Introduction
Diabetic nephropathy (DN) is a severe complication
of diabetes mellitus and is the major cause of end-stage renal
disease (1,2) Since the pathogenesis of DN remains to
be elucidated, there is currently no effective treatment.
Traditionally, it was hypothesized that glucose metabolism, renal
hemodynamics, oxidative stress, cytokines and genetic factors were
involved in the pathogenesis of DN (3). Previous studies have found that
numerous factors, including excess nutrients and free fatty acids,
high glucose, and oxidative stress in the diabetic condition can
cause endoplasmic reticulum stress (ERS) and initiate proapoptotic
pathways inducing apoptosis and tissue damage (4,5).
Thus, ERS is one mechanism responsible for the pathogenesis of DN
(6,7).
Histone deacetylation enzymes (HDACs) are a family
of enzymes that are important in gene transcription and chromatin
remodeling, with a dynamic balance between HDAC and histone
acetyltransferase (HAT). HDACs can regulate cell proliferation,
migration and death (8). Although
the regulation of ERS is more complex, several studies have
reported that histone acetylation modification can control the
transcription of proteins involved in ERS (9–11).
Valproate (VPA) is a histone deacetylation enzyme
inhibitor (HDACI) that increases the acetylation level of histones
and promotes gene transcription. VPA has previously been used in
antiepileptic and antitumor treatments. Currently, it has also been
applied in the treatment of nerve degeneration, cardiovascular
disease, autoimmune diseases and diabetes mellitus (12–14).
VPA is now also suggested for the treatment of kidney disease;
however, the therapeutic mechanism involved remains to be
elucidated (15–17).
The present study aimed to determine whether VPA
attenuates DN through the inhibition of ERS and the possible
mechanism involved.
Materials and methods
Animals
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Jilin University and
rats were handled in strict accordance with the guidelines of the
care and use of medical laboratory animals (Ministry of Health P.R.
China, 2011) and the guidelines of the laboratory animal ethical
standards of Jilin Medical University. A total of 60 adult male
Wistar rats weighing 290±20 g and aged 8 weeks obtained from the
Laboratory Animal Center of Jilin University (Changchun, China)
were used in the present study. All animals received normal rat
chow and tap water ad libitum in a constant environment
(room temperature, 24±3°C; room humidity, 55±5%) with a 12-h
light/12-h dark cycle. The animals were kept under observation for
2 weeks prior to the start of the experiments.
Induction of a DN model and study
design
A total of 60 Wistar rats were used in this
experiment. For the normal control group, 20 rats were used (n=20),
which received a single injection of 0.1 mol/l citrate buffer. A
group of 40 rats were intravenously injected with streptozotocin
(STZ; Sigma-Aldrich, St. Louis, MO, USA; 70 mg/kg body weight) in a
0.1 mol/l citrate buffer (pH 4.5). Only rats with blood glucose
>11.1 mmol/l after 7 days were considered diabetic in the
fasting state. Glucose measurement was performed using a OneTouch
Select Analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Rats
with blood glucose <7.8 mmol/l were excluded from the study (6
rats). A total of 34 diabetic rats were fed a high-fat diet (45%
kcal% fat) for 22 weeks. The normal control group was fed normal
rat chow. During the 22 weeks, two diabetic rats died. In total, 32
diabetic rats were randomly divided into two groups: DN group
(n=15) and the DN plus VPA treatment group (DN+VPA group; n=17).
For the normal control group, 20 rats were also randomly divided
into two groups: Normal group (N group; n=10) and normal plus VPA
group (N+VPA group; n=10). Rats in the appropriate groups underwent
intragastric administration of 200 mg/kg VPA (Sigma-Aldrich) in 50
µl of distilled water or 50 µl of distilled water
alone once daily for 6 weeks. At week 6, all rats were euthanized.
Body weight, blood glucose, plasma creatinine and 24-h urinary
protein levels were measured regularly (fortnightly interval) and
at the end of the experiment duration. Kidneys were dissected and
rinsed with ice cold normal saline and then weighed. An index of
renal hypertrophy was estimated by comparing the wet weight of the
kidney to the body weight.
Urinary albumin assay
Urine samples were collected at the end of the
experiment. Urine albumin (ELISA; IBL international GmbH, Hamburg,
Germany) and blood creatinine (ELISA; BioVision Inc., San
Francisco, CA, USA) were measured according to the manufacturer's
instructions of the kits used.
Pathological examination
The kidney tissue of each group was collected, fixed
with 40 g/l paraformaldehyde for 24 h, embedded in paraffin
(Beyotime Institute of Biotechnology, Haimen, China) and sectioned
at a thickness of 3–4 µm. The sections were deparaffinized
and pathological changes were examined following hematoxylin (1 g
hematoxylin dissolved in 100 ml distilled water) and eosin staining
(Beyotime Institute of Biotechnology, Nanjing, China).
Transmission electron microscopy
The renal cortex was fixed in 2% glutaraldehyde in
cacodylate buffer at 4°C, postfixed in 1% osmium-tetroxide and
stained with 2% uranyl acetate. The samples were dehydrated and
embedded in Poly/bed 812 araldite resin (Polysciences Inc.,
Eppelheim, Germany). Ultrathin sections (50–100 nm) were cut with
an ultramicro-tome (Ultracut; Reichert-Jung, Depew, NY, USA),
mounted on copper grids and examined with a Tecnai 10 transmission
electron microscope (Philips, Eindhoven, Netherlands). Digital
images were captured using a megaview G2 CCD camera (Soft Imaging
System GmbH, Münster, Germany) at ×6,200 magnification.
