Introduction
Adjuvants have been demonstrated to be key
components in vaccines and are capable of enhancing and/or shaping
antigen-specific immune responses (1). The majority of the existing vaccines
include a signal adjuvant, however, single adjuvants may be limited
to inducing immune responses of low potency or quality, therefore,
single adjuvants cannot elicit the optimal immune responses
required for certain vaccines (2).
Thus, the combined co-administration of different adjuvants with
antigens is a potential approach for improving immune responses to
vaccines. In previous years, numerous combination adjuvants have
been investigated, including alum and CpG oligodeoxynucleotides
(ODN), CpG ODN and polyphosphazenes, and CpG ODN and LL-37, which
demonstrate a synergistic response following vaccine immunization
(2,3). Furthermore, the combination adjuvant,
CpG-host defense peptide (HDP), has been demonstrated to exhibit a
synergistic immune response in mice when co-injected with ovalbumin
and leads to an improvement of innate immune in newborn piglets
(4,5). In the present study, CpG ODN was
combined with innate defense regulator (IDR) peptide, IDR-HH2, as a
novel adjuvant to evaluate its ability to enhance and modulate the
immune response when formulated with commercial hepatitis B (HBV)
vaccines, consisting of hepatitis B surface antigen (HBsAg) and
alum, which typically induce a T helper (Th)2-type immune response
(6).
IDRs are synthetic mimics of host defense peptides,
which are important components of the innate immune system and have
well-documented selective immune stimulatory activities (7). IDRs have also been reported to induce
the production of cytokines/chemokines by immune cells, stimulate
angiogenesis and wound healing, and modulate monocyte-macrophage
differentiation and monocyte-dendritic cell differentiation
(8). Our previous studies focused
on the antimicrobial and adjuvant activity of IDRs, designed a
novel IDR-DP7 and developed a combination adjuvant for tumor
vaccine (9,10). HH2 is a synthetic 12-aa IDR
optimized for immunomodulatory activity, which can form a complex
with CpG ODN via electrostatic interactions and stimulate potent
antibody production and B cell expansion (11). In addition, IDR-HH2 has been
demonstrated to recruit neutrophils (12).
CpG ODNs containing unmethylated CpG motifs have
been confirmed as potent stimulants of B cells and plasmacytoid
dendritic cells (13). They are
recognized by Toll-like receptor 9, and induce the proliferation of
the majority of B cells and the secretion of immunoglobulin (Ig)
and cytokines (14,15). CpG ODNs also directly activate a
number of other types of immune cells, including monocytes,
macrophages and dendritic cells, resulting in the secretion of
pro-inflammatory interleukin (IL)-1, IL-6, IL-18 and tumor necrosis
factor (TNF)-α, and IL-12 and interferon (IFN)-γ type 1 cytokines
(16). Previous studies have
demonstrated that when co-administered with several viral and
bacterial antigens, CpG ODNs elicit enhanced immune responses and
overcome the type 2 bias associated with conventional adjuvants,
including alum or oil-based adjuvants (17–19).
To date, the use of the CpG-HH2 complex as an
adjuvant of HBV vaccines has not been reported. The aim of the
present study was to investigate whether the CpG-HH2 complex is
able to overcome the Th2 immune response induced by alum when
co-administered with a commercial HBV vaccine, and induce mixed
Th1/Th2 responses. The resultant novel HBV vaccine may trigger more
potent and effective protection against the pathogen and indicate
that the CpG-HH2 complex may be used as a potential adjuvant
formulation.
Materials and methods
Materials
Phosphorothioate-stabilized CpG ODN
(5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) was synthesized by Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). IDR-HH2
(VQLRIRVAVIRA-NH2), the major histocompatibility complex (MHC)
class I-restricted HBsAg208–216 peptide (ILSPFLPL) and
MHC class II restricted HBsAg126–138 peptide
(RGLYFPAGGSSSG) were synthesized by Shang Hai Science Peptide
Biological Technology Co., Ltd (Shanghai, China). Aluminum
hydroxide (alum) was purchased from Brenntag Biosector
(Frederikssund, Denmark). HBsAg was purchased from American
Research Products, Inc. (Waltham, MA, USA).
Cell preparation and chemotaxis
assay
Neutrophils were prepared by density gradient
centrifugation using Polymorphprep™ (Axis-Shield Poc As, Oslo,
Norway), according to the manufacturer's protocol. Briefly, venous
blood (20 ml) was collected from 3 healthy volunteers (2 male, 1
female; aged 23–35), who had provided informed consent, at the West
China Hospital (Chengdu, China). The blood was layered onto an
equal volume of Polymorphprep, followed by centrifugation at 500 x
g for 30–35 min at room temperature. The plasma and mononuclear
cells were removed, and the lower band of polymorphonuclear
neutrophils were collected and washed twice in RPMI 1640 medium
(Gibco; Thermo Fisher Scientific, Inc.). The neutrophils were
resuspended in RPMI 1640 with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at a concentration of 1×107 cells/ml
for the chemotaxis assay.
