Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality worldwide (1). Non-small cell lung cancer (NSCLC)
constitutes up to 85% of all lung cancer cases (2) and is frequently diagnosed at the
advanced stages of disease; thus, curable surgical intervention is
not usually an option. Additionally, NSCLC is resistant to
chemotherapy (3,4) and radiation treatment, resulting in a
poor overall 5-year survival rate for patients with NSCLC of
<15% (2). Cisplatin is the most
widely used chemotherapeutic agent for NSCLC treatment; however,
the effect of cisplatin on NSCLC has several limitations, including
insensitivity in certain patients and high levels of toxicity
(5). Thus, there is a critical
requirement for the improved early diagnosis of lung cancer and
development of novel therapies and strategies for the treatment of
lung cancer. The development of anticancer therapeutic agents from
natural products is an area of considerable interest and importance
(5).
Podophyllotoxin (PPT) is isolated from the roots and
rhizomes of Podophyllum species, including Podophyllum
hexandrum and Podophyllum peltatum. The compound appears
to have useful antimitotic/cytotoxic activities and offers
potential as an agent for antitumor therapy. Semi-synthetic PPT
compounds, including etoposide (VP16), etopophos and teniposide,
have been developed and are currently being used clinically to
treat lung cancer and other neoplasms, including refractory
testicular tumors, lymphoma and nonlymphomatic leukemia (6,7).
However, due to cancer cell drug resistance and the side effects
associated with PPT and associated compounds, the identification of
more potent, but less toxic, anticancer analogues of PPT has become
an intense area of investigation (8). Deoxypodophyllotoxin (DPT) is a potent
antitumor and anti-inflammatory agent in vitro, which has a
close structural association with PPT (9,10).
In our previous study, nine novel derivatives of DPT were
synthesized (11). The results
identified one novel derivative, incorporating L-amino acid, which
demonstrated superior antitumor activity, compared with VP16 in
various cancer cell lines, including the NSCLC A549 cell line.
Therefore, the present study investigated the anticancer effects
and molecular activities of this derivative, N-(1-oxyl-4′-d
emethyl-4-deoxypodophyllic)-L -me thine-4′-piperazine carbamate
(LJ12; Fig. 1), in the human NSCLC
A549 cell line in vitro.
Materials and methods
Cell lines and culture
Human A549 NSCLC, HepG2 hepatocellular carcinoma,
and Hela and SiHa cervical cancer cell lines were obtained from the
Center of Experimental Medicine (Lanzhou, China) and maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), supplemented with 10% newborn calf serum (Hangzhou
Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou,
China), 100 µg/ml streptomycin and 100 IU/ml penicillin
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified
atmosphere of 5% CO2.
3-[4,5-Dimethylthiazo
l-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) assay of cell
viability
The effect of LJ12, synthesized by the School of
Pharmacy of Lanzhou University (Lanzhou, China) on the regulation
of cell viability was assayed using MTT (Sigma-Aldrich). The cells
were seeded at a density of 5×103 cells per well and
grown overnight, following which they were treated with LJ12 at
various doses (0.001–10 µM) and durations (12–60 h) at 37°C
in an atmosphere containing 5% CO2. LJ12 was synthesized
in the laboratory at the School of Pharmacy of Lanzhou University,
according to methods described previously (12), and was 8.59 mg LJ12 was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at a concentration of
100 mM as a stock solution. The final DMSO concentration used in
the culture medium was below 0.1% (v/v). Following incubation with
the drug, 20 µl MTT solution (5 mg/ml) was added to each
well, and the plates were further incubated for 4 h at 37°C.
Subsequently, 150 µl DMSO was added into each well to
dissolve the MTT-converted products, and the optical density of
each well was measured at 570 nm using a microplate reader (Bio-Rad
550; Bio-Rad Laboratories, Inc., Hercules, CA, USA). VP16, obtained
from Jiangsu Hengrui Medicine Co., Ltd. (Nanjing, China) was used,
at the same concentrations as in the treatment groups, as a
positive control. DMSO was used as the vehicle control.
Flow cytometric analysis of cell cycle
and apoptosis
The cells were seeded in culture flasks at a density
of 2.5×105 and treated with LJ12 or VP16 at various
concentrations for 12 or 24 h at 37°C in 5% CO2.
Following treatment, the cells were washed with phosphate-buffered
saline (PBS), fixed in ice-cold 70% ethanol at 4°C overnight and
stained with a propidium iodide (PI; Sigma-Aldrich) solution (80
µg/ml) containing Triton X-100 (0.1%; v⁄v; Sigma-Aldrich)
and RNase A (100 µg⁄ml; Sigma-Aldrich) in PBS. Following
staining, the DNA content was analyzed using a BD FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and CellQuest
Pro software (BD Biosciences).
For the assessment of cell apoptosis, the LJ12 or
VP16 treated cells were subjected to Annexin V-PI staining using an
Annexin V-fluorescein isothiocyanate/PI double staining apoptosis
detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). Briefly, 2×105 A549 cells were treated with LJ12
(0.05 µM) for 6, 12 and 24 h, harvested, washed twice with
PBS and resuspended in 500 µl 1X Hepes binding buffer
(Nanjing Jiancheng Bioengineering Institute). Subsequently, 5
µl Annexin V and 5 µl PI were added to the cell
solution, mixed and incubated at the room temperature in the dark
for 5 min, followed by analysis using the BD FACSCalibur flow
cytometer and CellQuest Pro 4.0 software (BD Biosciences, Franklin
Lakes, NJ, USA).
Hoechst 33258 staining and evaluation of
cell morphology
The apoptotic nuclei of the tumor cells were
visualized using Hoechst 33258 (10 µg⁄ml, Sigma-Aldrich)
staining. The A549 cells (2.5×105/well) were grown on
glass slides to ~70–80% confluency, and were then treated with 0.5
µM LJ12 or VP16 for 24 h at 37°C in an atmosphere containing
5% CO2. Following treatment, the cells were fixed,
washed twice with PBS and stained with Hoechst 33258 staining
solution, according to the manufacturer's protocol (Beyotime
Institute of Biotechnology, Jiangsu, China). Chromosomal
condensation and morphological changes were observed and quantified
using an Olympus BX61 fluorescence microscope (Olympus Corporation,
Tokyo, Japan).
