO2 incubator. The
medium was refreshed every 48 h, and the cells were sub-cultured
upon, or seeded into plates, on reaching 80% confluence.
Subsequently, control PDC315 or eukaryotic expression vector
PDC315-FAM172A plasmids were transfected into the cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. In the investigation of
the role of the p38 MAPK pathway in FAM172A-stimulated
proliferation, the IHH-4 cells were pre-incubated at 37°C for 2 h
in dimethyl sulfoxide (DMSO; control) or 20 µM SB202190
(dissolved in DMSO; Cell Signaling Technology, Inc., Danvers, MA,
USA), a selective inhibitor of p38 MAPK, prior to being transferred
with the plasmids.
MTT assay
An MTT assay was used to determine IHH-4 cell
proliferation and was performed, as described previously (11,12).
In brief, the IHH-4 cells (104 cells/well) were cultured
in 96-well plates and treated, as described above. Following
treatment 10 µl MTT (Beyotime Institute of Biotechnology,
Haimen, China) was added to each well for 4 h incubation at 37°C.
Following incubation, the medium was discarded and 150 µl
DMSO was added to each well and incubated for 5 min to dissolve the
purple-blue formazan precipitate. Subsequently, the optical density
(OD) was measured using an absorbance microplate reader (ELX800;
Bio-Tek Instruments, Inc., Winooski, VT, USA) at a wavelength of
490 nm. The data were obtained from each experiment with six
replicates.
Cell growth curve
The IHH-4 cells were seeded into 24-well plates at
5×104 cells per well and transfected in accordance with
methods as described above. Following intervention for 24, 48, 72
and 96 h, respectively, cell counting was performed and a cell
growth curve was constructed. Cell counting was performed using a
hemocytometer (Shanghai Qiujing Biochemical Reagents Instrument
Co., Ltd., Shanghai, China) under an XDS-1B model microscope
(Chongqing Mike Photoelectric Instruments Co., Ltd., Chongqing,
China).
Western blot analysis
The thyroid specimen proteins were extracted
following being ground in liquid nitrogen, lysed with cell lysis
buffer, containing radioimmunoprecipitation assay and
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology), and centrifuged at 12,000 × g for 20 min at 4°C.
The cell proteins were collected and the extracted protein (50
µg) was boiled for 5 min for denaturing and separated by 10%
SDS-polyacrylamide gel electrophoresis (Beyotime Institute of
Biotechnology) and then transferred onto a polyvinylidene fluoride
membrane (Pall Corporation, Port Washington, NY, USA). Following
blocking with 5% non-fat milk in 1X Tris-buffered saline with Tween
20 (Shanghai Ling Feng Chemical Reagent Co., Ltd.) for 1 h at room
temperature, the membrane was incubated with primary rabbit
antibodies against FAM172A (1:500; cat. no. ab121364; Abcam), GAPDH
(1:1,000; cat. no. ap0063; Bioworld Technology, Inc., St. Louis
Park, MN, USA) and antibodies associated with cell proliferation
pathways, including p85 PI3K (1:1,000; cat. no. 4292),
phosphorylated (p-)p85 PI3K (Tyr458; 1:1,000; cat. no. 4228), p38
MAPK (1:1,000; cat. no. 9212), p-p38 MAPK (Thr180/Tyr182; 1:1,000;
cat. no. 9211), AMPKα (1:1,000; cat. no. 2532) and p-AMPKα (Thr172;
1:1,000; cat. no. 2535) from Cell Signaling Technology, Inc., at
4°C overnight. Subsequently, the membranes were incubated with
anti-rabbit horseradish peroxidase (HRP)-conjugated IgG secondary
antibody (1:5,000; cat. no. bs10350; Bioworld Technology, Inc.) for
1 h at room temperature. The protein bands were visualized using an
enhanced chemiluminescence kit (Pierce Biotechnology, Inc.,
Rockford, IL, ISA) using Image Quant LAS 4000 mini
chemiluminescence (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA), and quantified using Gel-Pro Analyzer 4.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Each experiment was repeated at least three times
and the results are expressed as the mean ± standard deviation.
