Introduction
Lung cancer is the leading cause of
cancer-associated mortality in the world (1) and non-small cell lung cancer (NSCLC)
composes the majority of all lung carcinomas (2). Despite previous advances in diagnosis
and treatment, the 5-year survival rate has remained <15%, with
only a 5–10% survival rate for advanced NSCLC (3,4).
Therefore, identifying new therapeutic targets and agents to
improve the prognosis of NSCLC is urgently required.
There has been a growing interest in the use of
natural compounds as a new source of anti-tumor agent owing to
their wide range of biological activities, low toxicity and weak
side effects. Studies investigating the anti-tumor mechanisms of
traditional Chinese herbal medicine, which are predominantly
extracted from natural plants and animals, provide a theoretical
basis and new strategies for the treatment of cancer. Currently,
several anti-tumor components isolated from Chinese herbal
medicines, including cephalotaxine, paclitaxel, podophyllotoxin,
10-hydroxycamptothecin and vinblastine, are used in clinical
practice. Increasing attention has been paid to uncovering the
anti-tumor potential and mechanisms of Chinese herbal medicine.
Chinese herbs have been found to inhibit cell proliferation,
angiogenesis and tumor metastasis, induce cell apoptosis and
differentiation and regulate tumor-associated signaling pathways
and the immune system (5–7), thus exhibiting anti-tumor potential
in vitro and in vivo.
Maslinic acid (MA), a pentacyclic triterpene acid,
is widely present in dietary plants and is particularly abundant in
olive fruit skins. This compound has attracted significant interest
due to its pharmacological safety and its various biological
activities, including its anti-inflammatory, anti-bacterial,
anti-viral and anti-oxidative properties (8–10).
It has previously been reported that MA exerts anti-tumor effects
on HT29 colon cancer cells, DU145 human prostate cancer cells and a
mouse melanoma cell line, which were at least partially associated
with apoptotic induction (11–13).
However, the effects of MA on various types of lung
cancer remain to be elucidated. Thus, in the present study, the
effects of MA on the proliferation and apoptosis of A549 lung
cancer cells and the possible underlying mechanisms were
examined.
Materials and methods
Reagents
MA was purchased from Shanghai Pure One
Biotechnology Co., Ltd. (Shanghai, China). The extract used was a
white powder comprising 98% MA and 2% oleanolic acid. This extract
was stable when stored at 4°C. It was dissolved prior to its use at
10 mg/ml in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO,
USA). A stock solution was frozen and stored at −20°C.
Cell culture
The A549 lung cancer cell line was obtained from the
Department of Cell Biology (China Medical University, Shenyang,
China) and cultured in RPMI-1640 (Gibco-BRL, Carlsbad, CA, USA)
containing 10% fetal calf serum (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) at 37°C in 5% CO2.
The medium was changed daily and the cells were digested using
0.25% trypsin (Biological Industries, Beit HaEmek, Israel). Cells
were treated with different doses of MA (0, 9, 12, 15, 18 and 21
µg/ml) in their logarithmic growth phase.
MTT assay
A549 cells (1×105/well) were plated in
96-well plates and cultured overnight. Subsequently, cells were
incubated with different concentrations of MA (0, 9, 12, 15, 18 and
21 µg/ml) for 24 h, respectively. The corresponding culture
medium was used as an empty control. Briefly, 20 µl of 5
mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT; Nanjing KeyGen Biotech. Co., Ltd., Nanjing, China) solution
was added to each well and incubated for 4 h at 37°C. The
supernatant was then removed from each well and DMSO (150
µl) was added to dissolve the formazan crystals. Absorbance
was measured at 570 nm with a microplate reader (ELx808; BioTek
Instruments, Winooski, VT, USA). Each experiment was performed in
triplicate. The following formula was used to calculate the
inhibition ratio: Inhibition ratio (%) = (1 − M/C) × 100%, where M
is the absorbance of MA-treated cells and C is the absorbance of
control cells.
Flow cytometry
A549 cells (5×105/well) were plated in
6-well plates and cultured overnight. Subsequently, cells were
treated with different concentrations of MA (0, 9, 12, 15, 18 and
21 µg/ml) for 24 h and were harvested by 0.25% trypsin. The
corresponding culture medium was used as the empty control. For
Annexin V/propidium iodide (PI) apoptosis analysis, the cells were
resuspended in 500 µl of binding buffer and adjusted to
1×106/ml. Staining solution containing 5 µl
Annexin V/fluorescein isothiocyanate and 5 µl PI (Nanjing
KeyGen Biotech. Co., Ltd.) was added to the cells and then
incubated at 2–8°C for 15 min in the dark. Following this, the
cells were analyzed using a FACSCalibur flow cytometer (Becton
Dickinson, Franklin Lakes, NJ, USA). CellQuest version 5.1 software
(BD Biosciences, San Jose, CA, USA) was used to analyze the data.
Each experiment was performed in triplicate.