Immunofluorescence
The A549 cells (2.5×105/well) were plated
onto coverslips and then treated with or without 0.5 µM VP16
or LJ12 for 24 h at 37°C in an atmosphere containing 5%
CO2. Following treatment, the cells were fixed with 4%
paraformaldehyde (Tianjin Tuo Ou Li Yuan Chemical Co., Ltd.,
Tianjin, China) in PBS for 4 h at room temperature and
permeabilized in 0.1% Triton X-100 in PBS for 30 min at room
temperature. The cells were then incubated with primary mouse
anti-human α-tubulin monoclonal antibody (1:500; cat. no. sc-5286;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 90 min at
37°, followed by incubation with goat anti-mouse fluorescein
isothiocyanate-labeled IgG (1:100; cat. no. ZF-0312; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijin, China) for
30 min at 37°C. The cells were further stained for 5 min with 300
nM 4,6-dianidino-2-phenylindole (DAPI; Sigma-Aldrich). Images of
the stained cells were captured and data were quantified using an
Olympus BX61 fluorescence microscope (Olympus Corporation).
Protein extraction and western blot
analysis
The A549 cells were treated, as described
above, harvested and lysed with cell lysis buffer containing 1 mM
Tris-HCl (pH 7.5), 0.1 mM Na2EDTA, 15 mM NaCl, 0.1 mM
EGTA, 0.25 mM sodium pyrophosphate, 0.1% Triton X-100, 0.1 mM
Na3VO4, 0.1 mM β-glycerophosphate, 0.2 mM
phenymethylsul-fonyl fluoride and 0.1 µg/ml leupeptin (all
from Sigma-Aldrich). The lysates were centrifuged at 12,000 × g for
15 min at 4°C and the protein concentration in the supernatant was
determined using the Bradford method. Equal quantities of protein
(30–40 µg) were separated via 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and electrotransferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
MA, USA). For western blot analysis, the membranes were blocked in
5% dried skimmed milk in Tris-buffered saline-Tween 20 for 1 h,
followed by incubation overnight at 4°C with the indicated primary
antibody. The membranes were then incubated at 37°C for 2 h with
horseradish-peroxidase-conjugated secondary antibodies (1:100) and
the protein band was developed using an enhanced chemiluminescence
detection system (UVP Inc., Upland, CA, USA). β-actin was used as a
loading control. The primary and secondary antibodies used were as
follows: Goat anti-human polyclonal p-Cdc2 (p34; 1:500; cat. no.
sc-7989-R), mouse anti-human monoclonal caspase 3 (1:500; cat. no.
sc-271028), mouse anti-human monoclonal p53 (1:2,000; cat. no.
sc-126), rabbit anti-human polyclonal B cell lymphoma-2
(Bcl-2)-associated X protein (Bax; 1:1,000; cat. no. sc-493),
rabbit anti-human polyclonal Bcl-2 (1:1,000; cat. no. sc-492) and
goat anti-human polyclonal β-actin (1:1,000; cat. no. sc-1616) were
purchased from Santa Cruz Biotechnology, Inc. Mouse anti-human
monoclonal cyclin B (1:500; cat. no. 647901), and mouse anti-human
polyclonal cdc2 (p34) were purchased from Biolegend (BioLegend Inc.
San Diego. CA, USA). Horse anti-mouse IgG-AP (cat. no. ZB-2310),
goat anti-rabbit IgG-AP (cat. no. ZB-2308) and rabbit anti-goat
IgG-AP (cat. no. ZB-2311) (all 1:5,000) were from purchased from
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.
Statistical analysis
All statistical analyses were performed using SPSS
software version 17 (SPSS, Inc., Chicago, IL, USA). Statistical
significance was determined at the 95% confidence interval using
Student's t-test. All data are expressed as the mean ± standard
deviation, which was calculated automatically using SPSS. P<0.05
was considered to indicate a statistically significant value.
Results
Cytotoxic effects of LJ12 on cancer cell
lines in vitro
With the aim of identifying more potent antitumor
agents, the present study synthesized LJ12 in the laboratory and
investigated its effects on the viability of A549, HepG2 and Hela
cancer cells. The structure of LJ12, and how it compares with other
derivatives, is shown in Fig. 1.
In the present study, the cells were treated with various
concentrations of LJ12 for varying lengths of time (Fig. 2). Subsequent MTT assays showed that
LJ12 reduced the viability of the A549, HepG2 and Hela cells in a
dose- and time-dependent manner (Fig.
2A). The half maximal inhibitory concentration
(IC50) values of LJ12, determined following 48 h
treatment, were 0.183, 0.21 and 0.191 M for the A549, HepG2 and
Hela cells, respectively. The IC50 values determined for
LJ12 were markedly lower than the observed corresponding
IC50 values of VP16 (14.8, 28.4 and 50.6 µM for
the A549, HepG2 and Hela cells, respectively). In addition, it was
found that the cells treated with LJ12 exhibited morphological
changes, which were typical of tumor cell apoptosis (Fig. 2B), including cell shrinkage and
condensed chromatin. These findings suggested that LJ12 opposed the
proliferation of A549, HepG2 and Hela cells. The present study
focussed on A549 cells for the subsequent experiments, as VP16,
which is similar to LJL2 structurally, is currently used as a
first-line treatment in small cell lung cancer (13).