Immunohistochemical staining
The kidney tissue was fixed in 40 g/l
paraformaldehyde for 24 h, embedded in paraffin, and then sectioned
at a thickness of 3–4 µm. The sections were deparaffinized
and 3% hydrogen peroxide in methanol was used to block endogenous
peroxidase at room temperature for 30 min. Pre-incubation of
sections for 30 min with 2% bovine serum albumin in 0.01 mol/l
phosphate-buffered saline (PBS) was used to block nonspecific
reactivity. The sections were then incubated with polyclonal rabbit
anti-human GRP78 (ab21685), monoclonal mouse anti-human C/EBP
homologous protein (CHOP; ab11419) or polyclonal rabbit anti-human
caspase-12 antibodies at a dilution of 1:400 at 4°C overnight [all
antibodies were obtained from Abcam (Hong Kong) Ltd., Hong Kong].
The sections were washed with PBS, bound antibodies were detected
with the SP1 kit (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China) and immunoreactive products were
visualized in 0.05% 3,3′-diaminobenzidine and 0.03%
H2O2. The sections were counterstained with
hematoxylin, dehydrated, cleared, counted and observed under an
Olympus BX51 microscope (Olympus, Tokyo, Japan). In control
staining, the sections were incubated with goat anti-mouse
immunoglobulin G (IgG) [ab197767; Abcam (Hong Kong) Ltd.] instead
of test antibodies.
Immunofluorescence staining
Sections of kidney tissue were prepared as described
above. Following blocking with 3% normal goat serum in 0.1 M
phosphate buffer containing 0.1% Triton X-100 (Sigma-Aldrich) for
30 min at room temperature, the sections were incubated with
anti-GRP78, anti-CHOP or anti-caspase-12 antibodies [1:200; Abcam
(Hong Kong) Ltd.] overnight at 4°C. Following being washed with 0.1
M phosphate buffer, sections were reacted with Alexa Fluor
488-conjugated secondary antibody (1:400; Invitrogen; Thermo Fisher
Scientific, Rockford, IL, USA) for 1 h, stained with Hoechst 33342
(2 µg/ml) for 2 min and washed with PBS three times. The
sections were examined with a laser scanning confocal microscope
(FV1000; Olympus) at an excitation wavelength of 488 nm.
Western blot analysis
Kidney tissues were homogenized in RIPA buffer (50
mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM
EDTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM
β-glycerophosphate, 1 mM Na3VO4, 1 mM NaF, 1
µg/ml leupeptin and 1 mM PMSF; Beyotime Institute of
Biotechnology) and total protein was extracted. The homogenized
samples were boiled for 5 min and stored at −20°C. The protein
concentration was determined using a Pierce BCA kit (Thermo Fisher
Scientific). For western blot analysis, protein lysates (30–50
µg) were resolved by 12% SDS-polyacrylamide gel
electrophoresis (Beyotime Institute of Biotechnology) and
transferred onto immobilon-P transfer membranes (Millipore, Boston,
MA, USA). The membranes were blocked with 5% non-fat milk powder in
buffer [10 mM Tris-HCl, (pH 7.6), 100 mM NaCl and 0.1% Tween 20]
for 2 h at room temperature and then incubated with the desired
primary antibody, including polyclonal rabbit anti-human GPR78
(cat. no. ab21685), polyclonal rabbit anti-human caspase-12 (cat.
no. ab4051), rabbit polyclonal anti-caspase-3, mouse anti-human
monoclonal CHOP (cat. no. ab11419), monoclonal rat anti-human
B-cell lymphoma 2 (Bcl-2; cat. no. ab166652), rabbit monoclonal
anti-Bcl-2-associated X protein (Bax; cat. no. ab32503), polyclonal
rabbit anti-phosphorylated JNK (pJNK; cat. no. ab4821), monoclonal
mouse anti-activating transcription factor 4 (cat. no. ab23760) or
monoclonal mouse anti-β-actin [cat. no. ab6276; 1:1,000 dilution;
all obtained from Abcam (Hong Kong) Ltd.] overnight at 4°C,
followed by incubation with a horseradish peroxidase-conjugated
secondary antibody (Hangzhou HuaAn Biotechnology Co., Ltd.,
Hangzhou, China) at a 1:2,000 dilution for 1.5 h at room
temperature. The immunoreactive bands were visualized with
3,3′-diaminobenzidine (Sigma-Aldrich). The representative bands
were measured by a Tanon GIS gel imager system (Tanon Science &
Technology Co., Ltd., Shanghai, China) and analyzed. The levels of
proteins were normalized to those of β-actin and the ratios are
presented as the mean ± standard deviation of three independent
experiments. Protein levels were quantified by densitometry using
Quantity One 1-D software (version 4.4.02; Bio-Rad Laboratories,
Hercules, CA, USA).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)
Analysis of apoptosis was performed using an In Situ
Cell Death Detection kit (Roche Diagnostics, Indianapolis, IN, USA)
to identify DNA breaks according to the manufacturer's
instructions. Sections were incubated with the TUNEL reaction mix
containing 10 units of terminal deoxyribosyltransferase, 10 mM
dUTP-biotin and 2.5 mM cobalt chloride in 1X terminal transferase
reaction buffer for 1 h at 37°C in a humidified atmosphere.
Apoptotic cells with characteristic nuclear fragmentation (staining
green) were counted in six randomly selected fields. The experiment
was repeated three times.