Immunofluorescence
A549 cells (5×105/well) were seeded on
slides in 6-well plates and cultured overnight. Subsequently, the
cells were treated with 18 µg/ml MA at 37°C and 5%
CO2 for 24 h. Corresponding culture medium was used as
the empty control. Cells were washed twice with cold
phosphate-buffered saline (PBS), fixed with methanol and glacial
acetic acid (3:1) for 15 min, stained with Hoechst 33342 (5 mg/l)
at 37°C for 15 min (Sigma-Aldrich), and mounted with 1% glycerol.
Morphological alterations were observed using fluorescence
microscopy (BX53; Olympus, Tokyo, Japan). Each experiment was
performed in triplicate.
Western blot analysis
Cells were seeded in culture flasks, allowed to
attach overnight and incubated with 12 or 18 µg/ml MA for 24
h. An equal quantity of RPMI-1640 was added as a control. Following
that, cells were harvested (cell number >5×106/ml)
and washed twice with cold PBS. Western blot analysis was then
performed. Briefly, the cell pellets were resuspended in lysis
buffer (Nanjing KeyGen Biotech. Co., Ltd.) at 4°C for 1 h.
Following centrifugation at 12,000 × g for 20 min, the supernatant
was collected and stored at −80°C. The protein was quantified using
a bicinchoninic acid quantification kit (Beyotime Institute of
Biotechnology, Haimen, China). A total of 50 µg of protein
was separated by 10% SDS-PAGE (Beijing Solarbio Science &
Technology Co., Ltd.) and transferred onto polyvinylidene fluoride
membranes (Millipore, Billerica, MA, USA). The membranes were
blocked with 5% non-fat milk and incubated overnight at 4°C with
the following antibodies: Mouse monoclonal anti-caspase-3 (1:200;
cat. no. sc-7272), mouse monoclonal anti-caspase-8 (1:500; cat. no.
sc-81656), mouse monoclonal anti-caspase-9 (1:1,000; cat. no.
sc-73548), rabbit polyclonal anti-cleaved caspase-3 (1:500; cat.
no. sc-22171), rabbit polyclonal anti-cleaved caspase-8 (1:500;
cat. no. sc-7890), goat polyclonal anti-cleaved caspase-9 (1:500;
cat. no. sc-22182), mouse monoclonal anti-X-linked inhibitor of
apoptosis protein (XIAP; 1:500; cat. no. sc-55552), mouse
monoclonal anti-c-IAP1 (1:500; cat. no. sc-271419), rabbit
polyclonal anti-c-IAP2 (1:500; cat. no. sc-7944), rabbit polyclonal
anti-Survivin (1:500; cat. no. sc-10811), rabbit polyclonal
anti-Smac (1:500; cat. no. sc-22766) and rabbit polyclonal
anti-GAPDH (1:2,000; cat. no. sc-25778), all purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Following incubation
with peroxidase-conjugated anti-goat IgG (1:3,000; cat. no.
SC-2020; Santa Cruz Biotechnology, Inc.) and peroxidase-conjugated
anti-rabbit IgG (1:3,000; cat. no. SC-2004; Santa Cruz
Biotechnology, Inc.) at room temperature for 2 h, proteins were
visualized using enhanced chemiluminescence (Pierce Biotechnology,
Inc., Rockford, IL, USA) and detected using BioImaging Systems (UVP
Inc., Upland, CA, USA).
Statistical analysis
All data were analyzed with SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). All values are expressed as the
mean ± standard deviation. One-way analysis of variance and
Fisher's least significant difference test was used to compare the
differences between individual groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
MA treatment inhibits the proliferation
of A549 lung cancer cells
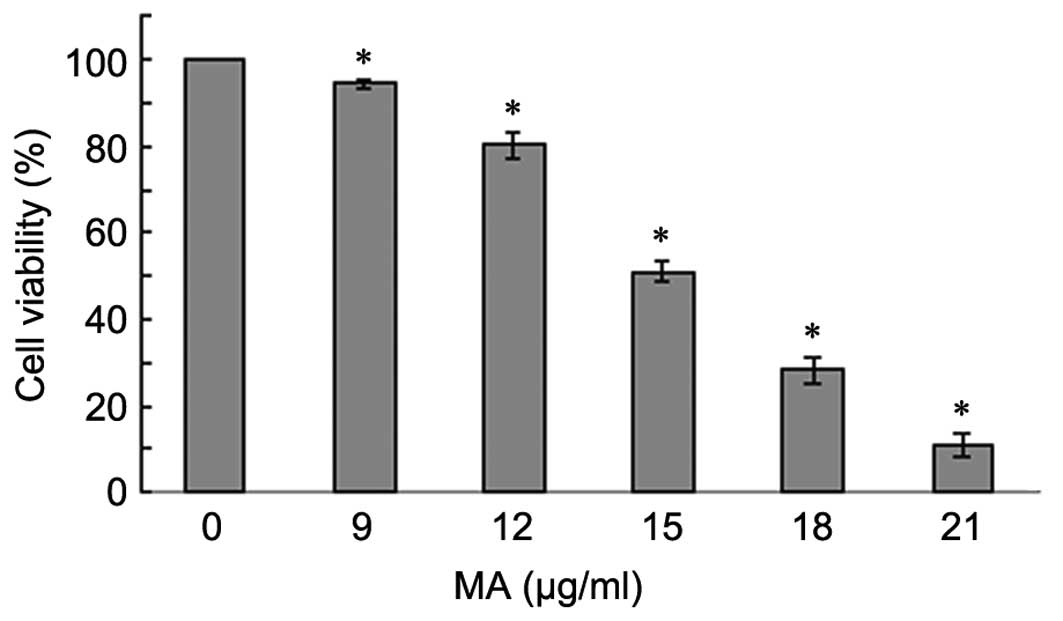
To examine the effect of MA on the proliferation of
A549 cells, an MTT assay was performed in cells treated with
different doses of MA (0, 9, 12, 15, 18 and 21 µg/ml) for 24
h. As the treatment dose increased, cell growth rate significantly
decreased (P<0.05; Fig. 1),
suggesting that MA treatment suppressed A549 cell proliferation in
a dose-dependent manner.
