Introduction
Hepatic injury, caused by misdirected immune
stimulation or viral infection, is a type of acute inflammatory
injury characterized by inflammatory infiltration of macrophages, T
cells, and neutrophils into liver (1). Although hepatitis represents a
ubiquitous human health problem, effective therapeutic strategies
with minimal side-effects are lacking and the precise mechanisms
underlying hepatitis are not fully understood. There are various
mouse models of inflammatory liver injury, which have been
established to facilitate functional studies on the mechanisms of
hepatic injury. For example, liver failure, induced by either
intravenous injection of concanavalin A or by sensitizing mice with
D-galactosamine, prior to lipo-polysaccharide (LPS) administration,
is commonly used as an experimental animal model for mimicking
human liver disease (2,3). Liver failure is characterized by an
accumulation and activation of macrophages, which are highlighted
for being involved in the inflammation process by producing large
quantities of tumor necrosis factor (TNF)-α, which leads to a
direct hepatotoxic potential (4,5). In
addition, TNF is known to mediate intrahepatic induction of
inducible NO synthase, which is also significant in acute hepatitis
(6).
Inflammation is a pathological condition in which
various signaling mechanisms control a complex network of cellular
and molecular interactions involving the crosstalk between
independent biochemical cascades, which terminate in the activation
of gene expression programs for cytokines and chemokines (7). In the canonical pathway, nuclear
factor κ-light-chain-enhancer of activated B cell (NF-κB) is
present as a latent, inactive IκB-bound complex in the cytoplasm,
which prevents it from entering nuclei. When these cells are
exposed to stimuli, including LPS, LPS binds to Toll-like
receptor-4 and phosphorylates IκB, resulting in its subsequent
degradation, which enables NF-κB to be released from IκB and enter
the nucleus (8). Numerous stimuli
activate NF-κB, including LPS, TNF-α and interleukin (IL)-1, and
other physiological and pathological stimuli (9,10).
IκB phosphorylation regulates the expression of numerous genes
involved in inflammatory responses, including genes encoding
proinflammatory cytokines, chemokines, enzymes that generate
mediators of inflammation, immune receptors and adhesion molecules
(11).
Phosphoinositide 3-kinase (PI3K) is a pivotal kinase
known to regulate inflammatory responses in various types of
disease (12,13). In recent years, there has been
increasing evidence regarding the pan-PI3K inhibitor, LY294002
ameliorating the severity of a series of models of autoimmune
diseases, including cecal ligation and puncture-induced sepsis,
idiopathic pulmonary fibrosis and colitis-induced cancer (14–16).
However, whether the PI3K inhibitor suppresses autoimmune hepatitis
remains unclear. Furthermore, pan-PI3K inhibition may promote
infarct resorption and prevents adverse cardiac remodeling
following myocardial infarction in mice (17). A study using PI3Kγ deficient mice
demonstrated a complex contribution of PI3Kγ to reparative
angiogenesis in myocardial infarction (18). Although previous research suggested
that PI3Kγ inhibitors, such as AS605240, were involved in liver
diseases (19), it remains unclear
whether pan-PI3K inhibition is essential for ameliorating the
severity of LPS-induced hepatitis. In the present study, the
therapeutic effect of the pan-PI3K inhibitor, LY294002 on acute
hepatitis was investigated using an LPS-induced murine hepatitis
model. Our results showed that LY294002 prevented the development
of hepatitis stimulated by LPS. These data may define an
anti-inflammatory role of LY294002 in immunologically mediated
hepatic diseases and may provide a foundation for a novel
therapeutic modality for treatment of inflammatory hepatic
diseases.
Materials and methods
Reagent and animals
A total of 80 female BALB/c mice (aged, 6–8 weeks)
were obtained from Hua Fukang Experimental Animal Center (Bejing,
China) and treated with humane care according to the National
Institutes of Health Guidelines of China. The mice were housed
(five mice per cage) at 23+2°C under a 12:12 light/dark cycle and
allowed free access to food and water. Following an acclimatization
period of 1 week, they were divided into four groups, according to
body weight. The pan-PI3K inhibitor, LY294002 (Fig. 1) was purchased from Sigma Aldrich
(St. Louis, MO, USA).
Establishment of a murine model of
LPS-induced hepatitis
Thhe present study was approved by the ethics
committee of Renmin hospital of Wuhan university (Wuhan, China).