One-way analysis of variance was used to analyze data using SPSS
19.0 software (IBM SPSS, Armonk, NY, USA). P<0.05 (two-sided)
was considered to indicate a statistically significant
difference.
Results
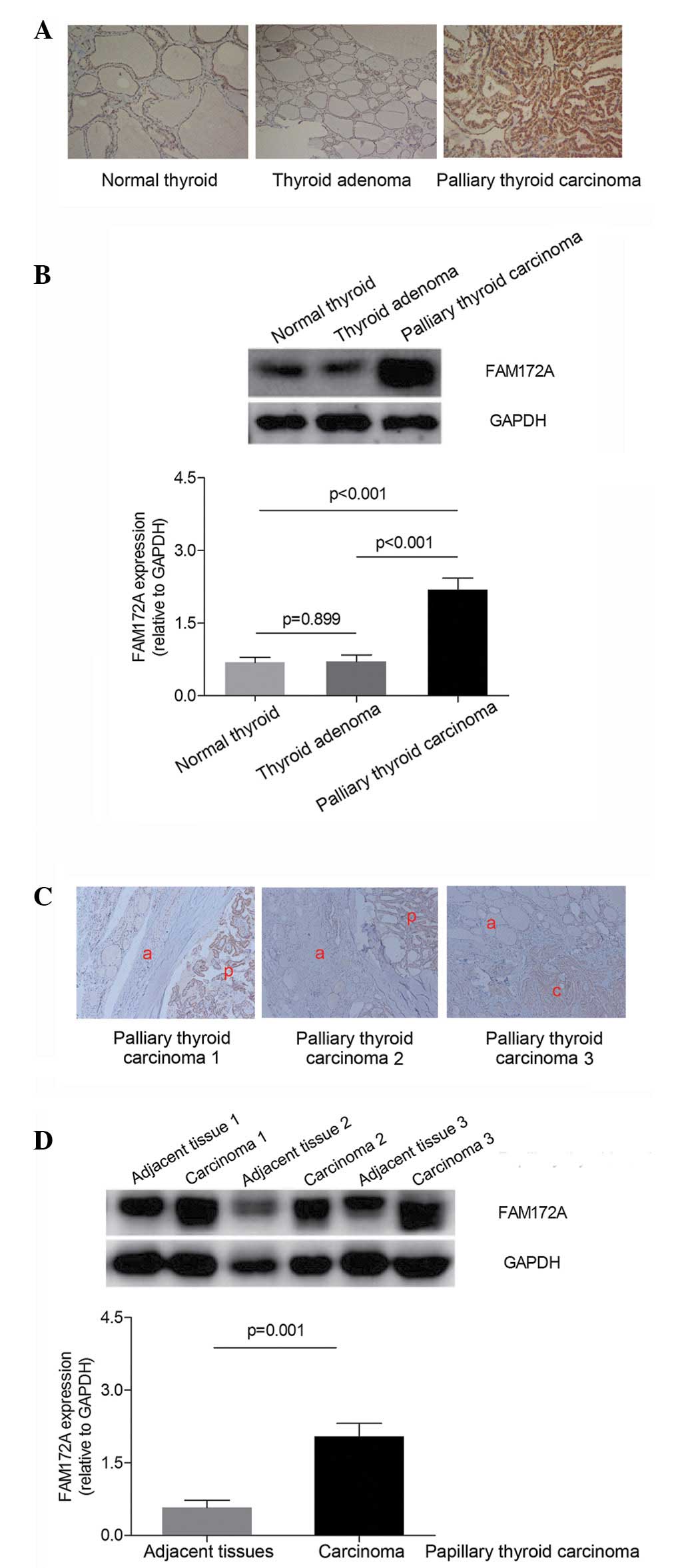
Protein expression levels of FAM172A are
increased in human PTC tissues
As shown in Fig. 1,
the expression levels of FAM172A among the human normal thyroid,
thyroid adenoma and PTC tissues were examined. The results of the
immunohistochemical staining and western blotting demonstrated that
the protein expression of FAM172A was highest in the human PTC
tissues, compared with the normal thyroid and thyroid adenoma
tissues (P<0.001; Fig. 1A and
B). Furthermore, in the patients with PTC, the protein
expression level of FAM172A in the PTC tissues was significantly
higher than that observed in the noncancerous tissues adjacent to
the carcinoma in the same patient (P=0.001; Fig. 1C and D).