MA treatment induces apoptosis of A549
lung cancer cells
MA was reported to have anti-tumor effects on HT29
colon cancer cells, DU145 human prostate cancer cells and B16F0
mouse melanoma cell line, due to its role in apoptosis induction
(12–14). Therefore, the effect of MA on the
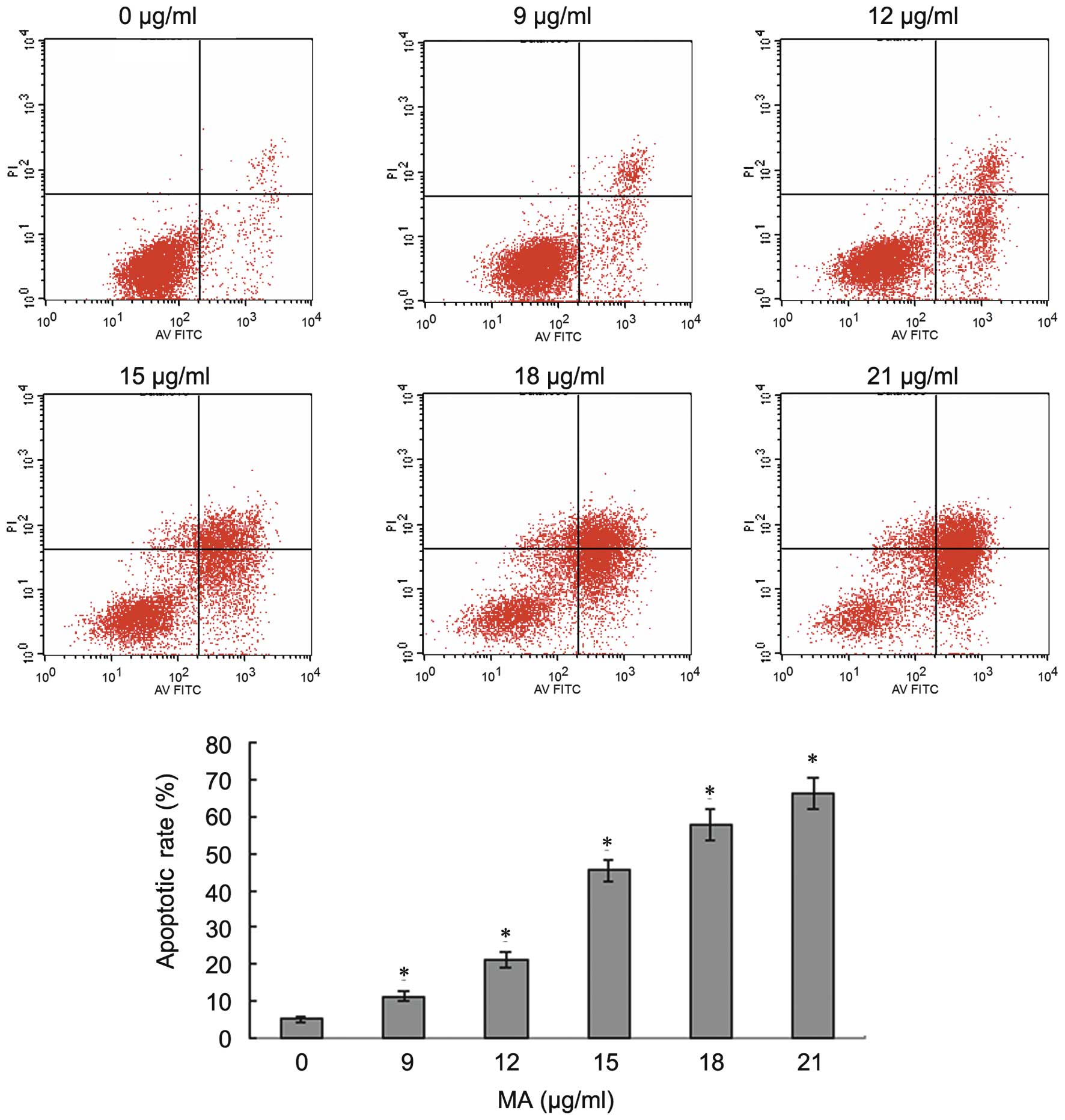
apoptosis of A549 cells was examined. A549 cells were incubated
with different doses of MA (0, 9, 12, 15, 18 and 21 µg/ml)
for 24 h and then Annexin V/PI flow cytometric analysis was
performed to investigate the effect of MA on the apoptosis of NSCLC
cells. As shown in Fig. 2, early
apoptosis and late apoptosis markedly increased as the
concentration of MA increased. The percentages of apoptotic cells
were 5.73, 11.31, 21.06, 44.72, 57.71 and 66.10% following
treatment with 0, 9, 12, 15, 18 and 21 µg/ml MA,
respectively. The apoptotic rates induced by different doses of MA