The chemotactic activities of THP-1 monocytes
(American Type Culture Collection, Rockville, MD, USA) and RAW264.7
cells (Type Culture Collection of the Chinese Academy of Sciences,
Shanghai, China) in response to IDR-HH2 were measured in a 24-well
Transwell plate (Nunc®; Thermo Fisher Scientific, Inc.)
with a 8.0-µm pore size polycarbonate membrane. The
neutrophils were assayed in a 24-well Transwell plate (Corning
Incorporated, Corning, NY, USA) with a 3.0-µm pore size
polycarbonate membrane. THP-1 cells were suspended in RPMI 1640
with 10% FBS, while RAW264.7 cells were suspended in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.)
with 10% FBS. Subsequently, 100 µl aliquots of the cell
suspensions at concentrations of 2×106 cells/ml (THP-1),
5×106 cells/ml (RAW264.7), 1×107 cells/ml
(neutrophils) were added into separate Transwell inserts of the
chemotaxis chambers, in which the lower compartment contained 600
µl of media containing IDR-HH2 (0, 2.5, 5, 10, 20 or 40
µg/ml). Following incubation at 37°C for 45, 90 and 180 min,
the inserts were removed, and the THP-1 cells, which had migrated
into the lower chamber, were scored using fluorescence-activated
cell sorting analysis within 2 min, whereas the chemotactic
migration of the two other types of cell, which were stained with
0.01% crystal violet (Beyotime Institute of Biotechnology,
Shanghai, China), was determined by counting the number of migrated
cells on the polycarbonate membrane in five randomly selected
microscope fields per well (magnification, ×400). The results were
expressed as the number of migrated cells in response to
IDR-HH2.
Isolation of bone marrow dendritic cells
(BMDCs)
The experimental protocol was approved by the Ethics
Review Committee for Animal Experimentation of Sichuan University.
Female C57BL/6J mice (6–8 weeks old) were purchased from the
Experimental Animal Center of Sichuan University (Sichuan, China)
and maintained at 23±1°C in a 12-h light/dark cycle with free
access to food and water in a pathogen-free animal facility. Mice
were sacrificed by cervical dislocation, and the femur and tibia
were removed. The ends of the femur and tibia were pierced using a
1 ml syringe, and the bone marrow washed 5 times with RPMI 1640.
The BMDCs were collected and centrifuged at 300 × g for 3 min at
room temperature, washed twice with RPMI 1640, followed by
incubation with red blood cell lysis buffer (Beyotime Institute of
Biotechnology) for 3–5 min at room temperature. Following two
washes, the cells were suspended in RPMI 1640 with 10%
heat-inactivated FBS, and cultured at 37°C in 5% CO2 for
subsequent experiments.
Flow cytometeric analysis
BMDCs were cultured for 5 days in RPMI 1640 complete
medium supplemented with 10 ng/ml each of granulocyte-macrophage
colony-stimulating factor and IL-4 (Shanghai PrimeGene Bio-Tech,
Shanghai, China). The BMDCs (2×106 cells/ml) were
incubated with either CpG ODN, IDR-HH2, the CpG-HH2 complex or
lipopolysaccharide (LPS; Sigma-Aldrich, St. Louis, MO, USA) for 16
h at 37°C and 5% CO2. The cells were washed twice in PBS
and stained with peridinin chlorophyll protein (PerCP)-conjugated
anti-mouse cluster of differentiation (CD)80, fluorescein
isothiocyanate-conjugated anti-mouse CD86 and
phycoerythrin-conjugated anti-mouse MHC II (BD Biosciences,
Franklin Lakes, NJ, USA) for 30 min on ice. Analysis was performed
using a FACSCalibur flow cytometer in conjunction with CellQuest™
Pro software, version 6.0 (BD Biosciences). The expression levels
of CD80, CD86 and MHC II in the BMDCs were defined as the mean
fluorescence intensity.
Isolation of human PBMCs and stimulation
with CpG-HH2 formulations
The PBMCs were prepared by density gradient
centrifugation of venous blood samples collected from healthy
volunteers, as previously reported (11). Briefly, a volume of venous blood
was centrifuged at 300 × g at room temperature for 20 min. The
buffy coat was collected and diluted in 2–3 times volumes of
complete RPMI 1640 medium. Following mixing, the mixture was
layered on an equal volume (5 ml) of Ficoll-Hypaque Plus (Tianjin
Haoyang Biological Manufacture Co., Ltd., Tianjin, China).
Following centrifugation at 400 × g at room temperature for 30 min,
the layer containing the PBMC fraction was obtained and suspended
in RPMI 1640 (Invitrogen; Thermo Fisher Scientific, Inc.) at a cell
concentration of 1×106 cells/ml. The PBMCs
(5×105) were then seeded into 24-well tissue culture
dishes and incubated for 1 h at 37°C in 5% CO2.
Subsequently, the PBMCs were stimulated with the CpG-HH2
formulations; ranging between 4:1 and 1:4 (wt/wt; with CpG ODN
constant at 10 µg) for 24 h at 37°C. All experiments were
repeated on at least three separate occasions.
Chemokine/cytokine induction
Following 24 h culture with the CpG-HH2
formulations, the culture supernatants were collected, and the
concentrations of monocyte chemotactic protein (MCP)-1, IFN-γ and
TNF-α were detected using Quantikine® Huma MCP-1, IFN-γ,
IL-4, IL-6 and TNF-α ELISA kits (R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's protocol.
Briefly, the culture supernatants were diluted 2-fold in assay
diluent, and 100 µl sample was added to each well and
incubated at room temperature for 2 h. Wells were washed with wash
buffer (400 µl) for a total of 5 times, and the antibodies
against human MCP-1 conjugated to horseradish peroxidase (or the
antibodies against human IFN-γ, IL-4, IL-6, TNF-α; 100 µl)
were added to each well followed by a 2 h incubation at room
temperature. Wells were washed and incubated with 100 µl
substrate solution added for 30 min at room temperature. The
reaction was allowed to develop for 30 min, and absorbance was read
as optical density (OD) at 450 nm using a Multiskan Mk3 (Thermo
Fisher Scientific, Inc.). Values were corrected for background
cytokine secretion by subtracting the concentrations in the control
(medium) wells. The concentration of the cytokines in the culture
medium was quantified by establishing a standard curve with serial
dilutions of recombinant human MCP-1 (or recombinant human IFN-γ,
IL-4, IL-6, TNF-α).