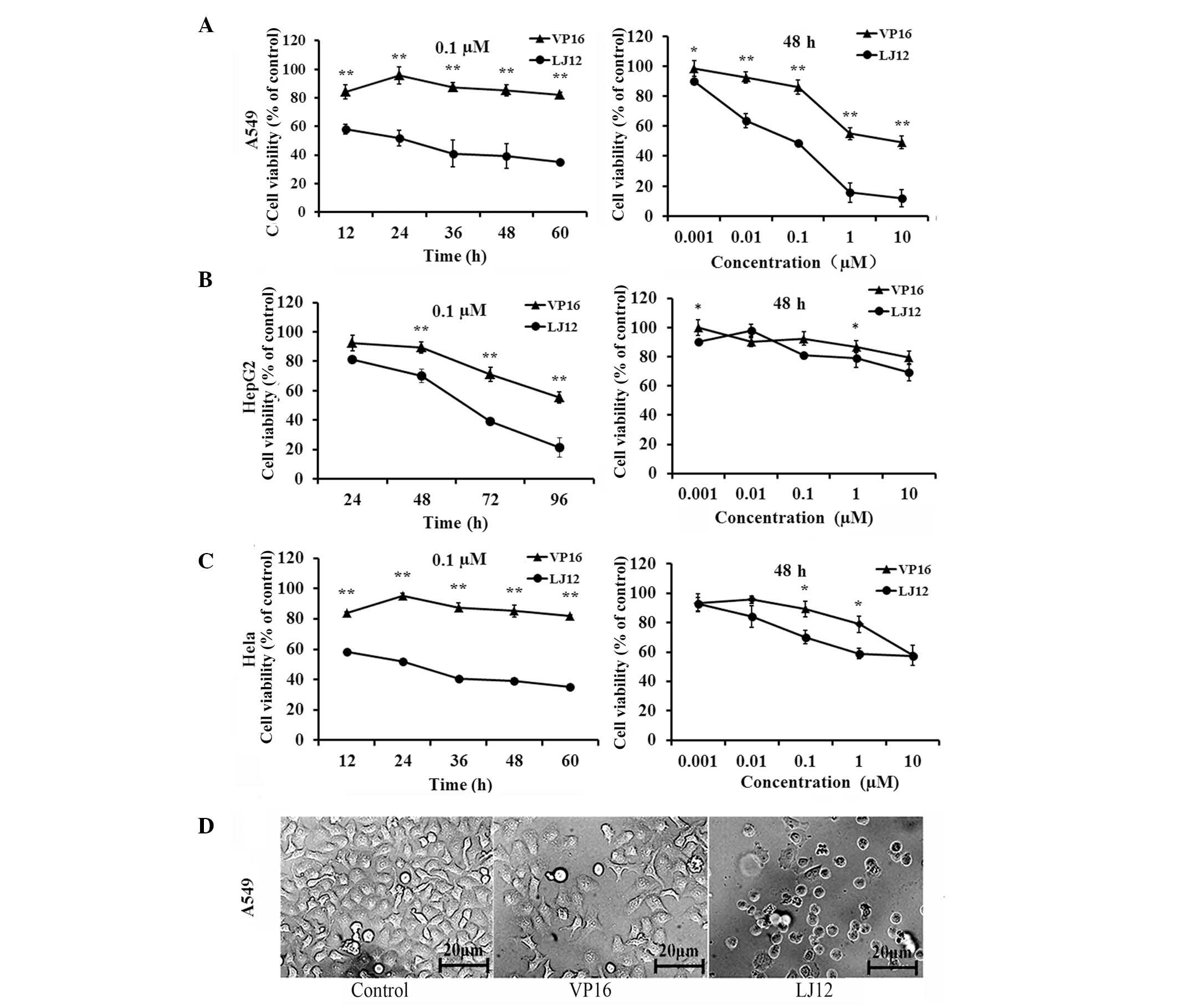 | Figure 2Antiproliferative effects of LJ12 and
VP16 in different cancer cells. (A) A549, (B) HepG2 and (C) Hela
cells were grown and treated with different concentrations of VP16
or LJ12 (0.001–10 µM) for varying periods of time (12–60 h).
Cell viability was assayed using a 3-[4,5-dimethylthiazo
l-2-yl]-2,5-diphenyl-tetrazolium bromide assay. (D) Morphological
analysis of A549 cells following 24 h treatment with 0.05 µM
VP-16 or 0.05 µM LJ12 (magnification, ×200). A549 cells
treated with LJ12 displayed typical morphological features of
apoptosis, including cell shrinkage and condensed chromatin. Values
are expressed as the mean ± standard error of the mean from at
least four independent experiments (n=4). *P<0.05,
vs. VP16, **P<0.01, vs. VP16. LJ12,
N-(1-oxyl-4′-demethyl-4-deoxypo dophyllic)-L-methine-4′-piperazine
carbamate; VP16, etoposide. |
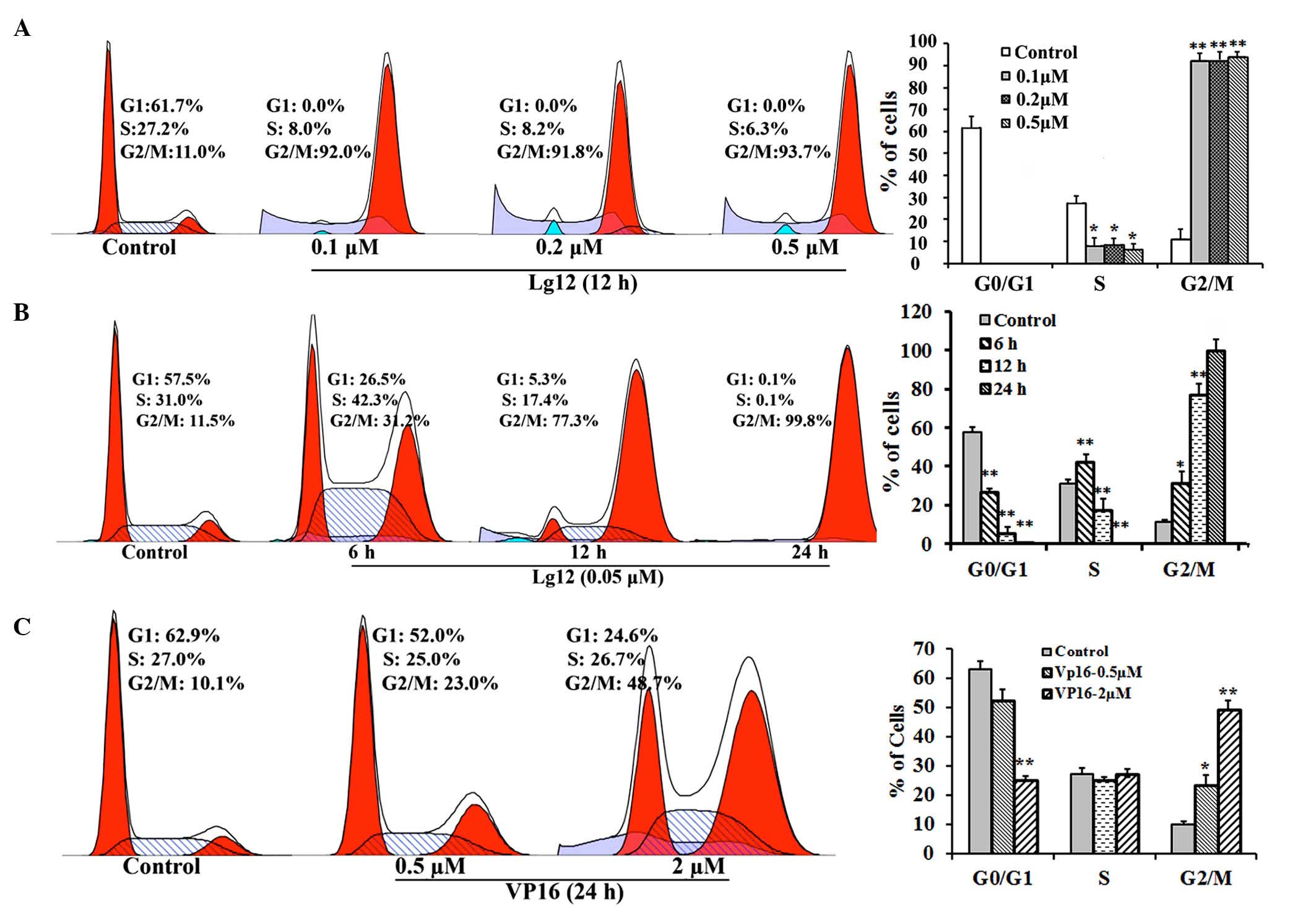
LJ12 treatment induces A549 cell cycle
arrest
In the initial cytotoxicity assessment, a
significant enlargement in the size of certain cells was observed
in the LJ12-treated A549 cells. To evaluate whether LJ12 affected
mitosis, the present study examined the effect of LJ12 treatment on
the cell cycle distribution of the A549 cells. The percentage of
A549 cells in the G1 phase was substantially lower following
treatment with LJ12, compared with the percentage of control cells
in the G1 phase (Fig. 3A–C). This