were significantly higher compared with that in untreated cells of
the control group (P<0.05). When the dose of MA increased, the
number of apoptotic cells increased. The results indicated that MA
induced apoptosis of A549 cells in a dose-dependent manner.
MA treatment induces apoptotic
morphological alterations in A549 lung cancer cells
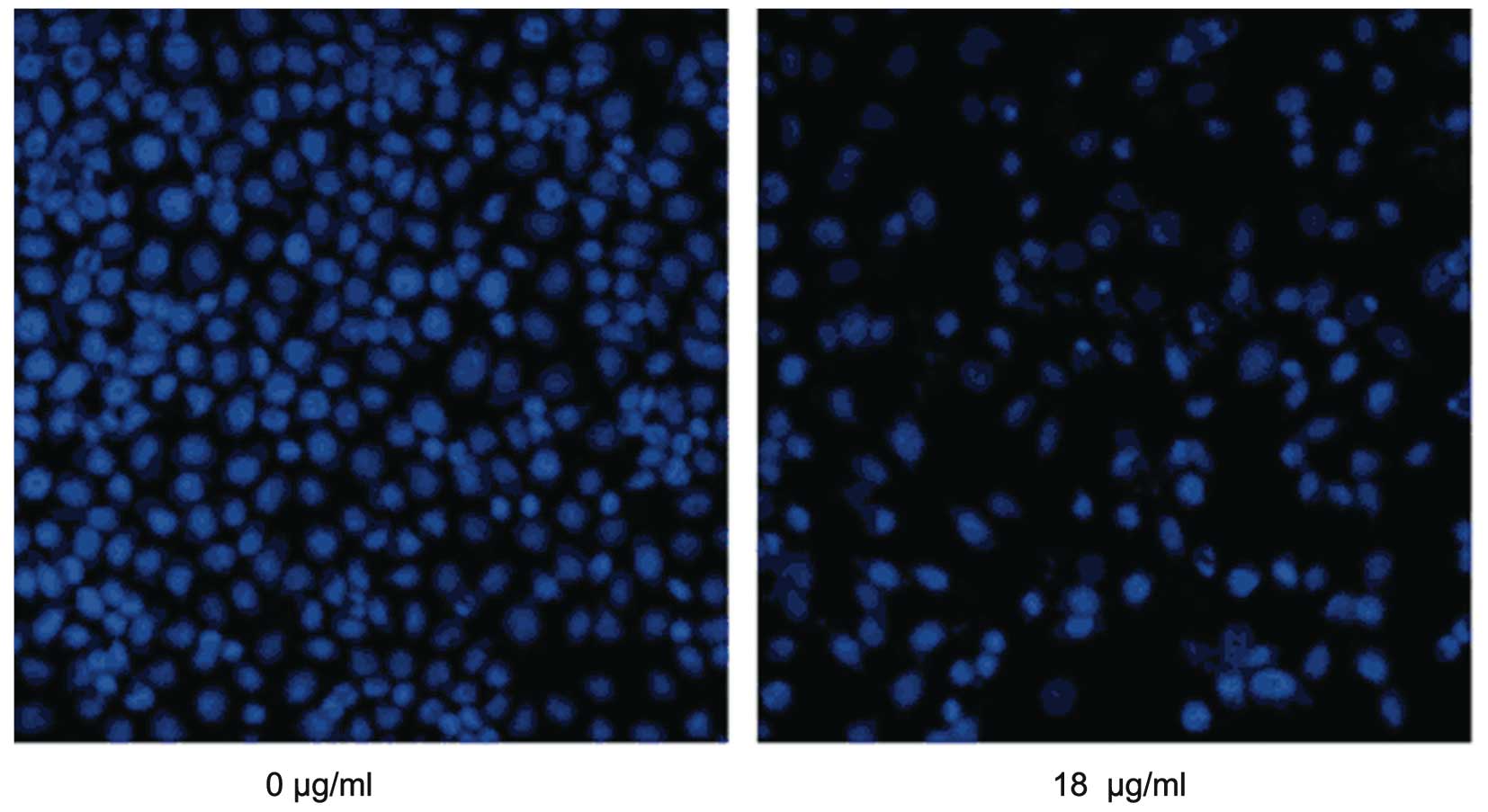
Morphological alterations of cells is another
important index of apoptotic detection. A549 cells were treated
with 18 µg/ml MA for 24 h, stained with Hoechst 33342 and
were then observed under fluorescence microscopy. It was found that
MA treatment caused marked morphological alterations, including
chromatin condensation, karyopyknosis and nuclear fragmentation,
which are characteristic features of apoptotic cells (Fig. 3). In accordance with flow
cytometric analysis, when the dose of MA increased, more apparent
morphological alterations and more apoptotic cells were
observed.