Chromatin immunoprecipitation assay
(ChIP)
ChIP assays using monoclonal rabbit
anti-acetylated-histone H4 antibodies [ab109463; Abcam (Hong Kong)
Ltd.] were performed to investigate the effects of VPA on the
acetylation of histone in the GRP78 and CHOP promoter. ChIP assays
were performed using a ChIP assay kit, according to the
manufacturer's instructions (EMD Millipore, Billerica, MA, USA).
Briefly, kidney tissues were treated as indicated and cross-linked
with formaldehyde, then sonicated. Resulting kidney tissue lysates
(input) were immunoprecipitated with anti-acetylated-histone H4
antibodies. The precipitated protein-DNA complexes (IP) were
subjected to proteinase treatment. The following primers were used:
GRP78, forward 5′-CTC GAG GAA GGG CATA AGAG CATCA-3′ and reverse
5′-CCG CTT CTC CTC AGG TTC CGG CTGT-3′; CHOP, forward 5′-ACT GAC
AAC GAC AAG ACCCC-3′ and reverse 5′-AGT CAC AGC CAG TAT CGAGC-3′;
GAPDH, forward 5′-ACA ACC TGG TCC TCA GTG TAGCC-3′ and reverse
5′-AAG GTC ATC CCA GAG CTG AACGG-3′.
Statistical analysis
Data are representative of three independent
experiments, each conducted in triplicate. The differences between
two groups were analyzed by the Kruskal-Wallis H non-parametric
test using SPSS 19.0 for Windows (SPSS, Inc., Chicago, IL, USA). A
two-sided P-value of P<0.05 was considered to indicate a
statistically significant difference.
Results
VPA reduces ERS in a rat model of DN
Immunohistochemistry and immunofluorescence
microscopy were used to determine the expression of the
ERS-associated proteins GRP78, CHOP and caspase-12. Compared with
the DN group, the expression of GRP78 in the DN+VPA group was
enhanced, while the expression of CHOP and caspase-12 was reduced
(Fig. 1A and B). Western blot
analysis was used to quantify the expression of GRP78, CHOP and
caspase-12. Similar to the immunohistology results, compared with
the DN group, the expression of GRP78 in the DN+VPA group was
enhanced, while the expression of CHOP and caspase-12 was
significantly reduced (Fig. 1C and
D). These results indicate that VPA can effectively reduce ERS
in a rat model of DN.
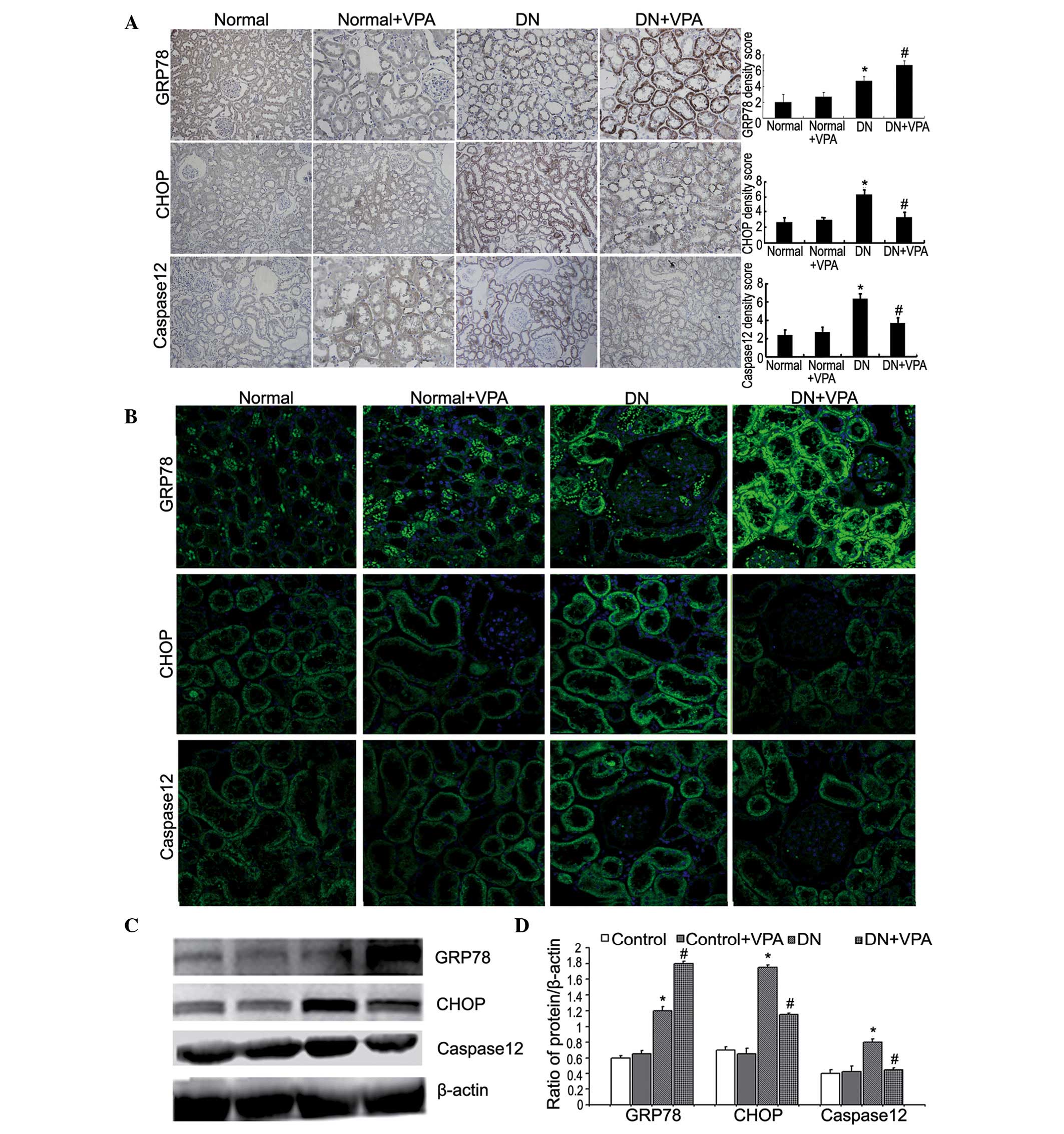 | Figure 1VPA relieves endoplasmic
reticulum-stress-mediated apoptosis in DN rats. (A) Rats were
divided into four groups: Normal group, normal+VPA group, DN group
and the DN+VPA group. The kidney tissues removed from four groups
were immunohistochemically stained with anti-GRP78, anti-CHOP and
anti-caspase-12 antibody (top, magnification, ×200). Quantitations
of GRP78, CHOP and caspase-12 density score (bottom). Data are
presented as the mean ± SD (n=3). *P<0.05 vs. the
normal group; #P<0.05 vs. the DN group. (B)
Expression levels of GRP78, CHOP and caspase-12 in kidney tissues