LPS dissolved in saline was administered at a total volume of 100
μl per mouse via i.p. injection. For therapeutic agent
treatment, LY294002 (concentration, 40 μM; volume, 10
μl) was administered once by i.p. injection 1 h prior to
treatment with the corresponding hepatotoxin in the murine model of
LPS-induced hepatitis. Following anesthetization of the mice with
45 mg/kg ketamine (Sigma-Aldrich), serum and liver tissue samples
were collected 8 h following LPS treatment. To examine survival
rate, the mice were challenged with LPS, however the mice were
pretreated with LY294002 1 h prior to LPS treatment. The mice were
subsequently monitored every 2 h for survival.
Analysis of liver enzymes
Liver injury in LPS-induced acute hepatitis was
quantified by measurement of the serum enzyme activities of alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) using
an automated procedure with an enzymatic assay (ALT-ASTl Kainos
Laboratories, Toyko, Japan).
Histologic examination
Four weeks after immunization, the mice were
sacrificed by anesthetization, and their hearts were perfused with
normal saline, removed, fixed in 4% buffered formaldehyde (BASO,
Taiwan, China), and processed for hematoxylin and eosin (H&E)
staining (BASO). Hepatic injury on LPS-induced acute hepatitis was
scored on the H&E-stained sections using grades from 0 to 4 as
follows: 0, No necrotic infiltrates; 1, small foci of necrotic
cells between hepatocytes or necrotic cells surrounding individual
hepatocytes; 2, larger foci of 100 necrotic cells or involving 30
hepatocytes; 3, 10% of a hepatocytic cross-section involved; and 4,
30% of a hepatic cross-section involved (20,21).
ELISA
The concentrations of TNF-α, IL-1β, IFN-γ and IL-6
in plasma samples or lymph node cell suspensions were analyzed by
ELISA using commercially available TNF-α (cat. no. F8005A), IL-1β
(cat. no. F10091A), IFN-γ (cat. no. F5980A) and IL-6 (cat. no.
F7699A) ELISA kits from JingMei Biotech. (Shenzhen, China),
according to the manufacturer's instructions.
Western blot analysis
The mice were pre-treated with LY294002
(concentration, 40 μM; volume, 10 μl) 1 h before
stimulation with LPS, respectively, for 15, 30, 60 or 90 min. The
mice were then sacrificed at different time points (15, 30, 60 or
90 min) to harvest the liver tissues. Protein (20 μg) from
each sample was mixed with an equal volume of 2X SDS sample buffer
(Beyotime Institute of Biotechnology, Haimen, China), boiled for 5
min and then separated by 10% SDS-polyacrylamide gel
electrophoresis. Following electrophoresis, proteins were
transferred to polyvinylidene fluoride membranes (EMD, Millipore,
Billerica, MA, USA). The membranes were incubated with monoclonal
rabbit anti-IκB antibody (cat. no. ab32518; 1:1,000; Abcam,
Cambridge, MA, USA), monoclonal mouse anti-phosphorylated (p)-IκB
antibody (cat. no. ab12135; 1: 1,000; Abcam) or monoclonal mouse
anti-GAPDH antibody (cat. no. G9295; 1:50,000; Sigma-Aldrich) at
4°C overnight. The total IκB and p-IκB protein signal was
quantified by scanning densitometry using a Quantity One image
analysis system (version 4.6.2; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
The Mann-Whitney U test was used for evaluation of
the severity scores. Parametric data were statistically analyzed by
Student's t-test or one-way analysis of variance. Data are
presented as means ± standard deviation and P<0.05 was
considered to indicate a statistically significant difference.
Results
LY294002 administration exerts
therapeutic effects in a mouse model of LPS-induced acute hepatic
injury
Injection of LPS causes hepatic injury, including
hepatic necrosis, steatosis and inflammatory infiltration. To
estimate the efficacy of LY294002 treatment on acute hepatic liver
injury, mice were sacrificed 8 h following an i.p. injection of
LPS. Routine histopathology confirmed hepatic injury had been
achieved in the murine model of LPS-induced acute hepatitis.