MA treatment regulates the expression of
apoptosis-associated proteins
To further examine the mechanism of MA-induced
apoptosis, the effects of MA on the protein expression of
caspase-3, -8 and -9 and cleaved caspase-3, -8 and -9, which are
important apoptosis-associated proteins, were examined. A549 cells
were treated with either 12 or 18 µg/ml MA for 24 h and then
the levels of caspase family proteins were analyzed by western blot
analysis. As shown in Fig. 4, MA
treatment suppressed the expression of caspase-3, -8 and -9, but
promoted the expression of cleaved caspase-3, -8 and -9. In
addition, as the doses increased, caspase-3, -8 and -9 decreased
and cleaved caspase-3, -8 and -9 increased, suggesting that MA
regulated the cleavage of caspase-3, -8 and -9 in a dose-dependent
manner.
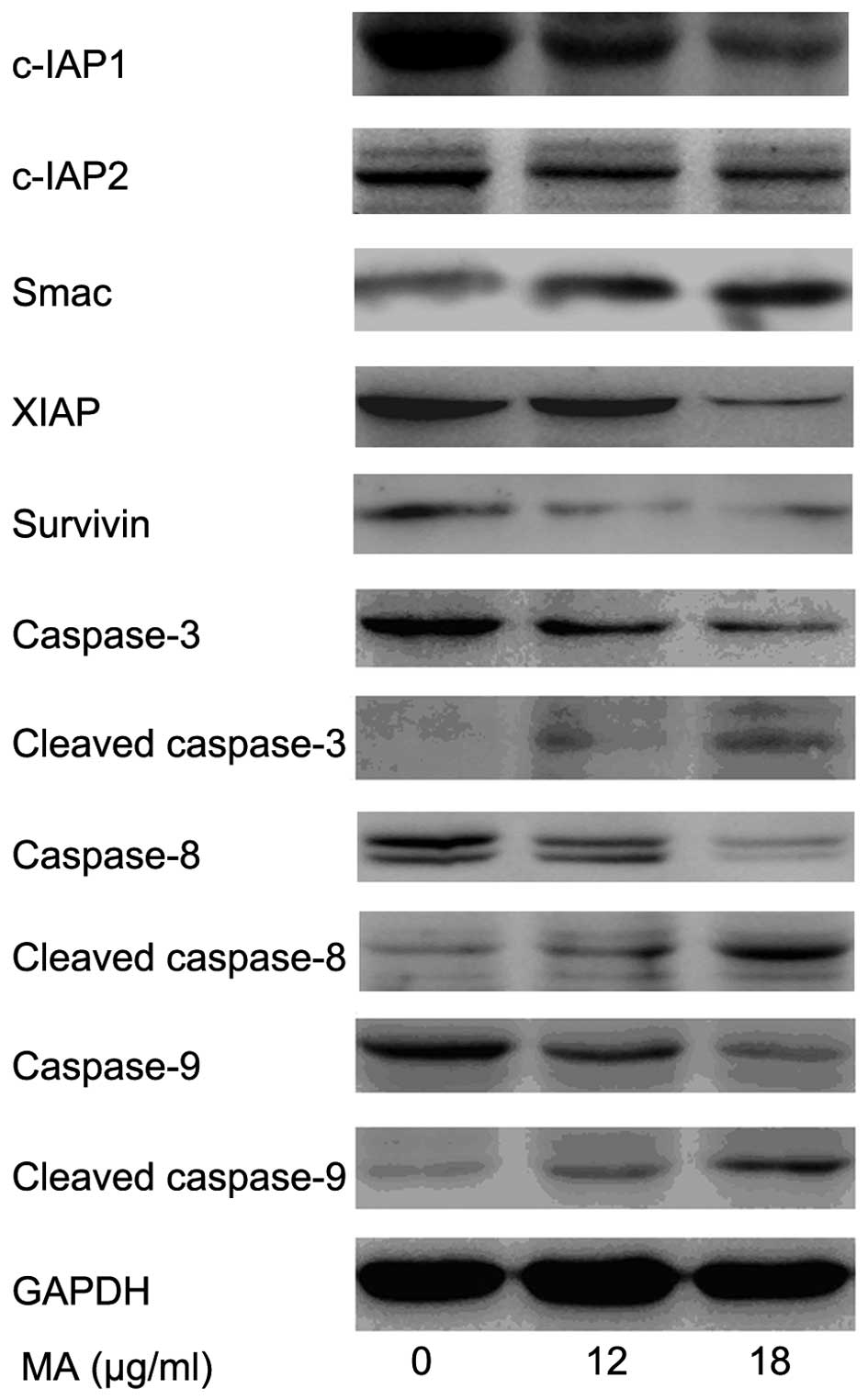 | Figure 4MA treatment regulates the expression
of apoptosis-associated proteins. A549 cells were treated with
different doses of MA (0, 12 and 18 µg/ml) for 24 h. MA
treatment increased the protein levels of Smac, cleaved caspase-3,
-8 and -9, and decreased the protein levels of c-IAP1, c-IAP2,
XIAP, Survivin and caspase-3, -8 and -9 in a dose-dependent manner.