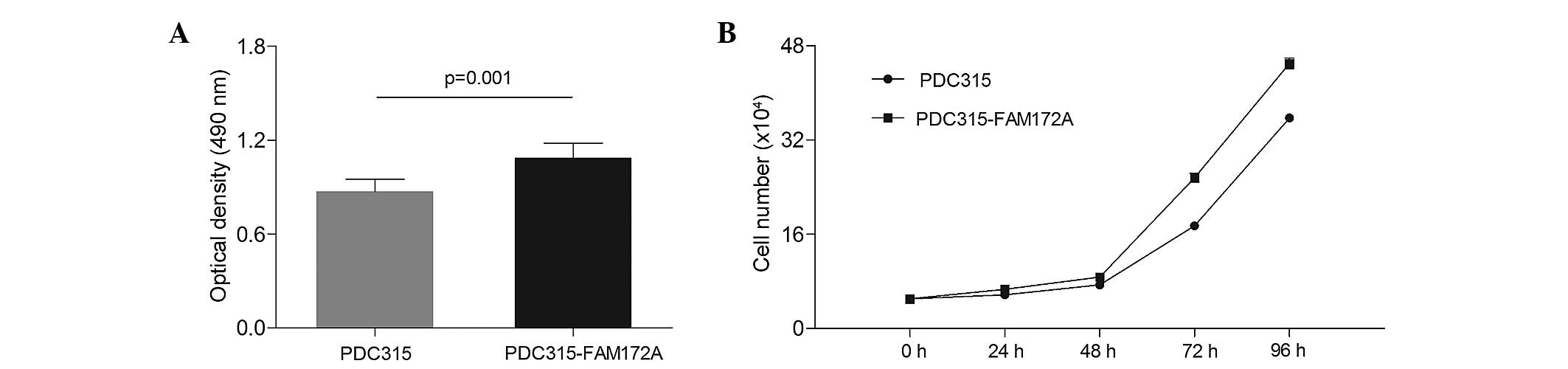
Overexpression of FAM172A accelerates
IHH-4 cell proliferation
To evaluate the proliferative effect of FAM172A on
IHH-4 cells, the numbers of cells were evaluated following
transfection of the cells with PDC315-FAM172A or PDC315 plasmids
using MTT assays and constructing a cell growth curve. As shown in
Fig. 2A, the OD measured for the
cells in the FAM172A group (1.09) was significantly higher than the
OD of the control group (0.87; P=0.001). As shown in Fig. 2B, the cell growth curve indicated
that the numbers of IHH-4 cells (×104) in the group
overexpressing FAM172A were 6.64±0.53, 8.71±0.13, 25.59±0.74 and
44.96±0.94 at 24, 48, 72 and 96 h, respectively, which were
significantly higher than those in the control group of 5.73±0.18,
7.38±0.35, 17.41±0.29 and 35.69±0.51 at 24, 48, 72 and 96 h,
respectively, at each time period (P=0.048 at 24 h; P=0.004 at 48
h; P<0.001 at 72 h and P<0.001 at 96 h; Fig. 2B).