Immunization of mice
Female C57BL/6J mice (6–8 weeks old) were purchased
from the Experimental Animal Center of Sichuan University (Sichuan,
China) and were maintained at 23±1°C in a 12-h light/dark cycle
with free access to food and water in the animal research facility.
Groups (n=5) of the female C57BL/6J mice were immunized by
intramuscular injection of either 0.1 or 1 µg HBsAg in
combination with 25 µg alum and/or 20 µg CpG ODN
(5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) and/or 40 µg IDR-HH2
(VQLRIRVAVIRA-NH2) in a total volume of 100 µl on weeks 0, 2
and 4. The mice were administered with a boost vaccine at the same
dose at week 31 post-immunization. Blood (50 µl) was
collected from the mice via a retro-orbital puncture at 0, 1, 3, 5,
7, 9, 13, 17, 21, 25, 30, 32, 34, 50, 68 and 76 weeks.
Subsequently, blood collected from the mice was clotted for 30 min
in an incubator at 37°C, and centrifuged at 2000 × g for 10 min at
4°C. Serum was transferred to the new tube, and stored at
-80°C.
Evaluation of anti-HBsAg titer
HBsAg specific total IgG, IgG1, IgG2a, IgG2b and
IgG3 immune responses were determined using end-point ELISA. In
brief, NUNC Maxisorp™ plates (Thermo Fisher Scientific, Inc.) were
coated with 0.1 µg/well HBsAg in 0.05 M sodium bicarbonate
(pH 9.6) overnight at 4°C. Plates were washed, blocked and then
incubated for 1 h at 37°C with two-fold serially diluted sera.
Specific total IgG antibodies were detected using horseradish
peroxidase (HRP)-conjugated goat anti-mouse IgG (1:5000; cat no.
ZB-2305; ZSGB-BIO, Beijing, China). IgG sub-types were detected
using HRP-conjugated goat anti-Mouse IgG1, IgG2a, IgG2b and IgG3
(1:400; cat nos. 5300–05; Southern Biotechnology, Inc., Birmingham,
AL, USA;), followed by the addition of substrate solution and
incubated for 30 min at room temperature. OD was measured using a
Multiskan Mk3 plate reader (Thermo Fisher Scientific, Inc.) The
end-point dilution titers for the total IgG and IgG isotypes in the
plasma were defined as the highest plasma dilution at an absorbance
of OD450, which was double that of non-immune plasma,
with a cut-off value of 0.05. Seroconversion was defined as a
dilution titer ≥100.
IFN-γ and IL-4 ELIspot assay
The spleens were removed from the mice 5 weeks
following immunization with HBsAg with alum and/or CpG ODN and/or
IDR-HH2 (n=3/group). Single splenocytes were obtained from the
spleens using Ficoll-Hypaque PLUS (EZ-Sep Mouse 1×; Dakewei Biotech
Co., Ltd., Shenzhen, China), and suspended in RPMI 1640 with 10%
heat-inactivated FBS at a concentration of 5×106
cells/ml. The IFN-γ and IL-4 ELISpot assays were assessed using a
mouse IFN-γ/IL-4 kit (R&D systems, Inc.), according to the
manufacturer's protocol. The splenocytes were added to triplicate
wells at a concentration of 5×105 cells/well, and were
cultured in the presence of 10 mg/ml HBsAg and the HBsAg-derived
peptides (S208–216 and S126–138) or medium
for 48 h at 37°C. The stained spots were counted using a
computer-assisted ELISpot image analyzer (CTL-ImmunoSpot S5;
Cellular Technology, Ltd., Cleveland, OH, USA).
Assays for lymphocyte proliferation
The HBsAg-specific lymphoproliferative responses
were assayed in the immunized mice (1 µg dose groups), as
described previously (20).
Briefly, the mice spleens were removed 5 weeks following
immuni-zation to produce single-cell suspensions for the assay. The
splenocytes (2×106 cells/ml) were suspended in complete
RPMI 1640 with 5% heat-inactivated FBS, and were added in
triplicate to each well of 96-well round-bottom plates in a total
volume of 100 µl. Subsequently, HBsAg was added at a
concentration of 3 µg/ml; and bovine serum albumin (30
µg/ml; Sigma-Aldrich) served as a negative control.
Following 4 days in culture at 37°C, the cells were pulsed with
[3H] thymidine (0.5 µCi/well) for 18 h, washed
twice in phosphate-buffered saline (PBS) and harvested for
measurement of the incorporated radioactivity on a Topcount
Microplate Scintillation and Luminescence Counter (Packard; Perkin
Elmer, Inc., Waltham, MA, USA). The stimulation index was
calculated based on the mean counts per minute (cpm) of the
stimulated wells divided by the mean cpm of the control wells.
Statistical analysis
All experiments were performed on groups of 3–5
individual mice. Statistical comparisons were made with SPSS
software, version 16.0.2 (SPSS, Inc., Chicago, IL, USA) using a
one-way analysis of variance followed by Tukey's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
IDR-HH2 induces chemotactic migration of
THP-1 cells, RAW264.7cells and neutrophils
IDR peptides are potent immunoregulatory agents
that, in conjunction with the innate and adaptive immune response,
are capable of activating immune cells, inducing the production of
cytokines and chemokines, and selectively modulating
immune-mediated inflammation (11,21).
A previous study demonstrated that IDR-HH2 induces the migration of
human neutrophils (12). In the
present study, IDR-HH2 was chemotactic for the THP-1 and RAW264.7
cells in addition to the neutrophils (Fig. 1A–C). The migration of these cell
types was observed to be typically bell-shaped and dose-dependent.