MA, maslinic acid; XIAP, X-linked inhibitor of apoptosis protein;
IAP, inhibitor of apoptosis. |
Smac and inhibitors of apoptosis (IAPs) family
proteins also have a critical role in the regulation of apoptosis
by inhibiting caspase family proteins. Thus, the expression of Smac
and IAP family proteins, including c-IAP1, c-IAP2, XIAP and
Survivin was investigated in cells treated with different
concentrations of MA (0, 12 and 18 µg/ml). MA treatment
increased the protein level of Smac and decreased the protein
levels of c-IAP1, c-IAP2, XIAP and Survivin in a dose-dependent
manner (Fig. 4).
Discussion
Apoptosis is an active form of cellular suicide
encoded by an endogenous program that can be triggered by either
internal or external cues. It is well established that resistance
to apoptosis is a hallmark of cancer (15) and suppression of apoptosis is
closely associated with the progression of various types of cancer,
including NSCLC.
Caspases are a family of cysteine-containing
proteolytic enzymes that have a central role in the execution-phase
of cell apoptosis. Currently, 14 mammalian caspases have been
found, which exist as inactive proenzymes distributed in different
cellular compartments. The caspases consist of two sub-groups,
initiator caspases, including caspase-2, -8, -9 and -10, and
executioner caspases, including caspase-3, -6 and -7, which form a
caspase-cascade system that has a central role in the induction,
transduction and amplification of intracellular apoptotic signals
(16). Caspase-3 is a major
caspase, which amplifies signals from intrinsic and extrinsic
pathways (17). Caspase-8 is
important in the death receptor-mediated extrinsic pathway.
Caspase-9 is regarded as the canonical caspase in the intrinsic
mitochondrial pathway that is regulated primarily by Bcl-2 family
and Bcl-2 homologous domain-3 only proteins (18). Caspase-3, -8 and -9 are synthesized
as inactive pro-enzymes that are activated by proteolytic cleavage
in cells undergoing apoptosis.
In addition, IAP family proteins are important in
the regulation of apoptosis by inhibiting caspases. This protein
family includes XIAP, cellular IAP1/2 and Survivin. IAPs are often
found to be overexpressed in several types of human cancer and
contribute to chemoresistance (19,20).
XIAP can inhibit apoptosis by binding and inactivating caspases,
including initiator caspase-9 and the effector caspase-3. XIAP is
an important member of the mammalian IAP protein family, as it is
the only member capable of inhibiting active caspases (21,22).
cIAP-1 and cIAP-2 are predominantly involved in the regulation of
the extrinsic pathway of apoptosis, through the inhibition of
caspase-8 activation (23,24). Survivin has been demonstrated to
inhibit caspase-dependent apoptosis through co-operation with XIAP
and interference with caspase-3/9 (25,26).
By contrast, Smac is a mitochondrial apoptogenic molecule that is
released from the mitochondria in response to apoptotic stress.
Smac is known to antagonize the function of IAPs (27–29).
Dysregulation of cell proliferation and apoptosis
has been verified to be closely associated with tumor progression,
and a number of anticancer drugs have been designed to induce
apoptosis of cancer cells by targeting cellular processes,
including cell growth, metabolism and proliferation (30). There has been a growing interest in
the use of traditional Chinese medicines as a new source of
anti-tumor agents owing to their wide range of biological
activities, low toxicity and side effects. It is reported that
certain terpenoids originating from Chinese medicine, particularly
certain triterpenoids, have potential anti-tumor activities, which
are often associated with apoptosis induction (31). MA is a pentacyclic triterpene acid,
which is present in several dietary plants. This compound has been
demonstrated to have abundant biological activities, including
anti-inflammatory, anti-bacterial, anti-viral, anti-oxidative,
anti-proliferative, anti-angiogeneic properties as well as the
ability to induce apoptosis (32).