removed from four groups were analyzed by confocal microscopy
(magnification, ×400). (C) Western blot analysis was used to
analyze the expression of GRP78, CHOP and caspase-12 in kidney
tissues removed from four groups (left). (D) Quantitations of
GRP78, CHOP and caspase-12 protein levels (right). Data are
presented as the mean ± SD (n=3). *P<0.05 vs. the
normal group; #P<0.05 vs. the DN group. VPA,
valproate; DN, diabetic nephropathy; SD, standard deviation; CHOP,
C/EBP homologous protein; GRP78, glucose-regulated protein. |
VPA reduces apoptosis in a rat model of
DN
The TUNEL assay was used to determine whether VPA
could modulate apoptosis in kidney tissue in DN. Compared with the
normal group, apoptosis was significantly increased in the DN
group. However, compared with the DN group, apoptosis in the DN+VPA
group was reduced following VPA treatment (Fig. 2A and B). Notably, apoptosis was
predominantly concentrated in the renal tubules. Western blot
analysis was then used to investigate the expression of
apoptosis-associated proteins. Compared with the control group, the
expression of pJNK, Bax and cleaved caspase-3 was enhanced in the
DN group, while the expression of Bcl-2 protein was decreased. By
contrast, compared with the DN group, the expression of pJNK, Bax
and cleaved caspase-3 was reduced in the DN+VPA group, while the
expression of Bcl-2 was enhanced (Fig.
2C and D). These results suggest that VPA reduces apoptosis in
renal tissue.
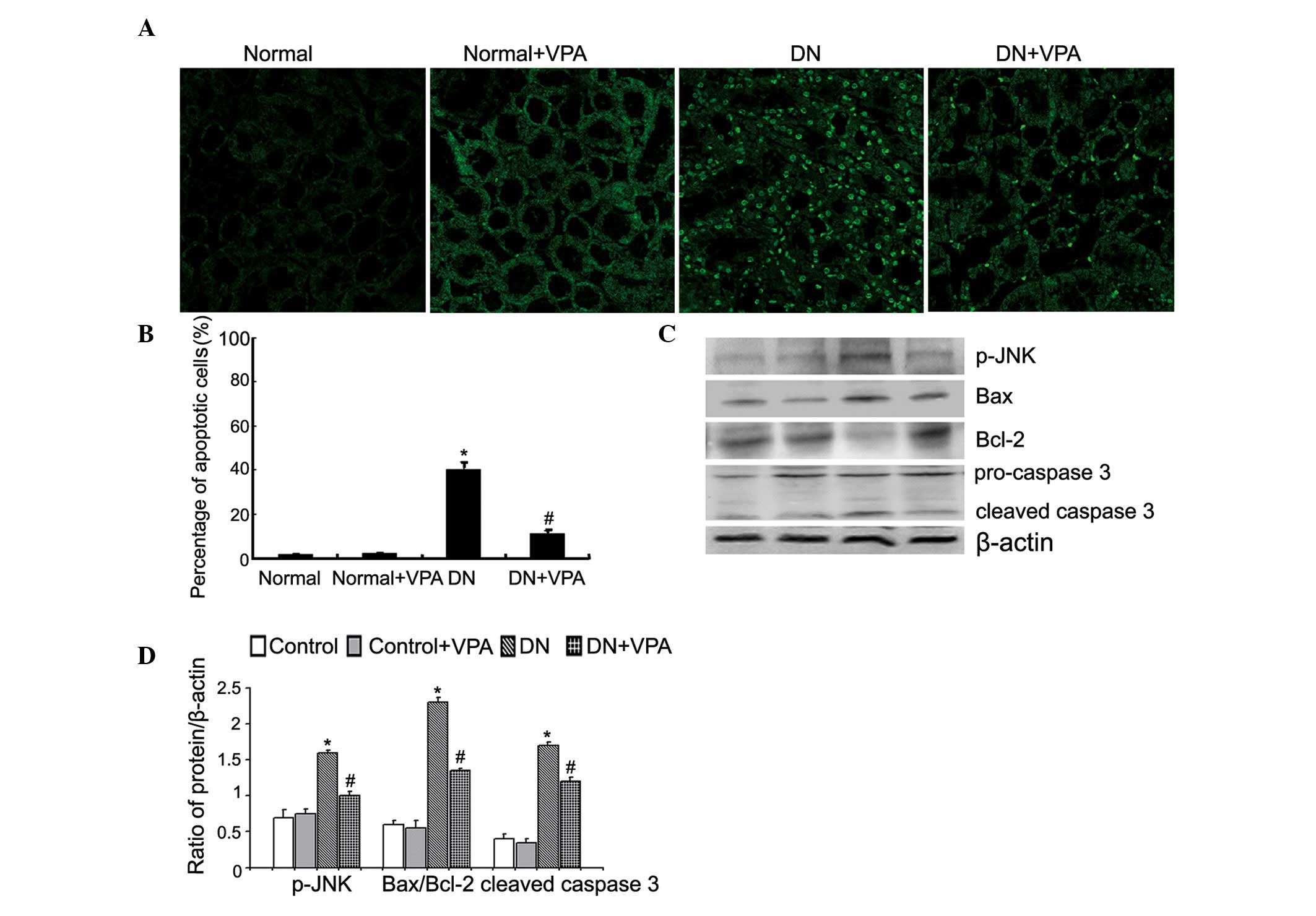 | Figure 2VPA reduces mitochondrial-mediated
apoptosis in diabetic nephropathy rats. (A) Rats were divided into
four groups: Normal group, normal+VPA group, DN group and the
DN+VPA group. The kidney tissues removed from four groups were
stained with TUNEL, followed by fluorescence microscopy at a
magnification of ×400 (top). (B) Quantitation of the percentage of
apoptotic cells in four groups (bottom). Data are presented as the
mean ± SD (n=3). *P<0.05 vs. the normal group;
#P<0.05 vs. the DN group. (C) The expression of
p-JNK, Bax, Bcl-2, procaspase-3 and cleaved caspase-3 in the four
groups was investigated by western blot analysis (left). (D)
Quantitation of p-JNK, Bax, Bcl-2, procaspase-3 and cleaved
caspase-3 protein levels (right). Data are presented as the mean ±
SD (n=3). *P<0.05 vs. the normal group;
#P<0.05 vs. the DN group. VPA, valproate; DN,
diabetic nephropathy; SD, standard deviation; Bax, Bcl-2-associated
X protein; Bcl-2, B-cell lymphoma 2; p-JNK, phosphorylated JNK;
TUNEL, terminal deoxynucleotidyl transferase dUTP nick end
labeling. |
VPA attenuates kidney injury in a rat
model of DN
Excessive apoptosis eventually leads to organ
dysfunction. Blood sugar, the ratio of kidney weight/body weight,