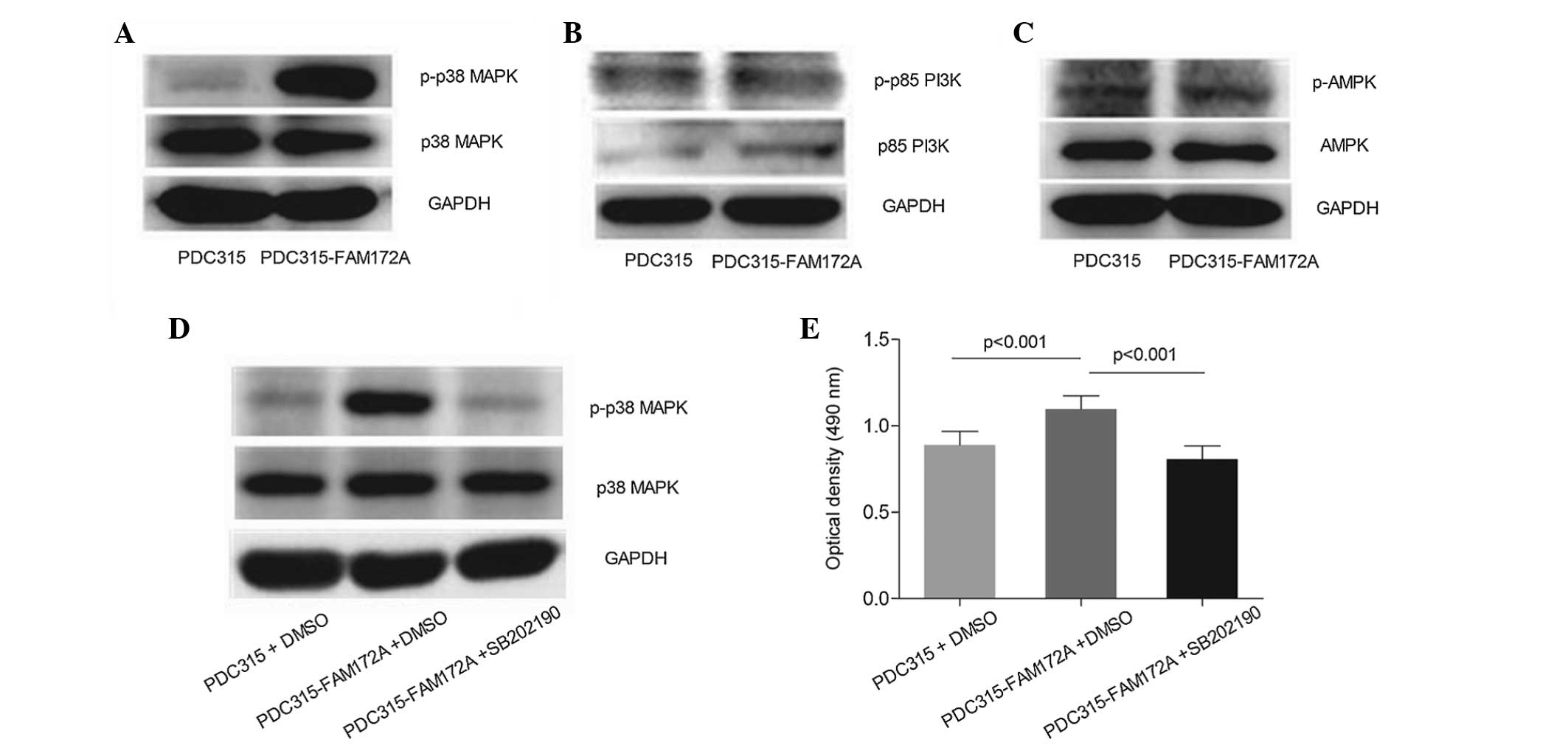
P38 MAPK pathway is involved in the
pro-proliferative effects of overexpressed FAM172A on IHH-4
cells
When FAM172A was transiently transfected into the
IHH-4 cells for 24 h, the overexpression of FAM172A was observed to
trigger robust phosphorylation of p38 MAPK activation, however, no
effect on the PI3K or AMPK signaling pathways were observed. As
shown in Fig. 3A, the expression
of p-p38 MAPK was prominently upregulated in the FAM172A group,
compared with the expression in the control group, whereas no
effect was observed on the expression of total p38 MAPK. No
activation of PI3K or AMPK was observed. As shown in Fig. 3B and C, compared with the control
group, the FAM172A-transfected group exhibited no significant
difference in the protein expression levels of p-p85 PI3K or
p-AMPK, and no changes were observed in the total protein levels of
p85 PI3K and AMPK.