The optimal chemotactic dose for the THP-1 cells and the
neutrophils was 5 µg/ml, whereas 10 µg/ml was the
optimal dose for the RAW264.7 cells. To evaluate whether the
combination of the CpG ODN formulation with IDR-HH2 enhanced
chemotactic activity, complexes of increasing concentrations of CpG
ODN (2.5–40 µg/ml) with 10 µg/ml IDR-HH2 (10
µg/ml) were assessed in these three cells. No significant
increases in the number of migrated cells were observed, compared
with the group administered with IDR-HH2 only in the chemotaxis
assays (data not shown).
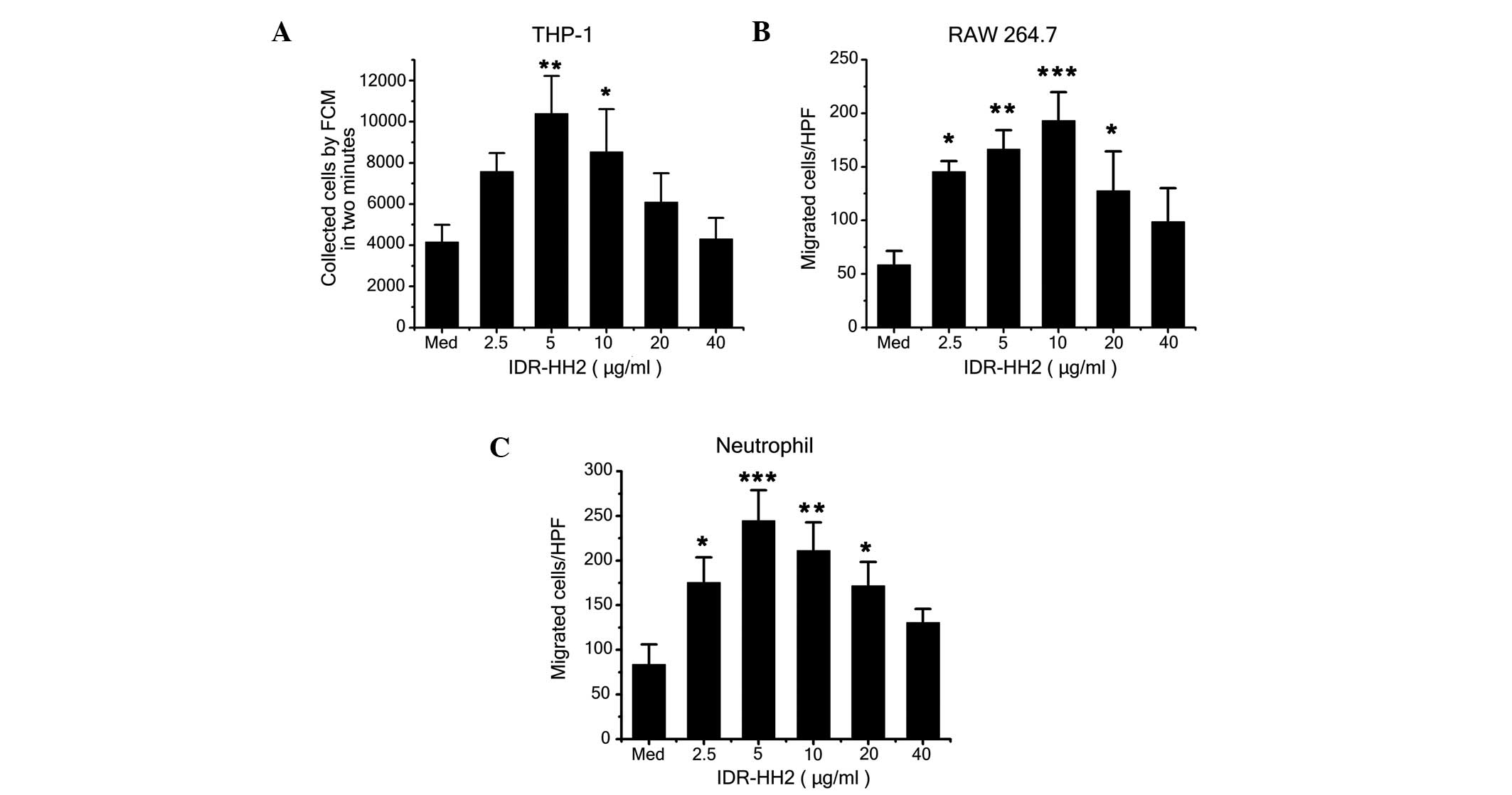 | Figure 1Effect of IDR-HH2 on the chemotactic
migration of THP-1 cells, RAW264.7 cells and neutrophils. THP-1
cells (2×105), RAW264.7 cells (5×105) and
neutrophils (5×106) were placed in the upper wells of a
chemotactic microchamber, and 600 µl of media containing
differing concentrations of IDR-HH2 (0–40 µg/ml) was added
to the lower wells. Folllowing incubation at 37°C for 45, 90 and
180 mins, chemotaxis was assessed. Results of the migration of (A)
THP-1 cells, (B) RAW264.7 cells and (C) neutrophils are presented.