Furthermore, the mice treated with LY294002 demonstrated a markedly
reduced severity of hepatic necrosis when compared with the
LPS-treated mice (Fig. 2A). The
pattern of infiltrate was assessed by scoring of H&E stained
sections. Treatment with LY294002 significantly alleviated
LPS-induced hepatitis in mice, as indicated by the reduction of
hepatic necrosis. The histological scores of inflammatory
infiltrates for individual mice are presented in Fig. 2B. LY294002 treatment significantly
reduced the LPS-induced histopathological hepatic injury in mice
(P<0.05). Therefore, treatment with LY294002 significantly
alleviated LPS-induced liver injury in mice, as indicated by the
reduction of serum aminotransferase (Fig. 2C; P<0.01). Furthermore,
reduction in liver enzyme levels by LY294002 treatment correlated
with enhanced survival. This protective effect of LY294002
pretreatment was further confirmed by analysis of the survival rate
of mice challenged with LPS. Mortality was observed as early as 2 h
following LPS administration. However, animals treated with
LY294002 showed a statistically significant enhancement in survival
rate (P<0.05; Fig. 2D).
 | Figure 2(A) Representative liver histology of
mice from each group. Liver sections were stained with hematoxylin
and eosin (original magnification, ×100). (B) Centrilobular
necrosis scores. The histological score of centrilobular necrosis
for each mouse (n=5) 8 h following LPS injection is presented.
Histology slides were evaluated by a board-certified veterinary
pathologist blinded to the treatment groups. Histopathological
changes were recorded and graded on a severity scale of 0 to 4 (0,
no necrotic infiltrates; 1, small foci of necrotic cells between
hepatocytes, or necrotic cells surrounding individual hepatocytes;
2, larger foci of 100 necrotic cells or involving 30 hepatocytes;
3, 10% of hepatic cross-section involved; 4, 30% of a hepatic
crosss-section involved). ♦Saline + LPS; •LY + LPS. (C)
Survival curve of mice following administration of LPS. Balb/c mice
(n=6) were administrated with saline or LY294002 1 h before and 8 h
after a lethal intraperitoneal dose of LPS. Animals were monitored
over a 7 day period for survival. Data is plotted as the percentage
of animals surviving. Saline + LPS; LY294002+LPS (D) Survival curve
of mice following administration of LPS. BALB/c mice (n=5) were
administrated with saline or LY 1 h prior to and 8 h following a
lethal intraperitoneal dose of LPS. Mice were monitored over a
seven-day period for survival. Data are plotted as the percentage
of surviving mice. *P<0.05 and #P<0.01.
LPS, lipopolysaccharide; ALT, alanine aminotransferase; AST,
aspartate aminotransferase; LY, LY294002. |
Pan-PI3K inhibitor, LY294002 reduces the
production of proinflammatory cytokines
Proinflammatory cytokines are important in human and
animal models of acute liver injury (22). In the present study, the effects of
pan-PI3K inhibitor, LY294002 on proinflammatory cytokines in
LPS-induced acute hepatitis were analyzed. As shown in Fig. 3A–D, mice injected with LPS
demonstrated increased secretion levels of TNF-α, IL-6, IL-1β and
IFN-γ in murine liver tissue. Administration of LY294002 in mice
following LPS injection resulted in a significant reduction of
TNF-α, IL-6, IL-1β and IFN-γ production (Fig. 3A–D; P<0.05). These results
indicate that LY294002 treatment suppresses the production of
cyto-kines during inflammation, which may protect the liver from
injury.
LY294002 treatment inhibits IκB
phosphorylation following LPS injection
Phosphorylation of the inhibitory protein, IκB
enables nuclear translocation and DNA binding of NF-κB during
inflammation (23). To gain
further insight into the mechanism of LY294002-mediated regulation
of inflammation, variation in IκB phosphorylation in the mouse
model of LPS-induced hepatitis was analyzed in the present study.
As shown in Fig. 4, LY294002
significantly inhibited IκB phosphorylation in the mouse model. The
mice were pre-treated with LY294002 (concentration, 40 μM;
volume, 10 μl) 1 h before stimulation with LPS,
respectively, for 15, 30, 60 or 90 min. Subsequently, it was
observed that LY294002 inhibited IκB phosphorylation from 15 to 90
min following LPS-injection. The difference was statistically
significant at 60 min following LPS-injection (Fig. 4; P<0.05).