In order to further clarify the role of p38 MAPK on
the proliferation of IHH-4 cells induced by FAM172A, the inhibitor
of p38 MAPK was used (SB202190). As shown in Fig. 3D, the activation of p38 MAPK, which
was induced by FAM172A in the IHH-4 cells, was inhibited by
SB202190. Correspondingly, the MTT assays demonstrated that FAM172A
transfection increased the OD of the IHH-4 cells by 23.4%, compared
with the cells in the control group (P<0.001), however, the
pro-proliferative effect of FAM172A on the IHH4 cells was
significantly attenuated by SB202190 (P<0.001; Fig. 3E).
Discussion
In the present study, the protein expression levels
of FAM172A in PTC were investigation, and it was found that the
protein expression levels of FAM172A in PTC tissues were not only
significantly higher than those in the noncancerous tissues
adjacent to the carcinoma tissues, but they were also markedly
higher than those in the normal thyroid and thyroid adenoma
tissues. To the best of our knowledge, the present study is the
first to confirm that high protein expression levels of FAM172A are
found in human PTC.
PTC is the most common type of malignant tumor of
the endocrine system, and statistics have shown that its incidence
has continuously and sharply increased over previous decades
worldwide (13,14). A previous study indicated that
thyroid cancer is the fifth most common type of cancer in women
(15) and, in Italy, thyroid
cancer is the second most frequent type of cancer in women <45
years of age (16). Therefore,
early detection and prevention is of crucial importance for thyroid
cancer. Various examination techniques, including thyroid
ultrasound, radionuclide imaging and fine needle aspiration
cytology have the potential to improve the accuracy of the
diagnosis of thyroid cancer (17,18),
however, there remains a lack of simple and effective methods to
enable early differential diagnoses for benign and malignant
thyroid nodules. In our previous study, it was demonstrated that
there is a signal peptide sequence in the N paragraph of the
FAM172A protein, determined using bioinformatics techniques
(3), and this signal peptide is a
noteworthy feature of the secreted protein (19).
Considering that uncontrolled cell growth has been
widely known as a fundamental factor for tumor occurrence and
progression (20,21), human PTC IHH-4 cells were used as a
model of PTC in the present study to further examine the underlying
role of the FAM172A protein in PTC. A noticeable increase (25%) in
the OD of the IHH-4 cells was observed following transfection of
the cells with the FAM172 overexpression vectors. The cell growth
curve also showed the same results, and these results suggested
that FAM172A may be important in the pathogenesis of PTC. Analogous
to the results of the present study, our previous study
demonstrated that HEK293 cells transfected with PDC315-FAM172A
vectors proliferated at a markedly faster rate, compared with those
transfected with PDC315 vectors (1).
Subsequently, the present study investigated the
effect of FAM172A on several common signaling pathways associated
with cell proliferation, including the PI3K, p38 MAPK and AMPK
signaling pathways, in IHH-4 cells (22–24).
The results demonstrated that the overexpression of FAM172A caused
marked activation of the p38 MAPK pathway, and this pathway
activation was inhibited following treatment with SB202190, a
selective inhibitor of p38 MAPK. Correspondingly, FAM172A-induced
cell proliferation was attenuated following treatment with
SB202190. The above results indicated that FAM172A may promote cell
proliferation via the p38 MAPK pathway and, thus be involved in the
disease course of PTC. Consistent with these results, a study by
Pomérance et al (25)
showed that p-p38-MAPK is markedly expressed in PTC cells; Huang
et al (26) reported that
norepinephrine stimulates pancreatic cancer cell proliferation
through activation of the p38 MAPK pathway, and Ayllón V et
al (27) demonstrated that
PBK/TOPK promotes tumour cell proliferation through the p38 MAPK
activity. However, Gao et al (28) reported that the p38 MAPK pathway is
involved in the pro-apoptotic effect of notoginsenoside Ft1 on
SH-SY5Y human neuroblastoma cells. The different results among
these previous studies may be due to p38 MAPK having have multiple
functions, including cell proliferation and apoptosis, which appear
to act differently depending on the types of cells and the specific
conditions (29).