Values were compared with unstimulated cells, in medium (Med)
alone). Data are expressed as the mean ± standard deviation of
three separate experiments. *P<0.05,
**P<0.01 and ***P<0.001, vs. Med. IDR,
innate defense regulator; FCM, flow cytometry; HPF, high-power
field. |
CpG-HH2 complex induces increased
activation of BMDCs
To investigate the effects of the CpG-HH2 complex on
the phenotypical changes of BMDCs, immature BMDCs were stimulated
in vitro with either CpG ODN (5 µg/ml) or IDR-HH2 (10
µg/ml), or with the formulated CpG-HH2 complex for 16 h. The
ratio of CpG:HH2 (wt/wt) was 1:2, as reported previously (11). LPS (1 µg/ml) served as a
positive control. The cells were analyzed for the surface
expression of MHC class II and costimulatory molecules, including
CD80 and CD86. Stimulation of the BMDCs with IDR-HH2 resulted in a
significant upregulation of CD80, compared with the cells incubated
with CpG ODN (Fig. 2A). Enhanced
expression of CD80 was induced by the CpG-HH2 complex, although the
difference was not significant, compared with IDR-HH2 alone. As
shown in Fig. 2B, all the
vaccination groups (CpG ODN, IDR-HH2, CpG-HH2 complex and LPS)
induced high expression levels of CD86. As with CD80, stimulation
with IDR-HH2 also resulted in a significant upregulation of MHC
class II, compared with the cells incubated with CpG ODN (Fig. 2C), and MHC class II was induced by
the CpG-HH2 complex, although the difference was not significant,
compared with IDR-HH2 alone. These data suggested that the CpG-HH2
complex may be a potent stimulation agent promoting the maturation
of BMDCs.
 | Figure 2BMDCs stimulated with the CpG-HH2
complex exhibit increased expression levels of CD80 and MHC II.
BMDCs were incubated with CpG ODN, IDR-HH2, CpG-HH2 complex or LPS
for 16 h and harvested. The expression levels of CD80, CD86 and MHC
class II molecules were examined using flow cytometry. The levels
of surface marker, defined as the MFI, were determined for. (A)
CD80; (B) CD86 and (C) MHC II. Data are expressed as the mean ±
standard deviation of three separate experiments.
*P<0.05, **P<0.01 and
***P<0.001. MFI, mean fluorescence intensity. BMDCs,
bone marrow dendritic cells; IDR, innate defense regulator; LPS,
lipopolysaccharide; CD, cluster of differentiation; MHC, major
histocompatibility complex. |
Synergistic effect of the CpG-HH2 complex
on the production of chemokines and cytokines
Chemokines, potent chemoattractants of inflammatory
cells, are critical in innate immunity (22). MCP-1, a member of the CC chemokine
family, is widely used as a chemotactic and activating factor for
monocytes or macrophages (23). In
the present study, CpG ODN and IDR-HH2 were pre-complexed with
serial dilutions of CpG:HH2 (wt/wt; 4:1–1:4, with CpG ODN at 10
µg) prior to simulation of the PBMCs. As shown in Fig. 3A, the marked induction in the
expression of MCP-1 ex vivo exhibited an increasing trend
with increasing concentration of the IDR-HH2 peptide. As cytokines
are important in the development of the immune response, the
concentrations of certain cytokines induced by the complexes,
including TNF-α, IFN-γ, IL-6 and IL-4, were measured using ELISA.
The induction in the expression of IFN-γ was similar to that of
MCP-1, which was dose-dependent with concentration of IDR-HH2
(Fig. 3B). However, the enhanced
induction of TNF-α was maximal at a 1:2 ratio of CpG:HH2 among the
four ratios (Fig. 3C). The
concentrations of IL-4 and IL-6 induced by the complexes were so
low that they were undected (data not shown). When a compound is
used as a vaccine adjuvant, its safety as well as potency requires
demonstration. A previous study determined that CpG-HH2 complexes
have almost no cytotoxic properties (11). In the present study, the 1:2
(wt/wt) ratio of CpG:HH2 was used to examine its adjuvant effect
for HBsAg.
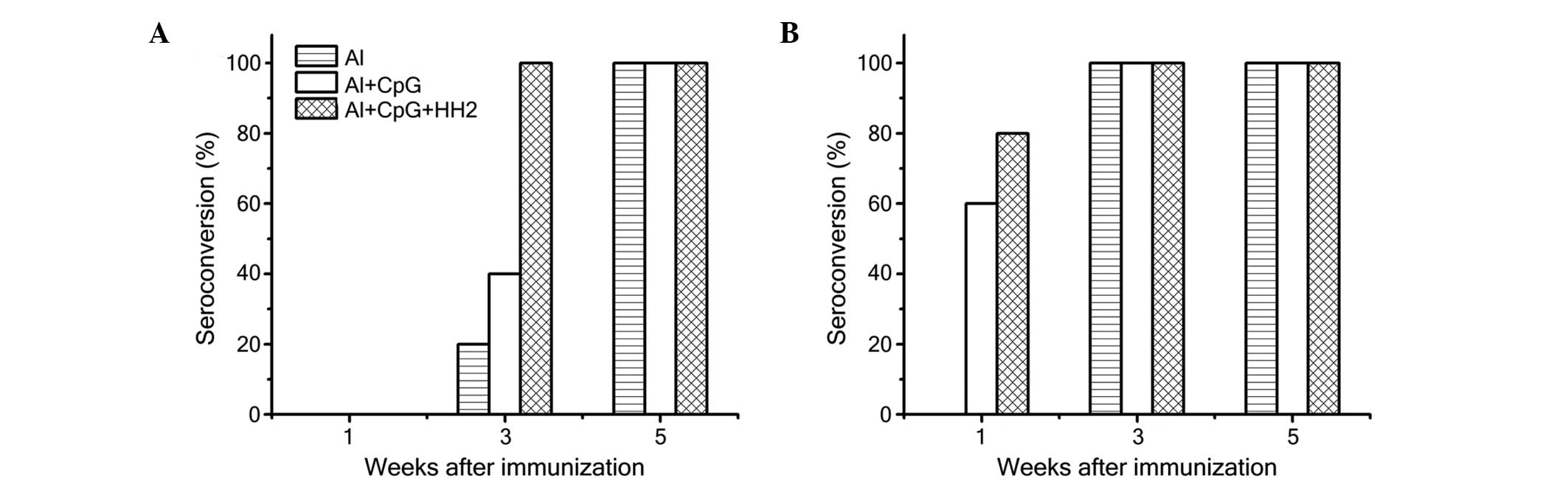
Al-CpG-HH2 induces earlier and increased
seroconversion
To further investigate the adjuvant effect of the
CpG-HH2 complex in vivo, mice were immunized with different
doses of HBsAg (1 µg or 0.1 µg) combined with Al,
Al-CpG or Al-CpG-HH2. The seroconversion and anti-HBs titer were
detected using ELISA. When 0.1 µg HBsAg was administered
with the adjuvant, no seroconversion was observed in any of the
three experimental groups after 1 week. (Fig. 4A). However, the rate of
seroconversion (titer ≥100) in the Al-CpG-HH2 group (80%) was 20%
higher than in the Al-CpG group (60%) at 1 week when the mice were
immunized with 1 µg HBsAg (Fig.