serum creatinine and 24-h urinary protein were all significantly
increased and body weight significantly reduced in the DN group
compared with the normal group. However, compared with the DN
group, in the DN+VPA group, 24-h urinary protein excretion and
serum creatinine level were significantly reduced, while the blood
sugar, body weight and kidney weight/body weight ratio exhibited no
pronounced alterations (Fig.
3A–F). Histopathological changes were also examined. Compared
with the normal group, in the DN group, the Bowman's space was
decreased, the glomerular basement membrane was thickened, the
mesangial matrix expanded and mesangial cells proliferated.
Compared with the DN group, these pathological alterations were
reduced in the DN+VPA group (Fig.
3G). Additionally, the ultrastructure of the kidney was
observed using transmission electron microscopy. Compared with the
normal group, the glomerular basement membrane became thickened,
the structure of the membrane filtration layers was unclear,
podocyte foot processes were disordered, the gap became broadened
and the mesangial matrix was increased in the DN group. These
findings were significantly attenuated in the DN+VPA group compared
with the DN group (Fig. 3H),
indicating that VPA can relieve renal injury in a rat model of
DN.
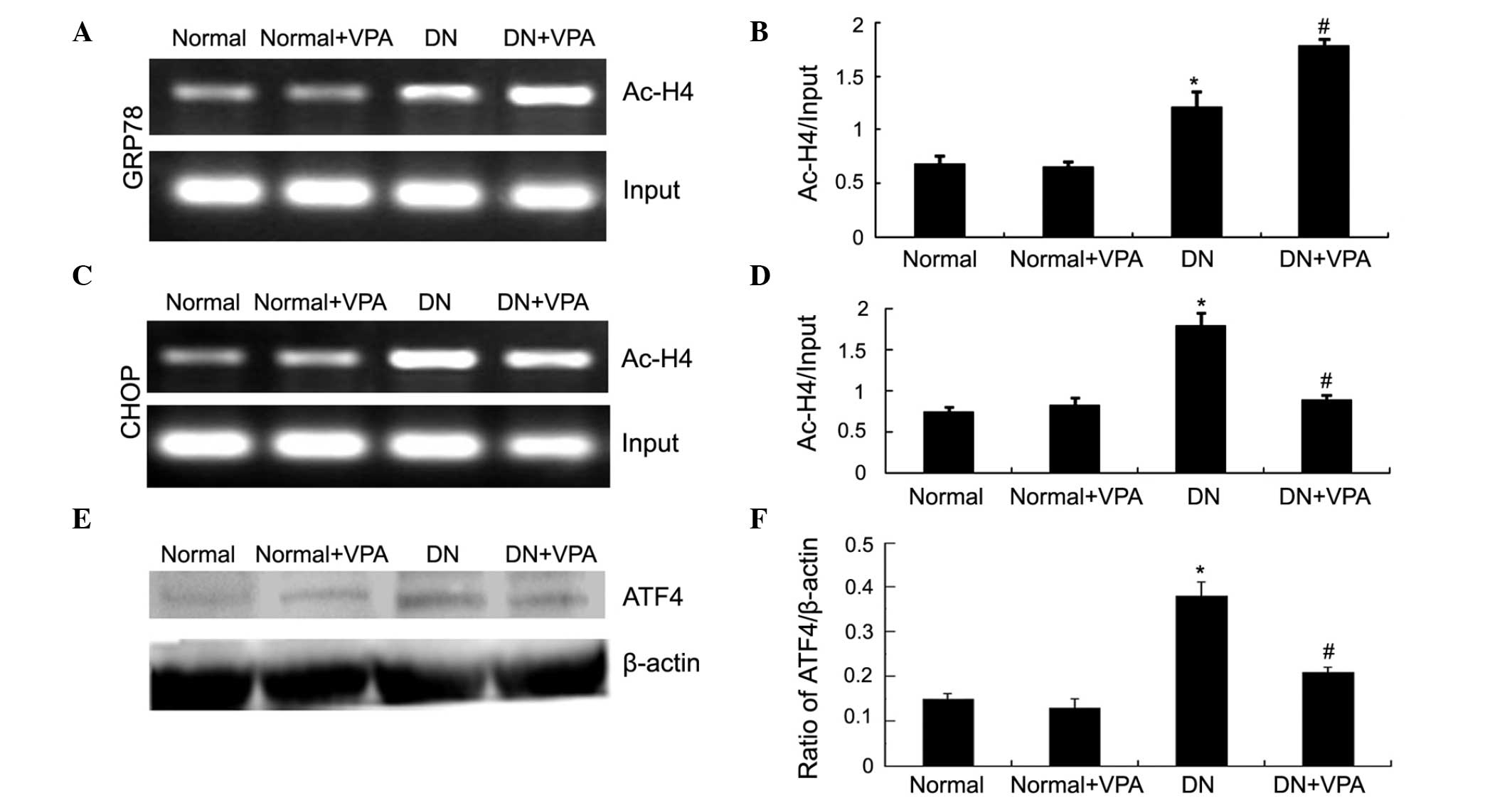
VPA increases histone H4 acetylation in
the GRP78 promoter and reduces histone H4 acetylation in the CHOP
promoter
ChIP assay was used to detect the acetylation level
of histone H4 in the GRP78 promoter. Compared with the normal
group, the acetylation level of histone H4 in GRP78 was increased
in the DN group. The acetylation level of H4 in the GRP78 promoter
was increased in the DN+VPA group compared with the DN group
(Fig. 4A and B). The present study
also assessed the acetylation level of H4 in the CHOP gene
promoter. Compared with the DN group, the acetylation level of H4
in the CHOP gene promoter was reduced in the DN+VPA group (Fig. 4C and D). To determine why the
acetylation of histone H4 in the CHOP promoter was altered, the
protein expression of ATF4 was investigated, which is upstream of
the CHOP gene. Compared with the normal group, the protein
expression of ATF4 was increased in the DN group. However, the
protein expression of ATF4 was reduced in the DN+VPA group compared
with the DN group (Fig. 4E and F).
These results indicated that VPA increases the acetylation level of
histone H4 in the GRP78 promoter and reduces the acetylation level
of histone H4 in the CHOP promoter.
Discussion
The functional role of the ER is the folding,
modification and degradation of secretory and transmembrane
proteins. Conditions, including oxidative stress,
ischemia-reperfusion injury, hypoxia and calcium metabolism