The present study has potential implications
regarding the pathogenesis and treatment of PTC. It was suggested
that FAM172A-p38 may be associated with the mechanisms underlying
the evolution and progression of PTC, since it was demonstrated
that FAM172A was able to activate the p38 signaling pathway, thus
promoting cell proliferation, which is an important mechanism
underlying tumor progression. In addition, FAM172A may be used to
identify benign and malignant thyroid tumors, as increased
expression of FAM172A was detected in PTC. The results of the
present study suggested that FAM172A and p38 may be used as
potential drug targets for the adjuvant treatment of PTC, since
FAM172A may activate the p38 signaling pathway and promote cell
proliferation.
In conclusion, the present study was the first, to
the best of our knowledge, to verify significantly high protein
expression levels of FAM172A in human PTC. The present study also
presented evidence that FAM172A may promote cell proliferation via
activating p38 MAPK signaling pathway and, therefore, be involved
in the pathogenesis of PTC. The results of the present study
extends current knowledge of FAM172A, a novel protein, and its role
in disease states, and supports its use as a novel and simple
biomarker for the early identification of PTC.
Acknowledgments
The authors would like to thank the Department of
Surgery of Shanghai Jiao Tong University Affiliated Sixth People's
Hospital for providing the human thyroid tissue samples, and would
also like to thank Professor Haixia Guan for provision of the IHH-4
human PTC cell line. This study was supported by grants from the
National Natural Science Foundation of China (grant. nos. 81170759
and 81502316), the Shanghai Science and Technology Commission
Funded Project (grant. no. 14411964100) and the Shanghai Scientific
Research Innovation Projects (grant. no. 1322015).
References
|
1
|
Li LX, Zhou WB, Tao Z, Deng WJ, Liang WC,
Yang ZH, Ye WW, Bao YQ, Jia WP and Hu RM: Effect of FAM172A protein
on apoptosis and proliferation in HEK293 cells. Zhonghua Yi Xue Za
Zhi. 90:2424–2427. 2010.In Chinese. PubMed/NCBI
|
|
2
|
Li L, Dong X, Leong MC, Zhou W, Yang Z,
Chen F, Bao Y, Jia W and Hu R: Identification of the novel protein
FAM172A and its up-regulation by high glucose in human aortic
smooth muscle cells. Int J Mol Med. 26:483–490. 2010.PubMed/NCBI
|
|
3
|
Li LX, Tao Z, Dong XH, Liang WC, Yang ZH,
Mou B, Bao YQ, Wang C, Jia WP and Hu RM: Molecular cloning of a
novel gene, C5orf21 gene and its roles in diabetic macroangiopathy.
Zhonghua Yi Xue Za Zhi. 89:2574–2577. 2009.In Chinese.
|
|
4
|
Harding JL, Shaw JE, Peeters A, Cartensen
B and Magliano DJ: Cancer risk among people with type 1 and type 2
diabetes: Disentangling true associations, detection bias and
reverse causation. Diabetes Care. 38:734–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ayturk S, Gursoy A, Kut A, Anil C, Nar A
and Tutuncu NB: Metabolic syndrome and its components are
associated with increased thyroid volume and nodule prevalence in a
mild-to-moderate iodine-deficient area. Eur J Endocrinol.
161:599–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gursoy A: Rising thyroid cancer incidence
in the world might be related to insulin resistance. Med
Hypotheses. 74:35–36. 2010. View Article : Google Scholar
|
|
7
|
Bae MJ, Kim SS, Kim WJ, Yi YS, Jeon YK,
Kim BH, Lee BJ, Lee JC, Kim IJ, Wang SG and Kim YK: High prevalence
of papillary thyroid cancer in Korean women with insulin
resistance. Head Neck. 8: View Article : Google Scholar : 2014.
|
|
8
|
Guo K and Wang Z: Risk factors influencing
the recurrence of papillary thyroid carcinoma: A systematic review
and meta-analysis. Int J Clin Exp Pathol. 7:5393–5403.
2014.PubMed/NCBI
|
|
9
|
Li Y, Nakamura M and Kakudo K: Targeting
of the BRAF gene in papillary thyroid carcinoma (review). Oncol
Rep. 22:671–681. 2009.PubMed/NCBI
|
|
10
|
Krawczyk-Rusiecka K,
Wojciechowska-Durczyńska K, Cyniak-Magierska A, et al: COX-2
expression in papillary thyroid carcinoma (PTC) in cytological
material obtained by fine needle aspiration biopsy (FNAB). Thyroid
Res. 4:32011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Meerloo J, Kaspers GJ and Cloos J:
Cell sensitivity assays: The MTT assay. Methods Mol Biol.