Previous studies have demonstrated that MA has anti-cancer capacity
in different cell types, including liver, breast, pancreatic and
prostate cancer (11,12,33–35).
Specifically in colon malignancy, MA induced apoptosis in HT29
human colon cancer cells through the mitochondrial apoptotic
pathway (35).
In the present study, the A549 lung cancer cell line
was treated with different doses of MA and it was found that MA
significantly inhibited A549 cell growth in a dose-dependent
manner. In addition, AnnexinV/PI flow cytometric analysis was
performed to investigate the effect of MA on the apoptosis of A549
cells. The results demonstrated that MA induced A549 apoptosis in a
dose-dependent manner. Similarly, it was confirmed that MA induced
apoptosis by observing the morphological alterations of cells,
which exhibited typical apoptotic morphological characteristics. In
addition, the effects of MA treatment on the protein expression of
caspase-3, -8 and -9 and cleaved caspase-3, -8 and -9, which are
important apoptosis-associated proteins, were examined. As shown in
the results, MA treatment suppressed the expression of caspase-3,
-8 and -9, but promoted the expression of cleaved caspase-3, -8 and
-9. As the dose increased, caspase-3, -8 and -9 decreased and
cleaved caspase-3, -8 and -9 increased, suggesting that MA
regulated caspase cleavage in a dose-dependent manner.
IAP family proteins are considered to regulate
apoptosis by inhibiting caspases, while Smac is known to antagonize
the function of IAPs. In order to examine the possible mechanism by
which MA regulated the activity of caspase-3, -8 and -9, the levels
of IAPs and Smac, which are possible upstream regulators of
caspases, were examined. The present study demonstrated that MA
treatment increased the protein level of Smac and decreased the
protein levels of c-IAP1, c-IAP2, XIAP and Survivin in a
dose-dependent manner.
Taken together, the results indicated that MA
treatment inhibited proliferation and induced apoptosis of A549
lung cancer cells. MA promoted apoptosis by regulating the cleavage
of caspase-3, -8 and -9 in a dose-dependent manner. Furthermore,
the results suggested that MA increases the expression of Smac and
decreases the expression of c-IAP1, c-IAP2, XIAP and Survivin,
which leads to caspase cleavage. MA treatment inhibited the
proliferation of A549 lung cancer cells in a dose-dependent manner
by inducing cell apoptosis. MA induced apoptosis via regulating the
cleavage of caspase-3, -8 and -9, by increasing Smac expression and
decreasing c-IAP1, c-IAP2, XIAP and Survivin expression.
Acknowledgments
This study was supported by the Liaoning Province
Science and Technology Plan project (grant no. 2013225021) and the
Outstanding Scientific Fund of Shengjing Hospital (grant no.
201205).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raponi M, Zhang Y, Yu J, Chen G, Lee G,
Taylor JM, Macdonald J, Thomas D, Moskaluk C, Wang Y and Beer DG:
Gene expression signatures for predicting prognosis of squamous
cell and adenocarcinomas of the lung. Cancer Res. 66:7466–7472.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mountain CF: The international system for
staging lung cancer. Semin Surg Oncol. 18:106–115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu B, Wang SS and Du Q: Traditional
Chinese medicine for prevention and treatment of hepatocarcinoma:
From bench to bedside. World J Hepatol. 7:1209–1232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CF, Lin SS, Liao PH, Young SC and
Yang CC: The immunopharmaceutical effects and mechanisms of herb
medicine. Cell Mol Immunol. 5:23–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu HB, Zheng LP, Li L, Xu LZ and Fu J:
Elemene, one ingredient of a Chinese herb, against malignant
tumors: A literature-based meta-analysis. Cancer Invest.
31:156–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mokhtari K, Rufino-Palomares EE,
Pérez-Jiménez A, Reyes-Zurita FJ, Figuera C, García-Salguero L,
Medina PP, Peragón J and Lupiáñez JA: Maslinic acid, a triterpene
from olive, affects the antioxidant and mitochondrial status of
B16F10 melanoma cells grown under stressful conditions. Evid Based
Complement Alternat Med. 2015:2724572012.
|
|
9
|
Yan SL, Yang HT, Lee HL and Yin MC:
Protective effects of maslinic acid against alcohol-induced acute
liver injury in mice. Food Chem Toxicol. 74:149–155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin X, Qui C and Zhao L: Maslinic acid
protects vascular smooth muscle cells from oxidative stress through
Akt/Nrf2/HO-1 pathway. Mol Cell Biochem. 390:61–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reyes-Zurita FJ, Pachón-Peña G, Lizárraga
D, Rufino-Palomares EE, Cascante M and Lupiáñez JA: The natural
triterpene maslinic acid induces apoptosis in HT29 colon cancer
cells by a JNK-p53-dependent mechanism. BMC cancer. 11:1542011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SY, Nho CW, Kwon DY, Kang YH, Lee KW
and Park JH: Maslinic acid inhibits the metastatic capacity of
DU145 human prostate cancer cells: Possible mediation via
hypoxia-inducible factor-1α signalling. Br J Nutr. 109:210–222.