disorders can all induce ERS. ERS can lead to disorders of protein
folding and cause the accumulation of unfolded and misfolded
proteins. In order to restore ER homeostasis, a variety of cell
signaling pathways are activated to maintain cell survival.
However, when the stimulus inducing ERS persists, the ERS response
eventually ends in apoptosis (5,7).
Islet β-cells are particularly sensitive to alterations in the ER
(18). High blood glucose, free
fatty acids (19) and
over-nutrition in diabetes mellitus are ERS-inducing factors. These
factors can induce ERS in renal tubular epithelial cells or other
renal cells (10,20), and if persistent may cause
apoptosis (19,21). ERS can also lead to glomerular
epithelial cell injury (22) with
the subsequent proteinuria inducing ERS and apoptosis in renal
tubular epithelial cells (23).
Previous studies have indicated that suppression of the expression
of the ERS-associated protein X-box binding protein 1 reduces
fibrosis caused by kidney mesangial cells (24,25).
Notably, Morse et al found that ERS was active in
nephropathy (26). These data
indicate that ERS is one mechanism involved in the pathogenesis of
DN (7,21). In the present study, it was found
that the expression of ERS-associated proteins GRP78, CHOP and
caspase-12 were all significantly increased in DN. This suggests
that apoptosis in renal tissue in a rat model of DN is upregulated
via an ERS-induced pathway.
The acetylation and deacetylation of histones is an
important and common pathway in the regulation of gene expression.
The dynamic balance between HDAC and HAT is critical in gene
transcription and chromatin remodeling. Several studies have
demonstrated that histone acetylation modification is associated
with ERS (9,27). Zhang et al demonstrated that
VPA increases the expression of GRP78 and reduces the expression of
CHOP and caspase-12 by increasing the acetylation level of histone
H3, attenuating retinal ischemia-reperfusion injury (27). Yu et al has demonstrated
that trichostatin A (TSA), another HDACI, increases the expression
of GRP78 in a myocardial ischemia-reperfusion injury model, and
also reduces the expression of CHOP and caspase-12, therefore
reducing apoptosis (9). In the
present study, it was found that VPA increases the expression of
GRP78 and reduces the expression of CHOP and caspase-12 and
decreases apoptosis in a rat model of DN. This suggests that VPA
can relieve ERS in DN and thus decrease ERS-induced apoptosis. It
was also found that 24-h urinary protein excretion and serum
creatinine were reduced in the DN+VPA group compared with the DN
group. Kidney histopathology and ultrastructure were also improved,
thus VPA can also attenuate overall renal injury in DN.