decrease was reflected in an increase in the population of cells in
the G2/M phase, with ~77.3% of the cells in the G2/M phase
following 12 h treatment with 0.05 µM LJ12 (Fig. 3B). In the untreated control cells,
the G2/M phase population was ~11.5%. Furthermore, the G2/M phase
distribution of cells treated for 12 h with 0.1, 0.2 and 0.5
µM LJ12 (92.0–93.7%) were 3.4- to 4-fold higher, compared
with the G2/M phase distribution of cells treated for 24 h with 0.5
µM VP16 (23.0%). However, the G2/M phase distribution of the
VP16-treated cells was significantly increased, compared with that
of the control cells (10.1%). In additionally, even the cells
treated with a 2.0 µM dose of VP16 for 24 h had
significantly fewer cells in the G2/M phase, compared with the
cells treated for 12 h with just 0.05 µM LJ12 (Fig. 3C).
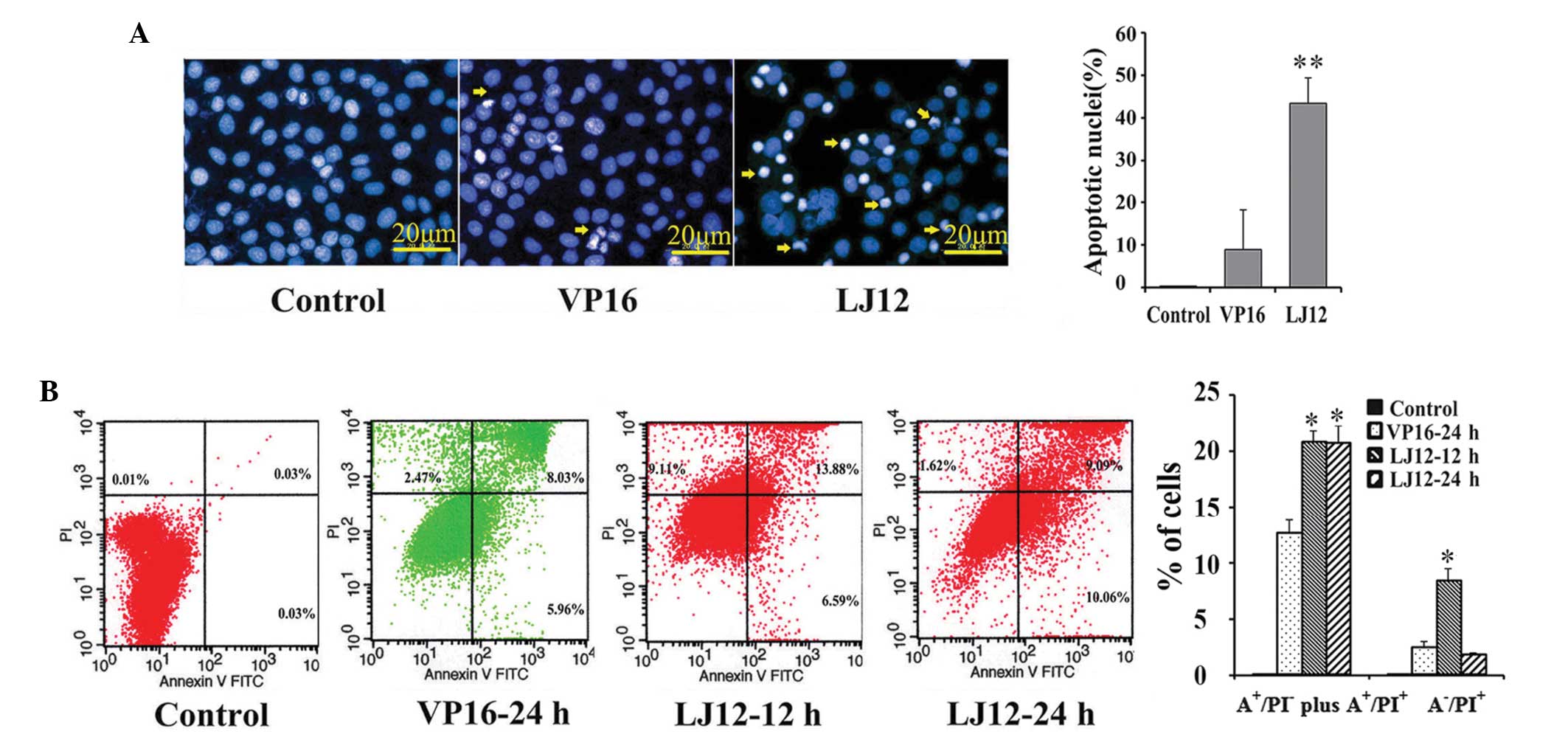
LJ12 induction of tumor cell
apoptosis
The Hoechst 33258 staining performed in the present
study revealed that LJ12 treatment evoked typical apoptotic
features in cells, including nuclear condensation and
fragmentation, cell shrinkage and detachment (Fig. 4A). These observations were
confirmed using a flow cytometric Annexin V-PI assay (Fig. 4B). LJ12 induced apoptosis at a dose
of 0.05 µM. The rate of early apoptosis in the LJ12-treated
cells was significantly increased, after 12 and 24 h, compared with
the control cells (20.47 and 19.15%, vs. 0.06%, respectively). The
rate of apoptosis following treatment with 0.05 µM LJ12 for