2013. View Article : Google Scholar
|
|
13
|
Parra A, Rivas F, Martin-Fonseca S,
Garcia-Granados A and Martinez A: Maslinic acid derivatives induce
significant apoptosis in b16f10 murine melanoma cells. Eur J Med
Chem. 46:5991–6001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peragón J, Rufino-Palomares EE,
Muñoz-Espada I, Reyes-Zurita FJ and Lupiáñez JA: A new HPLC-MS
method for measuring maslinic acid and oleanolic acid in HT29 and
HepG2 human cancer cells. Int J Mol Sci. 16:21681–21694. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuribayashi K, Mayes PA and El-Deiry WS:
What are caspases 3 and 7 doing upstream of the mitochondria?
Cancer Biol Ther. 5:763–765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lakhani SA, Masud A, Kuida K, Porter GA
Jr, Booth CJ, Mehal WZ, Inayat I and Flavell RA: Caspases 3 and 7:
Key mediators of mitochondrial events of apoptosis. Science.
311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuentes-Prior P and Salvesen GS: The
protein structures that shape caspase activity, specificity,
activation and inhibition. Biochem J. 384:201–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
LaCasse EC, Baird S, Korneluk RG and
MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging
role in cancer. Oncogene. 17:3247–3259. 1998. View Article : Google Scholar
|
|
20
|
Deveraux QL and Reed JC: IAP family
proteins-suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deveraux QL, Leo E, Stennicke HR, Welsh K,
Salvesen GS and Reed JC: Cleavage of human inhibitor of apoptosis
protein XIAP results in fragments with distinct specificities for
caspases. EMBO J. 18:5242–5251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riedl SJ, Renatus M, Schwarzenbacher R,
Zhou Q, Sun C, Fesik SW, Liddington RC and Salvesen GS: Structural
basis for the inhibition of caspase-3 by XIAP. Cell. 104:791–800.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rothe M, Pan MG, Henzel WJ, Ayres TM and
Goeddel DV: The TNFR2-TRAF signaling complex contains two novel
proteins related to baculoviral inhibitor of apoptosis proteins.
Cell. 83:1243–1252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-kappaB antiapoptosis: Induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li F: Survivin study: What is the next
wave? J Cell Physiol. 197:8–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiozaki EN and Shi Y: Caspases, IAPs and
Smac/DIABLO: Mechanisms from structural biology. Trends Biochem
Sci. 29:486–494. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Srinivasula SM, Hegde R, Saleh A, Datta P,
Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y
and Alnemri ES: A conserved XIAP-interaction motif in caspase-9 and
Smac/DIABLO regulates caspase activity and apoptosis. Nature.
410:112–116. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song Z, Yao X and Wu M: Direct interaction
between survivin and Smac/DIABLO is essential for the
anti-apoptotic activity of survivin during taxol-induced apoptosis.
J Biol Chem. 278:23130–23140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Feng Y, Wang N, Cheung F, Tan HY,
Zhong S, Li C and Kobayashi S: Chinese medicines induce cell death:
The molecular and cellular mechanisms for cancer therapy. Biomed
Res Int. 2014:5303422014.PubMed/NCBI
|
|
31
|
Leake I: Colorectal cancer:
Chemopreventive action of synthetic triterpenoids in CRC. Nat Rev
Gastroenterol Hepatol. 11:3952014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yap WH and Lim YH: Mechanistic
perspectives of maslinic acid in targeting inflammation. Biochem
Res Int. 2015:2793562015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin CC, Huang CY, Mong MC, Chan CY and Yin
MC: Antiangiogenic potential of three triterpenic acids in human
liver cancer cells. J Agric Food Chem. 59:755–762. 2011. View Article : Google Scholar
|
|
34
|
Rufino-Palomares EE, Reyes-Zurita FJ,
García-Salguero L, Mokhtari K, Medina PP, Lupiáñez JA and Peragón
J: Maslinic acid, a triterpenic anti-tumoural agent, interferes
with cytoskeleton protein expression in HT29 human colon-cancer
cells. J Proteomics. 83:15–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reyes-Zurita FJ, Rufino-Palomares EE,
Lupiáñez JA and Cascante M: Maslinic acid, a natural triterpene
from Olea europaea L., induces apoptosis in HT29 human colon-cancer
cells via the mitochondrial apoptotic pathway. Cancer Lett.
273:44–54. 2009. View Article : Google Scholar
|