In order to elucidate how VPA regulates ERS, the
present study further investigated the acetylation level of histone
H4 in ERS-associated protein promoters. The acetylation level of
histone H4 in the GRP78 promoter was increased in the DN+VPA group,
suggesting that VPA can inhibit HDAC indirectly to increase the
acetylation level of histones in the GRP78 gene promoter H4, which
promotes the transcription of the GRP78 gene. Baumeister et
al reported that ERS-induced binding of the histone
acetyltransferase p300 to the GRP78 promoter and histone H4
acetylation of the ER-bound bZIP family transcription factor is
activated upon ERS, inducing histone H3 and H4 acetylated promoter
gene transcription (28). The
acetylation of histone H3 and H4 in the activating transcription
factor 6α (ATF6α) promoter regulates ATF6α gene transcription
(29). These data indicate that
histone acetylated modification is associated with ERS. Bown et
al also found that VPA can regulate the transcription of
ERS-associated proteins (30).
Following ERS induction, SGF29 is required for increased H3K14
acetylation in the ERS genes GRP78 and CHOP promoters, which then
results in full transcriptional activation (11). This suggests HDACI may be
associated with the level of histone H3 and H4 acetylation in
ERS-targeted protein promoters. It was found that VPA can increase
the acetylation of histone H4 in the GRP78 promoter, which promotes
the protein expression of GRP78. Notably, it was found that VPA
reduces the acetylation of histone H4 in the CHOP promoter. Yu
et al found that TSA can also reduce the acetylation of
histone H3 in the CHOP promoter in myocardial ischemia-reperfusion
injury (9). Shan et al
found that following amino acid deprivation, a primary function of
ATF4 was to recruit HAT to target genes. ATF4 bound to the genes
prior to histone acetylation, thus ATF4 functions as a pioneering
factor to alter chromatin structure to enhance transcription in a
gene-specific manner (31). ATF4
is upstream of the CHOP gene and is also the main transcription
factor promoting CHOP gene transcription. Fawcett et al
identified that ATF4 can bind to the CHOP gene promoter at
C/EBP-ATF sites to promote CHOP mRNA expression in arsenite-treated
PC12 cells (32). In the present
study, it was found that VPA reduces the protein expression of ATF4
in the DN+VPA group. Therefore, it was hypothesized that VPA may
reduce the expression of ATF4, through inhibition of the binding of
ATF4 to the CHOP promoter, thereby inhibiting the recruitment of
HAT and eventually decreasing the acetylation of histone H4 in the
CHOP promoter.
In conclusion, the present study demonstrated that
VPA can relieve ERS in a rat model of DN and reduces ERS-induced
apoptosis, thereby attenuating diabetes-induced renal injury. This
may be performed through the regulation of histone H4 acetylation
in the promoters of the ERS-associated proteins GRP78 and CHOP.
These results provide initial experimental evidence for the
treatment of DN with VPA.
Acknowledgments
The authors would like to thank Professor Lining
Miao and Dr Yangwei Wang from the Second Clinical Hospital of Jilin
University for their assistance with the animal models, and Mr.
Liwen Bianji (Edanz Group China) for the editing of the
manuscript.
References
|
1
|
Grace BS, Clayton P and McDonald SP:
Increases in renal replacement therapy in Australia and New
Zealand: Understanding trends in diabetic nephropathy. Nephrology
(Carlton). 17:76–84. 2012. View Article : Google Scholar
|
|
2
|
Maric C and Hall JE: Obesity, metabolic
syndrome and diabetic nephropathy. Contrib Nephrol. 170:28–35.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higa A and Chevet E: Redox signaling loops
in the unfolded protein response. Cell Signal. 24:1548–1555. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W
and Duan H: Role of endoplasmic reticulum stress in apoptosis of
differentiated mouse podocytes induced by high glucose. Int J Mol
Med. 33:809–816. 2014.PubMed/NCBI
|
|
7
|
Cunard R and Sharma K: The endoplasmic
reticulum stress response and diabetic kidney disease. Am J Physiol
Renal Physiol. 300:F1054–F1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choudhary C, Kumar C, Gnad F, Nielsen ML,
Rehman M, Walther TC, Olsen JV and Mann M: Lysine acetylation
targets protein complexes and co-regulates major cellular
functions. Science. 325:834–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu L, Lu M, Wang P and Chen X:
Trichostatin A ameliorates myocardial ischemia/reperfusion injury
through inhibition of endoplasmic reticulum stress-induced
apoptosis. Arch Med Res. 43:190–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Zhao Y, Chu Q, Wang ZY, Li H and
Chi ZH: Zinc modulates high glucose-induced apoptosis by
suppressing oxidative stress in renal tubular epithelial cells.
Biol Trace Elem Res. 158:259–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schram AW, Baas R, Jansen PW, Riss A, Tora
L, Vermeulen M and Timmers HT: A dual role for SAGA-associated
factor 29 (SGF29) in ER stress survival by coordination of both
histone H3 acetylation and histone H3 lysine-4 trimethylation. PloS
One. 8:e700352013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HJ, Rowe M, Ren M, Hong JS, Chen PS
and Chuang DM: Histone deacetylase inhibitors exhibit
anti-inflammatory and neuroprotective effects in a rat permanent
ischemic model of stroke: Multiple mechanisms of action. J
Pharmacol Exp Ther. 321:892–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren M, Leng Y, Jeong M, Leeds PR and
Chuang DM: Valproic acid reduces brain damage induced by transient
focal cerebral ischemia in rats: Potential roles of histone
deacetylase inhibition and heat shock protein induction. J
Neurochem. 89:1358–1367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Leng Y, Tsai LK, Leeds P and
Chuang DM: Valproic acid attenuates blood-brain barrier disruption
in a rat model of transient focal cerebral ischemia: The roles of
HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 31:52–57.