4B). All vaccines were immunogenic in 100% of the mice
immunized with 1 µg of HBsAg at 3 and 5 weeks (Fig. 4B). However, when the mice were
immunized with 0.1 µg HBsAg, the rate of seroconversion in
the Al-CpG-HH2 group at 3 weeks was 100%, which was 60 and 80%
higher than in the Al-CpG and Al groups, respectively (Fig. 4A). All samples successfully
seroconverted at 5 weeks following immunization.
Al-CpG-HH2 triggers a robust and
long-term HBsAg-specific antibody response
The humoral immune responses evoked by each of the
vaccine formulations were detected by measuring the HBsAg-specific
serum IgG titers. In all groups, the peak antibody titers occurred
7 weeks following immunization. When the mice were administered
with 0.1 µg HBsAg, the anti-HBs titers in the Al-CpG-HH2
group were significantly higher than those in the Al and Al-CpG
groups between weeks 5 and 25 post-immunization (Fig. 5A). When the dose of HBsAg was
increased to 1 µg, the anti-HBs titers of the Al-CpG-HH2
group were also enhanced, compared with those in the Al and Al-CpG
groups (Fig. 5B). Although the
titers decreased between weeks 21 and 30 post-immunization, the
levels of HBsAg-specific antibodies in the mice immunized with the
Al-CpG-HH2 vaccine remained higher than those in the mice immunized
with the Al vaccine. Following administration of the mice with the
same dose of vaccines at week 31, the anti-HBs titers in the
Al-CpG-HH2 group were marginally higher than, or similar to, those
of the Al-CpG group, but were markedly higher than those of the Al
group (Fig. 5A and B). These
results indicated that both groups of Al-CpG-HH2 and Al-CpG
triggered potent immune responses, which may have the same effects
in memory recall. Of note, the anti-HBs titers elicited by the
Al-CpG-HH2 complex and 0.1 mg of HBsAg were markedly higher than
those evoked by alum with 1 mg HBsAg (Fig. 5A).
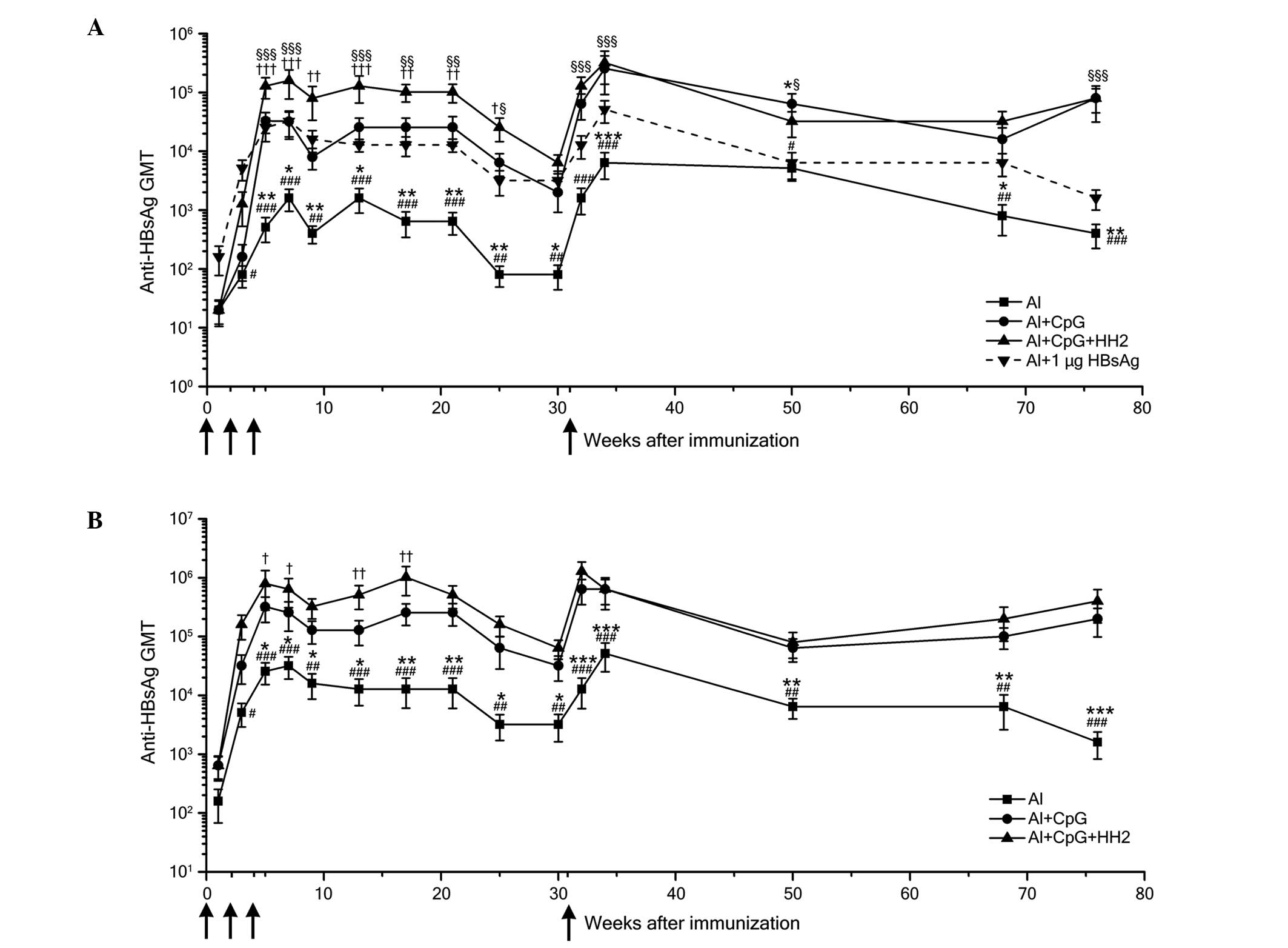 | Figure 5HBsAg-specific antibody responses in