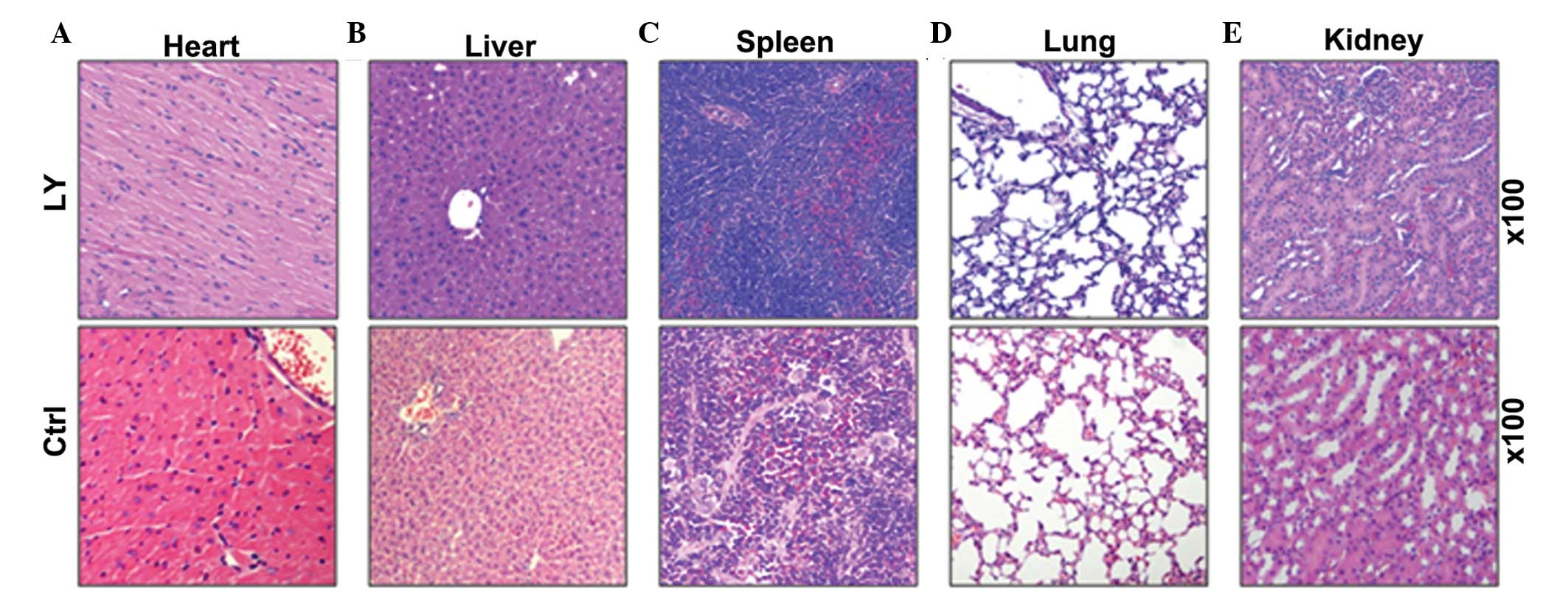
Toxicity of LY294002
To examine the toxicity of LY294002, the mice were
sacrificed following 4 weeks of daily injections of LY294002
(concentration, 40 μM; volume, 10 μl) and
histo-pathological analysis of heart, liver, spleen, lung and
kidney tissue was performed. As shown in Fig. 5, no significant difference was
observed in the histopathology between the LY294001 and the normal
control groups (Fig. 5).
Discussion
The current report demonstrates the in vivo
role of LY294002 in protecting mice from fulminant hepatitis and
investigates the possible underlying mechanisms. In the present
report, LY294002 was observed to protect the liver from injury by
reducing the activities of ALT and AST, and by improving the
histological architecture of the liver. In the mouse model of
LPS-induced hepatitis, treatment of LY294002 markedly inhibited
intrahepatic synthesis of various disease-relevant proinflammatory
cytokines (TNF-α, IL-6, IL-1β and IFN-γ). Furthermore, LY294002
significantly inhibited IκB phos-phorylation in the mouse model of
LPS-induced hepatitis. Therefore, it was hypothesized that LY294002
may protect the liver from LPS-induced injury by inhibition of the
IκB-NF-κB dependent signaling pathway.