2011. View Article : Google Scholar :
|
|
15
|
Van Beneden K, Geers C, Pauwels M,
Mannaerts I, Verbeelen D, van Grunsven LA and Van den Branden C:
Valproic acid attenuates proteinuria and kidney injury. J Am Soc
Nephrol. 22:1863–1875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H
and Lee HB: Histone deacetylase-2 is a key regulator of diabetes-
and transforming growth factor-beta1-induced renal injury. Am J
Physiol Renal Physiol. 297:F729–F739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Advani A, Huang Q, Thai K, Advani SL,
White KE, Kelly DJ, Yuen DA, Connelly KA, Marsden PA and Gilbert
RE: Long-term administration of the histone deacetylase inhibitor
vorinostat attenuates renal injury in experimental diabetes through
an endothelial nitric oxide synthase-dependent mechanism. Am J
Pathol. 178:2205–2214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Scheuner D, Ron D, Pennathur S and
Kaufman RJ: Chop deletion reduces oxidative stress, improves beta
cell function, and promotes cell survival in multiple mouse models
of diabetes. J Clin Invest. 118:3378–3389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tao JL, Wen YB, Shi BY, Zhang H, Ruan XZ,
Li H, Li XM, Dong WJ and Li XW: Endoplasmic reticulum stress is
involved in podocyte apoptosis induced by saturated fatty acid
palmitate. Chin Med J (Engl). 125:3137–3142. 2012.
|
|
20
|
Anil Kumar P, Welsh GI, Saleem MA and
Menon RK: Molecular and cellular events mediating glomerular
podocyte dysfunction and depletion in diabetes mellitus. Front
Endocrinol (Lausanne). 5:1512014.
|
|
21
|
Qi W, Mu J, Luo ZF, Zeng W, Guo YH, Pang
Q, Ye ZL, Liu L, Yuan FH and Feng B: Attenuation of diabetic
nephropathy in diabetes rats induced by streptozotocin by
regulating the endoplasmic reticulum stress inflammatory response.
Metabolism. 60:594–603. 2011. View Article : Google Scholar
|
|
22
|
McAlpine CS, Bowes AJ and Werstuck GH:
Diabetes, hyperglycemia and accelerated atherosclerosis: Evidence
supporting a role for endoplasmic reticulum (ER) stress signaling.
Cardiovasc Hematol Disord Drug Targets. 10:151–157. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lindenmeyer MT, Rastaldi MP, Ikehata M,
Neusser MA, Kretzler M, Cohen CD and Schlöndorff D: Proteinuria and
hyperglycemia induce endoplasmic reticulum stress. J Am Soc
Nephrol. 19:2225–2236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimura K, Jin H, Ogawa M and Aoe T:
Dysfunction of the ER chaperone BiP accelerates the renal tubular
injury. Biochem Biophys Res Commun. 366:1048–1053. 2008. View Article : Google Scholar
|
|
25
|
Shao D, Liu J, Ni J, Wang Z, Shen Y, Zhou
L, Huang Y, Wang J, Xue H, Zhang W and Lu L: Suppression of XBP1S
mediates high glucose-induced oxidative stress and extracellular
matrix synthesis in renal mesangial cell and kidney of diabetic
rats. PloS One. 8:e561242013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morse E, Schroth J, You YH, Pizzo DP,
Okada S, Ramachandrarao S, Vallon V, Sharma K and Cunard R: TRB3 is
stimulated in diabetic kidneys, regulated by the ER stress marker
CHOP, and is a suppressor of podocyte MCP-1. Am J Physiol Renal
Physiol. 299:F965–F972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Tong N, Gong Y, Qiu Q, Yin L, Lv
X and Wu X: Valproate protects the retina from endoplasmic
reticulum stress-induced apoptosis after ischemia-reperfusion
injury. Neurosci Lett. 504:88–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baumeister P, Luo S, Skarnes WC, Sui G,
Seto E, Shi Y and Lee AS: Endoplasmic reticulum stress induction of
the Grp78/BiP promoter: Activating mechanisms mediated by YY1 and
its interactive chromatin modifiers. Mol Cell Biol. 25:4529–4540.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Misra J, Kim DK, Choi W, Koo SH, Lee CH,
Back SH, Kaufman RJ and Choi HS: Transcriptional cross talk between
orphan nuclear receptor ERRγ and transmembrane transcription factor
ATF6α coordinates endoplasmic reticulum stress response. Nucleic
Acids Res. 41:6960–6974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bown CD, Wang JF, Chen B and Young LT:
Regulation of ER stress proteins by valproate: Therapeutic
implications. Bipolar Disord. 4:145–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shan J, Fu L, Balasubramanian MN, Anthony
T and Kilberg MS: ATF4-dependent regulation of the JMJD3 gene
during amino acid deprivation can be rescued in Atf4-deficient
cells by inhibition of deacetylation. J Biol Chem. 287:36393–36403.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fawcett TW, Martindale JL, Guyton KZ, Hai
T and Holbrook NJ: Complexes containing activating transcription
factor (ATF)/cAMP-responsive-element-binding protein (CREB)
interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF
composite site to regulate Gadd153 expression during the stress
response. Biochem J. 339:135–141. 1999.PubMed/NCBI
|