731:237–245. 2011. View Article : Google Scholar
|
|
12
|
Lee SB, Li DQ, Tan DT, Meller DC and Tseng
SC: Suppression of TGF-beta signaling in both normal conjunctival
fibroblasts and pterygial body fibroblasts by amniotic membrane.
Curr Eye Res. 20:325–334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schlumberger MJ: Papillary and follicular
thyroid carcinoma. N Engl J Med. 338:297–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
LiVolsi VA: Papillary thyroid carcinoma:
An update. Mod Pathol. 24(Suppl 2): S1–S9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dal Maso L, Lise M, Zambon P, Falcini F,
Crocetti E, Serraino D, Cirilli C, Zanetti R, Vercelli M, Ferretti
S, et al: Incidence of thyroid cancer in Italy, 1991–2005: Time
trends and age-period-cohort effects. Ann Oncol. 22:957–963. 2011.
View Article : Google Scholar
|
|
17
|
Multanen M, Haapiainen R, Leppäniemi A,
Voutilainen P and Sivula A: The value of ultrasound-guided
fine-needle aspiration biopsy (FNAB) and frozen section examination
(FS) in the diagnosis of thyroid cancer. Ann Chir Gynaecol.
88:132–135. 1999.PubMed/NCBI
|
|
18
|
Naswa N, Sharma P, Suman Kc S, Lata S,
Kumar R, Malhotra A and Bal C: Prospective evaluation of
68Ga-DOTA-NOC PET-CT in patients with recurrent medullary thyroid
carcinoma: Comparison with 18F-FDG PET-CT. Nucl Med Commun.
33:766–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joly DL, Feau N, Tanguay P and Hamelin RC:
Comparative analysis of secreted protein evolution using expressed
sequence tags from four poplar leaf rusts (Melampsora spp). BMC
Genomics. 11:4222010. View Article : Google Scholar
|
|
20
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
López-Sáez JF, de la Torre C, Pincheira J
and Giménez-Martín G: Cell proliferation and cancer. Histol
Histopathol. 13:1197–1214. 1998.PubMed/NCBI
|
|
22
|
Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y,
Hou KZ, Jiang YH, Yang XH and Liu YP: Gastric cancer exosomes
promote tumour cell proliferation through PI3K/Akt and MAPK/ERK
activation. Dig Liver Dis. 41:875–880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hardwick JC, van den Brink GR, Offerhaus
GJ, van Deventer SJ and Peppelenbosch MP: NF-kappaB, p38 MAPK and
JNK are highly expressed and active in the stroma of human colonic
adenomatous polyps. Oncogene. 20:819–827. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pomérance M, Quillard J, Chantoux F, Young
J and Blondeau JP: High-level expression, activation and
subcellular localization of p38-MAP kinase in thyroid neoplasms. J
Pathol. 209:298–306. 2006. View Article : Google Scholar
|
|
26
|
Huang XY, Wang HC, Yuan Z, Huang J and
Zheng Q: Norepinephrine stimulates pancreatic cancer cell
proliferation, migration and invasion via β-adrenergic
receptor-dependent activation of P38/MAPK pathway.
Hepatogastroenterology. 59:889–893. 2012.
|
|
27
|
Ayllón V and O'connor R: PBK/TOPK promotes
tumour cell proliferation through p38 MAPK activity and regulation
of the DNA damage response. Oncogene. 26:3451–3461. 2007.
View Article : Google Scholar
|
|
28
|
Gao B, Shi HL, Li X, Qiu SP, Wu H, Zhang
BB, Wu XJ and Wang ZT: P38 MAPK and ERK1/2 pathways are involved in
the pro-apoptotic effect of notoginsenoside Ft1 on human
neuroblastoma SH-SY5Y cells. Life Sci. 108:63–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signaling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|