mice immunized with HBsAg and the Al, Al-CpG or Al-CpG-HH2
adjuvant. C57BL/6J mice were immunized three times intramuscularly
with HBsAg (0.1 or 1 µg) formulated with Al (25 µg
Al3+), Al-CpG (20 µg) or Al-CpG-HH2 (40
µg) at 0, 2, 4 weeks (black arrow). To detect the memory
immune response, the mice were boosted with the same HBV vaccine as
for their primary immunization at 31 weeks. (A) Anti-HBs titer
kinetics of mice immunized with 0.1 µg HBsAg with the
various adjuvants (black solid line) and 1 mg of HBsAg with Al
(dotted line). (B) Anti-HBs titer kinetics of mice immunized with 1
µg HBsAg with the various adjuvants. Data are expressed as
the mean ± standard deviation in the group (n=5) titers, as
determined using ELISA. *P<0.05,
**P<0.01 and ***P<0.001, Al group vs.
Al+CpG group; #P<0.05, ##P<0.01 and
###P<0.001, Al group vs. Al+CpG+HH2 group;
†P<0.05, ††P<0.01 and
†††P<0.001, Al+CpG+HH2 group vs. Al+CpG group;
§P<0.05, §§P<0.01 and
§§§P<0.001, Al+CpG+HH2 group vs. Al+1 µg HBsAg
group. Al, alum; HBsAg, hepatitis B surface antigen; HBV, hepatitis
B virus; GMT, geometric mean titer. |
Al-CpG-HH2 induces a balanced Th1/Th2
immune response
To evaluate the type of Th response evoked by the
vaccine, anti-HBs antibody isotypes (IgG1, IgG2a, IgG2b and IgG3)
were detected 7 weeks post-immunization using ELISA (Fig. 6A and B). Alum produced a clear Th2
response with a predominantly IgG1 titer, regardless if it was
administered with 0.1 µg (Fig.
6A) or 1 µg (Fig. 6B)
HBsAg. When CpG ODN was administered with alum, the combination
evoked a Th1-biased response, with a more marked improvement in the
IgG2a titer. Finally, the CpG-HH2 complex suspended in alum
provided a balanced Th1/Th2 response, with similar improvements in
the IgG1 and IgG2a titers, as the ratios of IgG2a/IgG1 in the mice
immunized with 0.1 and 1 µg HBsAg were 1 and 0.8,
respectively (data not shown).