24 h (19.15%) was also elevated, compared with the rate of
apoptosis observed following treatment with 0.5 µM VP16 for
24 h (13.99%; Fig. 4B).
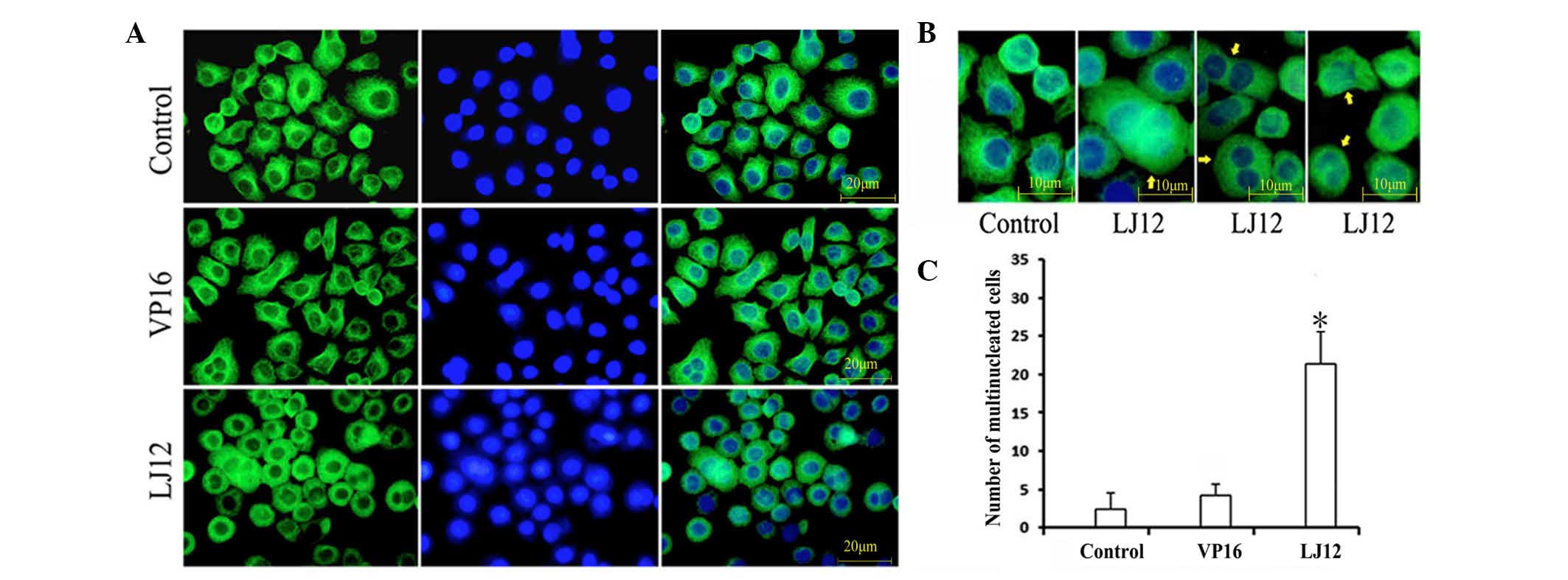
LJ12 treatment and the regulation of
microtubule structure and mitotic catastrophe
Mitotic catastrophe is a type of cell death that
occurs during abnormal mitosis. It usually leads to the formation
of large multiple nuclear cells with de-condensed chromatin
(14). To assess the morphology of
tumor cells following LJ12 treatment, the present study used
indirect immunofluorescence with anti-tubulin antibody staining of
microtubules and DAPI staining of nuclei. The results revealed
elongated, thin bundles of microtubules distributed throughout the
cytoplasm in the untreated control cells and in the VP16-treated
cells (Fig. 5A). By contrast,
following 24 h LJ12 treatment, the cells became round and shrinkage
was observed, but they contained short, dense microtubule networks.
LJ12 treatment also induced multipolar cell division and cells that
containing multiple nuclei were observed (Fig. 5B). Cells with double nuclei and
giant multinuclear cells are hallmarks of mitotic catastrophe and
were frequently observed in the LJ12-treated A549 cells (Fig. 5B). The numbers of giant
multinuclear cells were 21.4, 4.2 and 2.4 in the LJ12-treated
cells, VP16-treated cells and control cells, respectively, with a
significant increase in the LJ12-treated cells.