As a ubiquitous transcription factor that regulates
various genes involved in inflammation and immune responses, NF-κB
is normally sequestered in the cytoplasm where it associates with a
family of inhibitory proteins, known as IκB (24). In response to external signals, IκB
is phosphorylated by the IκB kinase complex, and subsequently
degraded through ubiquitin-dependent proteolysis (25). Notably, in the present study,
LY294002 administration was observed to decrease the cytoplasmic
level of IκB protein in sections of mouse liver tissue following
LPS injection. These data indicate that LY294002 may bind to IκB
and, therefore, may be involved in stabilizing the NF-κB-IκB
complex and tethering NF-κB in the cytosol. Thus, establishing
whether LY294002 binds to IκB during LPS-induced NF-κB activation
may be significant. In our future studies, the focus will be on the
mechanism by which LY294002 regulates NF-κB activity via IκB
phosphorylation inhibition.
Proinflammatory cytokines are critical in the
process of inflammation, and the increased production of TNF-α,
IL-6, IL-1β and IFN-γ has previously been reported to be associated
with autoimmune cardiac diseases (26–28).
In the current study, injection with LPS resulted in marked
intrahepatic increases of TNF-α, IL-6, IL-1β and IFN-γ, which was
consistent with previous reports (29). Subsequent LY294002 treatment
clearly inhibited the serum protein levels of TNF-α, IL-6, IL-1β
and IFN-γ; the T cell-produced cytokines in LPS-injected mice. The
results indicate that LY294002 may protect mice from LPS-induced
acute hepatic injury by inhibition of proinflam-matory
cytokines.
The PI3K/AKT signaling pathway has been shown to be
involved in LPS-induced immune cell proliferation, accumulation in
the liver and consequently liver damage (30,31).
In addition, the activation of PI3K/AKT signaling is key in various
autoimmune, inflammatory and allergic processes (32–34).
The pan-PI3K inhibitor, LY294002 has demonstrated its favorable
anti-inflammatory effects in murine models of certain inflammatory
diseases via selectively prohibiting activation of the PI3K/AKT
signaling pathway in various immune cell types, which was reflected
by the reduction of chemokine-induced AKT phosphorylation in
immunological cells following LY294002 treatment (35,36).
The present study confirmed that LY294002 effectively mediates the
induction and development of LPS-induced hepatitis, which was
accompanied by a significant reduction in histopathological hepatic
necrosis.
In conclusion, the present study demonstrated the
hepatoprotective activity of the compound, LY294002 in a mouse
model of LPS-induced liver injury, resembling autoimmune hepatitis.
The present study demonstrates that the pan-PI3K inhibitor,
LY294002 effectively protects and treats LPS-induced murine
hepatitis by targeting PI3K activity and consequently suppressing
leukocyte infiltration, as well as immunoregu-lating the unbalance
between pro- and anti-inflammation. The major mechanism of this
hepatoprotective efficacy appears to be the inhibition of
intrahepatic IκB phosphorylation, which prevents the subsequent
synthesis of TNF-α, IL-6, IL-1β and IFN-γ. The findings of the
present study may be significant in the development of LY294002 as
a therapeutic agent for the prevention and treatment of human
myocarditis. Consequently, the present study provides a novel
insight into the treatment of inflammatory liver diseases, such as
autoimmune hepatitis. However, further preclinical and clinical
studies using a PI3K inhibition compound, such as LY294002, are
required and may be valuable towards the treatment of hepatic
injury.
Acknowledgments
The present study was supported by Wuhan Science and
Technology, Wuhan, China (grant no. 2013062301010818).
References
|
1
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tiegs G, Hentschel J and Wendel A: A T
cell-dependent experimental liver injury in mice inducible by
concanavalin A. J Clin Invest. 90:196–203. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moebius U, Manns M, Hess G, Kober G, Meyer
zum Büschenfelde KH and Meuer SC: T cell receptor gene
rearrangements of T lymphocytes infiltrating the liver in chronic
active hepatitis B and primary biliary cirrhosis (PBC):
Oligoclonality of PBC-derived T cell clones. Eur J Immunol.
20:889–896. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schumann J, Wolf D, Pahl A, Brune K,
Papadopoulos T, van Rooijen N and Tiegs G: Importance of Kupffer
cells for T-cell-dependent liver injury in mice. Am J Pathol.
157:1671–1683. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gantner F, Leist M, Lohse AW, Germann PG
and Tiegs G: Concanavalin A-induced T-cell-mediated hepatic injury
in mice: The role of tumor necrosis factor. Hepatology. 21:190–198.
1995.PubMed/NCBI
|
|
6
|
Koerber K, Sass G, Kiemer AK, Vollmar AM
and Tiegs G: In vivo regulation of inducible NO synthase in
immune-mediated liver injury in mice. Hepatology. 36:1061–1069.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duran A, Rodriguez A, Martin P, Serrano M,
Flores JM, Leitges M, Diaz-Meco MT and Moscat J: Crosstalk between
PKCzeta and the IL4/Stat6 pathway during T-cell-mediated hepatitis.
EMBO J. 23:4595–4605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siebenlist U, Franzoso G and Brown K:
Structure, regulation and function of NF-kappa B. Annu Rev Cell
Biol. 10:405–455. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baeuerle PA and Baltimore D: NF-kappa B:
Ten years after. Cell. 87:13–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cimino F, Esposito F, Ammendola R and
Russo T: Gene regulation by reactive oxygen species. Curr Top Cell
Regul. 35:123–148. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wright FL, Moore EE, Nydam TL, et al:
QS406. Hypertonic saline inhibits pro-inflammatory effects of
cytokine stimulation on pulmonary epithelium at the common pathway
of IKB phosphorylation. J Surg Res. 144:4292008. View Article : Google Scholar
|
|
12
|
Ruckle T, Schwarz MK and Rommel C:
PI3Kgamma inhibition: Towards an 'aspirin of the 21st century'? Nat
Rev Drug Discov. 5:903–918. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng XD, Wu XH, Chen LJ, Wang ZL, Hu XH,
Song LF, He CM, Luo YF, Chen ZZ, Jin K, et al: Inhibition of
phosphoinositide 3-kinase ameliorates dextran sodium
sulfate-induced colitis in mice. J Pharmacol Exp Ther. 332:46–56.
2010. View Article : Google Scholar
|
|
14
|
Kim HS, Park EJ, Park SW, Kim HJ and Chang
KC: A tetrahy-droisoquinoline alkaloid THI-28 reduces LPS-induced
HMGB1 and diminishes organ injury in septic mice through p38 and
PI3K/Nrf2/HO-1 signals. Int Immunopharmacol. 17:684–692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stahl M, Schupp J, Jäger B, Schmid M,
Zissel G, Müller-Quernheim J and Prasse A: Lung collagens
perpetuate pulmonary fibrosis via CD204 and M2 macrophage
activation. Plos One. 8:e813822013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan MW, Keshavarzian A, Gounaris E,
Melson JE, Cheon EC, Blatner NR, Chen ZE, Tsai FN, Lee G, Ryu H, et
al: PI3K/AKT signaling is essential for communication between
tissue-infiltrating mast cells, macrophages and epithelial cells in
colitis-induced cancer. Clin Cancer Res. 19:2342–2354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seropian IM, Abbate A, Toldo S, Harrington
J, Smithson L, Ockaili R, Mezzaroma E, Damilano F, Hirsch E and Van
Tassell BW: Pharmacologic inhibition of phosphoinositide 3-Kinase
gamma (PI3Kγ) promotes infarct resorption and prevents adverse
cardiac remodeling after myocardial infarction in mice. J
Cardiovasc Pharmacol. 56:651–658. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siragusa M, Katare R, Meloni M, Damilano
F, Hirsch E, Emanueli C and Madeddu P: Involvement of
phosphoinositide 3-kinase gamma in angiogenesis and healing of
experimental myocardial infarction in mice. Circ Res. 106:757–768.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang ZL, Wu XH, Song LF, Wang YS, Hu XH,
Luo YF, Chen ZZ, Ke J, Peng XD, He CM, et al: Phosphoinositide
3-kinase gamma inhibitor ameliorates concanavalin A-induced hepatic
injury in mice. Biochem Biophys Res Commun. 386:569–574. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rangachari M, Mauermann N, Marty RR,
Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM and Eriksson U:
T-bet negatively regulates autoimmune myocarditis by suppressing
local production of interleukin 17. J Exp Med. 203:2009–2019. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eriksson U, Ricci R, Hunziker L, Kurrer
MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M and
Penninger JM: Dendritic cell-induced autoimmune heart failure
requires cooperation between adaptive and innate immunity. Nat Med.
9:1484–1490. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Affò S, Morales-Ibanez O, Rodrigo-Torres
D, Altamirano J, Blaya D, Dapito DH, Millán C, Coll M, Caviglia JM,
Arroyo V, et al: CCL20 mediates lipopolysaccharide induced liver
injury and is a potential driver of inflammation and fibrosis in
alcoholic hepatitis. Gut. 63:1782–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao XQ and Liu GT: Induction of
overexpression of the 27- and 70-kDa heat shock proteins by
bicyclol attenuates concanavalin A-Induced liver injury through
suppression of nuclear factor-kappaB in mice. Mol Pharmacol.