LJ12 treatment and regulation of the
expression levels of p53, Bax, caspase-3 and cyclin-dependent
kinase 2 (cdc2)/cyclin B1
The present study also assessed the effect of LJ12
treatment on the expression levels of various proteins. The results
revealed a marked increase in the protein expression levels of
cyclin B1 and cdc2 in the LJ12-treated A549 cells, compared with
the control cells. However, the protein expression of p-cdc2 was
decreased relative to the control cells (Fig. 6A). Following 0.1–5 µM LJ12
treatment, the expression levels of p53, Bax and caspase 3 were all
induced in the A549 cells (Fig.
6B). The effect of LJ12 treatment on the expression of
cell-cycle regulator proteins was also dose-dependent.
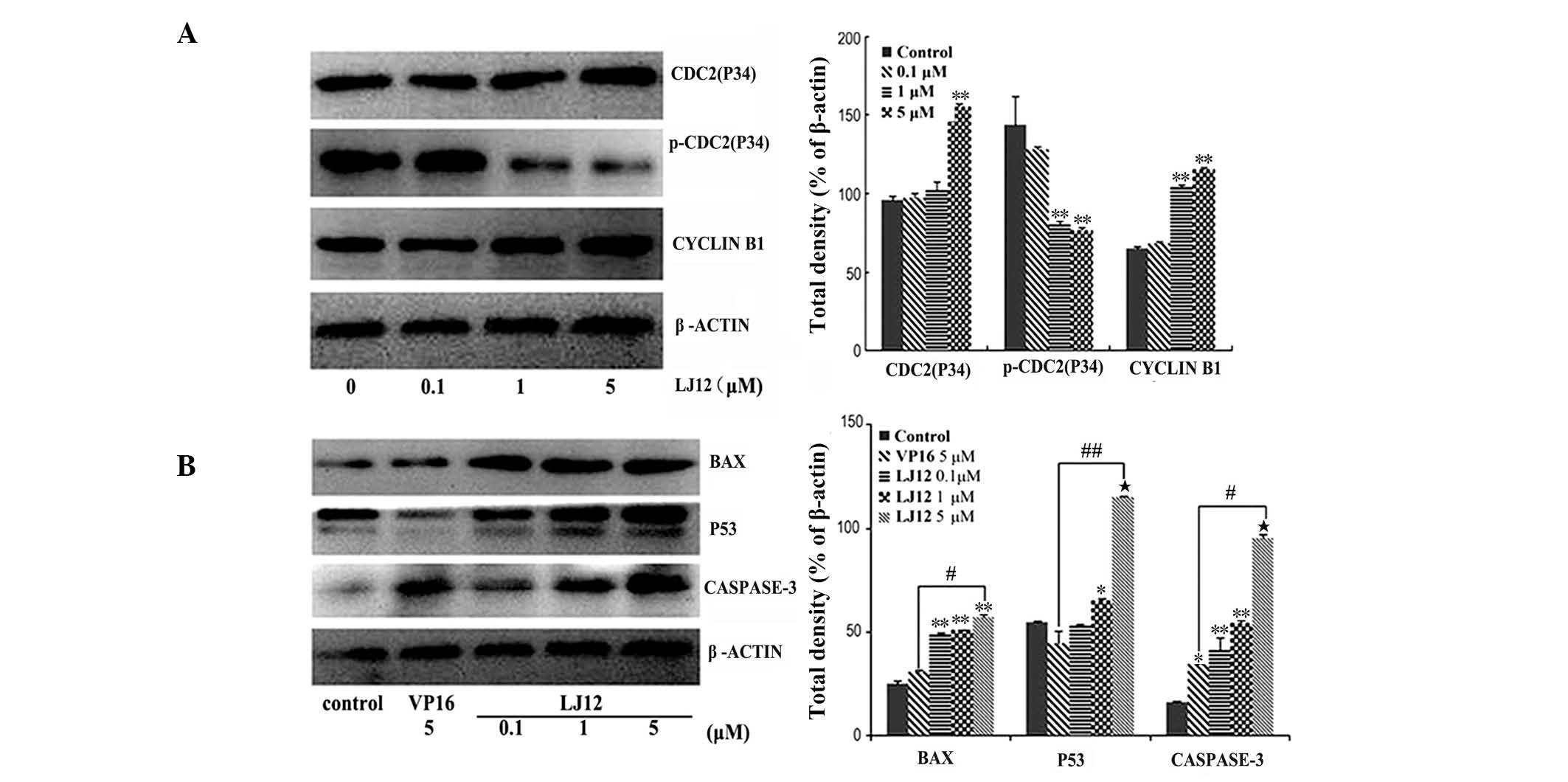 | Figure 6LJ12 regulates gene expression in
A549 cells. The A549 cells were treated with 0, 0.1, 1 or 5
µM LJ12 for 24 h and subjected to (A) western blot analyses
of apoptosis-associated proteins or (B) cell cycle-associated
proteins. The graphs show the quantified data of the corresponding
western blot. Data are expressed as the mean ± standard error of
the mean (n=3; *P<0.05, **P<0.01 and
***P<0.001, vs control; #P<0.01, vs.
VP16 and ##P<0.001, vs. VP16). LJ12,
N-(1-oxyl-4′-demethyl-4-deoxypo dophyllic)-L-methine-4′-piperazine
carbamate; VP16, etoposide. Bax, B cell lymphoma-2-associated X
protein; p-cdc2, phosphorylated-cyclin-dependent kinase 2. |
Discussion
In the present study, the in vitro antitumor
effects of LJ12 in NSCLC cells were examined. The data revealed
that LJ12 treatment significantly reduced A549 cell viability, and
that the antitumor activity of LJ12 was more marked, compared with
that of the closely associated VP16. It was observed that LJ12
treatment regulated the expression levels of various genes,
including cell cycle and apoptosis-associated genes. The expression
profiles of these genes following LJ12 treatment were different
from the expression profiles following VP16 treatment. These
findings indicated that LJ12 may offer potential as an anti-NSCLC
drug. However, in vivo investigations are required prior to
this drug being considered for testing in Phase I or II clinical
trials.
To evaluate the potential effectiveness of LJ12 as
an antitumor therapeutic agent, the present study investigated the
time- and dose-dependent regulation of the lung carcinoma A549 cell
line by LJ12. The IC50 of LJ12 was 0.1 µM, which
was favorable, compared with the VP16 DPT derivative,
(IC50, 10 µM). Apoptosis is a type of programmed
cell death with common identifiable characteristics, which include
membrane blebbing, cell shrinkage, chromosome condensation and
specific biochemical changes, including activation of the
caspase-cascade (15). The present
study demonstrated LJ12 treatment induced >40% of the A549 cells
to undergo apoptosis, compared with < 10% in the VP16-treated
tumor cells. This lower IC50 value and elevated
apoptotic induction indicated that LJ12 may have more marked
antitumor activity and lower toxicity, compared with VP16.
Mitotic catastrophe is a type of cell death, which
usually occurs during mitosis in response to DNA damage or
anti-mitotic agents (16). In
addition to inducing tumor cell death via apoptosis, LJ12 treatment
also induced mitotic catastrophe, supporting previous evidence that
LJ12 may be an ideal anti-tumor therapeutic agent (17,18).
Although several biochemical changes associated with mitotic
catastrophe have been found, there remains no specific mitotic
catastrophe marker (19).
Therefore, the identification of mitotic catastrophe depends on
cell morphology. Anticancer agents can induce cytokinesis failure,
which results in multinucleation and may lead to cell death
(20). Multinuclear cells,
premature chromosome condensation, aberrant mitotic figures and the
accumulation of affected cells in the G2/M phase are characteristic
of mitotic catastrophe (20). In
the present study, it was found that LJ12 treatment of the A549
cells induced multinucleate giant cells and cells with double
nuclei, indicating that LJ12-induced cell death occurred partially
through mitotic catastrophe. It was also demonstrated that the
LJ12-treated cells expressed apoptotic and mitotic
catastrophe-associated biochemical markers, providing further
evidence that LJ12 induces apoptosis and mitotic catastrophe in
A549 cells. Certain previous studies have shown that mitotic
catastrophe and apoptosis are independent pathways, whereas others
indicated that mitotic catastrophe may be a specific type of
apoptosis, or a precursor of apoptosis or necrosis (21,22).
However, the present study was unable not delineate whether
apoptosis induced by LJ12 was an independent event, or whether
apoptosis occurred due to LJ12-induced mitotic catastrophe.
Therefore, further investigation is required to clarify which
LJ12-induced cell death pathway is dominant in A549 cells. However,
despite this point of controversy, cell death is always the final
result.
The suppression of microtubule dynamics by
microtubule-targeting drugs, including vinca alkaloids and taxol,
can engage the mitotic spindle checkpoint and suppress cell cycle
progression, eventually inducing apoptosis (23). However, the direct inhibition of
microtubule dynamics may also disrupt a number of normal cellular
processes, including the transportation of intracellular cargo or
organelles within cells (24).
Substantial effort is being made to identify, design and develop
antimitotic agents, which bind indirectly to tubulin and alter
microtubule dynamics with minimal toxicity to normal tissues.
Tubulin is a basic microtubule component, which is involved in
several important cellular processes, including cell division,
chromosome segregation and cell shape maintenance (25). Tubulin polymerization is a key
mechanism of normal microtubule function. Therefore, agents, which
affect tubulin polymerization can induce cell death (26). The induction or inhibition of
tubulin polymerization can affect the dynamic instability, proper
attachment and movement of chromosomes during the various stages of
the mitotic phase, leading to mitotic arrest and cell death
(26). Thus, highly proliferative
cancer cells can be selectively eliminated by drugs, which affect
the dynamics of tubulin polymerization (27). A number of anticancer agents
targeting this mechanism have been developed and used to treat
human cancer (28). However,
further investigation is required to investigate how LJ12 treatment
targets tubulin polymerization in tumor cells, and whether this
mechanism contributes to LJ12 antitumor activity.
The results of the present study are only
'proof-of-principle' and a substantial further investigation is
required prior to LJ12 treatment being used in a clinical trials.
In addition, the present study has certain limitations to be
considered. Firstly, the present study only examined the effects of
LJ12 in vitro, thus the in vivo toxicity of LJ12 is
unknown. Secondly, the present study used a limited number of cell
lines, and the majority of the investigations were confined to just
one human cancer cell line. In addition, although the present study
showed that LJ12 regulated the expression of certain proteins, how
these mediate the antitumor activities of LJ12 remain to be
elucidated. However, the preliminary data demonstrated the
potential usefulness of LJ12 as an anticancer therapeutic agent. In
addition, LJ12 demonstrates more marked antitumor activity and
lower toxicity towards NSCLC cells in vitro, compared with
the closely associated PPT derivative, VP16.
Abbreviations:
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
DMSO
|
dimethyl sulfoxide
|
|
DPT
|
deoxypodophyllotoxin
|
|
ECL
|
enhanced chemiluminescence
|
|
PBS
|
phosphate-buffered saline
|
|
VP16
|
etoposide
|
|
IC50
|
half maximal inhibitory
concentration
|
|
PPT
|
podophyllotoxin
|
|
TBST
|
Tris-buffered saline-Tween 2
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
Acknowledgments
This study was supported, in part, by grants from
the National Natural Science Foundation of China (grant nos.
81372177 and 21372110), the Medical Scientific Research Projects of
Lanzhou Military Area Command of the Chinese People's Liberation
Army (grant no. CLZ12JB04) and the Natural Science Foundation of
Gansu Province (grant no. 1107RJZA106).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumor-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar
|
|
3
|
Newman DJ, Cragg GM and Snader KM: Natural
products as sources of new drugs over the period 1981–2002. J Nat
Prod. 7:1022–1037. 2003. View Article : Google Scholar
|
|
4
|
Imbert TF: Discovery of podophyllotoxins.