75:1180–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banan A, Shaikh M, Zhang L, et al:
Upregulation of NF-κB, IκBα phosphorylation (IκBα.P), iNOS and
cytoskeletal protein oxidation and dysfunction in colonic mucosa of
patients with inflammatory bowel disease (IBD). Gastroenterology.
124:A1982003. View Article : Google Scholar
|
|
25
|
Yamaoka S, Courtois G, Bessia C, Whiteside
ST, Weil R, Agou F, Kirk HE, Kay RJ and Israël A: Complementation
cloning of NEMO, a component of the IkappaB kinase complex
essential for NF-kappaB activation. Cell. 93:1231–1240. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie JH, Yamniuk AP, Borowski V, Kuhn R,
Susulic V, Rex-Rabe S, Yang X, Zhou X, Zhang Y, Gillooly K, et al:
Engineering of a novel anti-CD40L domain antibody for treatment of
autoimmune diseases. J Immunol. 192:4083–4092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watanabe R, Azuma RW, Suzuki J, Ogawa M,
Itai A, Hirata Y, Komuro I and Isobe M: Inhibition of NF-κB
activation by a novel IKK inhibitor reduces the severity of
experimental autoimmune myocarditis via suppression of T-cell
activation. Am J Physiol Heart Circ Physiol. 305:H1761–H1771. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dinarello CA, Simon A and van der Meer JW:
Treating inflammation by blocking interleukin-1 in a broad spectrum
of diseases. Nat Rev Drug Discov. 11:633–652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knulst AC, Tibbe GJ, Bril-Bazuin C,
Breedland EG, van Oudenarena, Benner R and Savelkoul HF: Cytokine
detection and modulation in acute graft vs. host disease in mice.
Mediators Inflamm. 3:33–40. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van de Laar L, Buitenhuis M, Wensveen FM,
Janssen HL, Coffer PJ and Woltman AM: Human CD34-derived myeloid
dendritic cell development requires intact phosphatidylinositol
3-kinase-protein kinase B-mammalian target of rapamycin signaling.
J Immunol. 184:6600–6611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rao J, Qian X, Li G, Pan X, Zhang C, Zhang
F, Zhai Y, X and Lu L: ATF3-mediated NRF2/HO-1 signaling regulates
TLR4 innate immune responses in mouse liver ischemia/reperfusion
injury. Am J Transplant. 15:76–87. 2015. View Article : Google Scholar
|
|
32
|
Yoo SY, Le TK, Jeong JJ and Kim DH:
Poligapolide, a PI3K/Akt inhibitor in immunodeficiency virus type 1
TAT-transduced CHME5 cells, isolated from the rhizome of Polygala
tenuifolia. Chem Pharm Bull (Tokyo). 62:467–471. 2014. View Article : Google Scholar
|
|
33
|
Liu SQ, Jiang S, Li C, Zhang B and Li QJ:
miR-17–92 cluster targets phosphatase and tensin homology and
ikaros family zinc finger 4 to promote TH17-mediated inflammation.
J Biol Chem. 289:12446–12456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goodwin CB, Li XJ, Mali RS, Chan G, Kang
M, Liu Z, Vanhaesebroeck B, Neel BG, Loh ML, Lannutti BJ, et al:
PI3K p110δ uniquely promotes gain-of-function Shp2-induced GM-CSF
hypersensitivity in a model of JMML. Blood. 123:2838–2842. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang E, Feng X, Liu F, Zhang P, Liang J
and Tang X: Roles of PI3K/Akt and c-jun signaling pathways in human
papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF and IL-8
expression and in vitro angiogenesis in non-small cell lung cancer
cells. Plos One. 9:e1034402014. View Article : Google Scholar
|
|
36
|
Eräsalo H, Laavola M, Hämäläinen M,
Leppänen T, Nieminen R and Moilanen E: PI3K Inhibitors LY294002 and
IC87114 reduce inflammation in carrageenan-induced paw oedema and
down-regulate inflammatory gene expression in activated
macrophages. Basic Clin Pharmacol Toxicol. 116:53–61. 2015.
View Article : Google Scholar
|