Biochimie. 80:207–222. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen K, Sun L, Zhang H, Xu Y, Qian X, Lu
Y, Li Q, Ni L and Liu J: A ROS-mediated lysosomal-mitochondrial
pathway is induced by a novel Amonafide analogue, 7c, in human Hela
cervix carcinoma cells. Cancer lett. 333:229–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eberhardt WE, Gauler TC, Lepechoux C,
Stamatis G, Bildat S, Krbek T, Welter S, Grunenwald D, Fischer B,
Rodrigo Hde L, et al: 10-year long-term survival (LTS) of induction
chemotherapy with three cycles cisplatin/paclitaxel followed by
concurrent chemoradiation cisplatin/etoposide/45 Gy (1.5 Gy bid)
plus surgery in locally advanced non-small-cell lung cancer
(NSCLC)-a multicenter phase-II trial (CISTAXOL). Lung Cancer.
82:83–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wood WA, Whitley J, Goyal R, Brown PM,
Sharf A, Irons R, Rao KV, Essenmacher A, Serody JS, Coghill JM, et
al: Effectiveness of etoposide chemomobilization in lymphoma
patients undergoing auto-SCT. Bone Marrow Transplant. 48:771–776.
2013. View Article : Google Scholar
|
|
8
|
Kumar A, Kumar V, Alegria AE and Malhotra
SV: Synthetic and application perspectives of azapodophyllotoxins:
Alternative scaffolds of podophyllotoxin. Curr Med Chem.
18:3853–3870. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khaled M, Jiang ZZ and Zhang LY:
Deoxypodophyllotoxin: A promising therapeutic agent from herbal
medicine. J Ethnopharmacol. 149:24–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang Z, Wu M, Miao J, Duan H, Zhang S,
Chen M, Sun L, Wang Y, Zhang X, Zhu X and Zhang L:
Deoxypodophyllotoxin exerts both anti-angiogenic and vascular
disrupting effects. Int J Biochem Cell Biol. 45:1710–1719. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carter GT: Natural products and Pharma
2011: Strategic changes spur new opportunities. Nat Prod Rep.
28:1783–1789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin Y, Liu J, Huang WT, Chen SW and Hui L:
Synthesis and biological evaluation of derivatives of
4-deoxypodophyllotoxin as antitumor agents. Eur J Med Chem.
46:4056–4061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Filloux F and Townsend JJ: Pre- and
postsynaptic neurotoxic effects of dopamine demonstrated by
intrastriatal injection. Exp neurol. 119:79–88. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vitale I, Galluzzi L, Castedo M and
Kroemer G: Mitotic catastrophe: A mechanism for avoiding genomic
instability. Nature Rev Mol Cell Biol. 12:385–392. 2011. View Article : Google Scholar
|
|
15
|
Shin SY, Bahk YY, Ko J, Chung IY, Lee YS,
Downward J, Eibel H, Sharma PM, Olefsky JM, Kim YH, et al:
Suppression of Egr-1 transcription through targeting of the serum
response factor by oncogenic H-Ras. EMBO J. 25:1093–1103. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bacso Z, Everson RB and Eliason JF: The
DNA of annexin V-binding apoptotic cells is highly fragmented.
Cancer Res. 60:4623–4628. 2000.PubMed/NCBI
|
|
17
|
Roy RV, Suman S, Das TP, Luevano JE and
Damodaran C: Withaferin A, a steroidal lactone from Withania
somnifera, induces mitotic catastrophe and growth arrest in
prostate cancer cells. J Nat Prod. 76:1909–1915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cotugno R, Fortunato R, Santoro A,
Gallotta D, Braca A, De Tommasi N and Belisario MA: Effect of
sesquiterpene lactone coronopilin on leukaemia cell population
growth, cell type-specific induction of apoptosis and mitotic
catastrophe. Cell Prolif. 45:53–65. 2012. View Article : Google Scholar
|
|
19
|
Wang X, Wu E, Wu J, Wang TL, Hsieh HP and
Liu X: An anti-mitotic and antivascular agent BPR0L075 overcomes
multidrug resistance and induces mitotic catastrophe in
paclitaxel-resistant ovarian cancer cells. PloS One. 8:e656862013.
View Article : Google Scholar
|
|
20
|
Caruso R, Fedele F, Lucianò R, Branca G,
Parisi C, Paparo D and Parisi A: Mitotic catastrophe in malignant
epithelial tumors: The pathologist's viewpoint. Ultrastruct pathol.
35:66–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vakifahmetoglu H, Olsson M and Zhivotovsky
B: Death through a tragedy: Mitotic catastrophe. Cell Death Differ.
15:1153–1162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castedo M, Perfettini JL, Roumier T,
Valent A, Raslova H, Yakushijin K, Horne D, Feunteun J, Lenoir G,
Medema R, et al: Mitotic catastrophe constitutes a special case of
apoptosis whose suppression entails aneuploidy. Oncogene.
23:4362–4370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gerdes JM and Katsanis N: Microtubule
transport defects in neurological and ciliary disease. Cell Mol
Life Sci. 62:1556–1570. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prinz H: Recent advances in the field of
tubulin polymerization inhibitors. Expert Rev Anticancer Ther.
2:695–708. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei JH and Seemann J: Nakiterpiosin
targets tubulin and triggers mitotic catastrophe in human cancer
cells. Mol Cancer Ther. 9:3375–3385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jackson JR, Patrick DR, Dar MM and Huang
PS: Targeted anti-mitotic therapies: Can we improve on tubulin
agents? Nat Rev Cancer. 7:107–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho RJ, Campbell MJ, Winzeler EA,
Steinmetz L, Conway A, Wodicka L, Wolfsberg TG, Gabrielian AE,
Landsman D, Lockhart DJ and Davis RW: A genome-wide transcriptional
analysis of the mitotic cell cycle. Mol Cell. 2:65–73. 1998.
View Article : Google Scholar : PubMed/NCBI
|