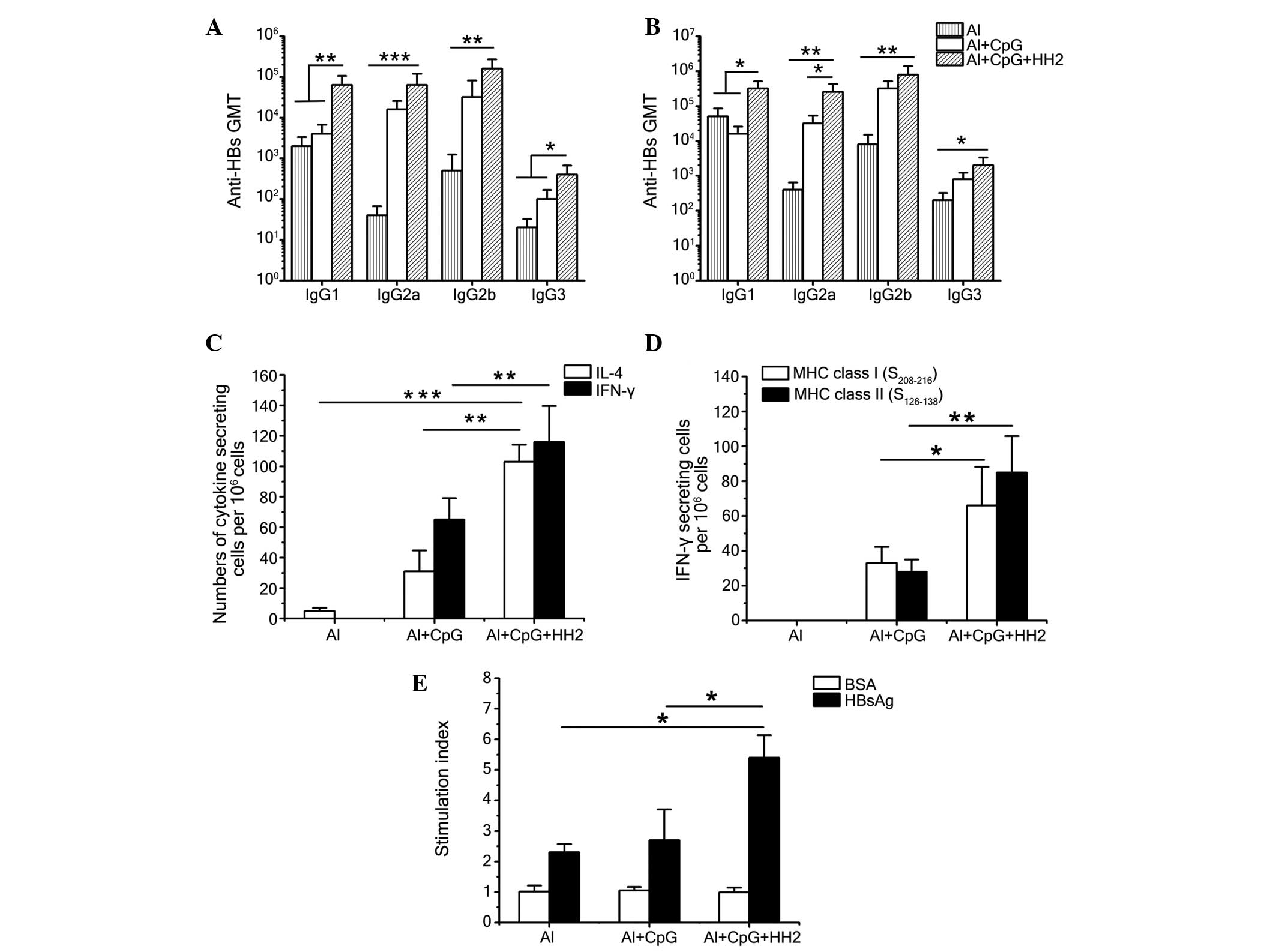 | Figure 6CpG-HH2 evokes a balanced Th1/Th2
immune response. Antibody isotypes in C57BL/6J mice 7 week
following immunization with (A) 0.1 µg HBsAg or (B) 1
µg HBsAg and Al, Al-CpG or Al-CpG-HH2. Data are expressed as
the mean ± standard deviation of the group (n=5) titers, as
determined using ELISA. (C and D) Groups of C57BL/6J mice were
immunized intramuscularly with 1 mg HBsAg and either Al, Al-CpG or
Al-CpG-HH2 at 0, 2, 4 weeks. Splenocytes were obtained 5 week
following immunization, followed by re-stimulation with (C) 10
µg/ml HBsAg or the (D) HBsAg-derived peptides,
S208–216 and S126–138. The numbers of
cytokine-secreting cells were detected using an ELISpot assay. (E)
T-cell proliferation assays. Splenocytes were prepared, as
described above and re-stimulated with HBsAg and BSA at final
concentrations of 10 µg/ml and 30 µg/ml,
respectively. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001. HBsAg, hepatitis B surface antigen; Th, T
helper; AL, alum; BSA, bovine serum albumin; Ig, immunoglobulin;
GMT, geometric mean titer; IL, interleukin; IFN, interferon; MHC,
major histocompatibility complex. |
To further characterize the immune response induced
by each of the formulations, the production of IL-4/IFN-γ by
HBsAg-exposed splenocytes from immunized mice was examined using
ELISpot following re-stimulation of splenocytes with HBsAg and
epitopes HBsAg208–216 and HBsAg126–138
epitopes. Following re-stimulation with HBsAg, significantly higher
numbers of IL-4-secreting cells and IFN-γ-secreting cells were
found in the mice of the Al-CpG-HH2 group, compared with the Al-CpG
group (P<0.01). In addition, the number of IFN-γ-secreting cells
was marginally higher than the number of IL-4-secreting cells in
the Al-CpG-HH2 group (Fig. 6C).
Furthermore, immunization with a combination of HBsAg and
Al-CpG-HH2 significantly increased IFN-γ secretion in response to
the HBsAg208–216 and HBsAg126–138 peptides
(Fig. 6D), suggesting that the
co-formulation of HBsAg with Al-CpG-HH2 induced a balanced Th1/Th2
immune response to HBsAg, which was MHC class I- and II-restricted.
These results suggested that the co-formulation of HBsAg with alum
and the CpG-HH2 complex induced significant increases in the
antibody- and cell-mediated immune responses.
Al-CpG-HH2 enhances the T cell
proliferative response
To further evaluate the vaccine enhancing effects on
T cells, a T cell proliferative response assay was evaluated, as
previously described (20). Groups
of C57BL/6J mice were administered with intramuscular injections of
normal saline, or with a mixture of HBsAg with alum, Al-CpG, or
Al-CpG-HH2. At 5 weeks post-immunization, the splenocytes of the
mice were examined for proliferation in response to specific
(HBsAg) and nonspecific (BSA) stimulation. The mice immunized with
the Al-CpG-HH2 vaccine developed a significant T cell-proliferative
response to HBsAg, but not to the control BSA protein. Compared
with the Al and Al-CpG groups, immunization with Al-CpG-HH2
resulted in significant (P<0.05) cellular proliferation, with an
average peak stimulation index of 5.4 (Fig. 6E). The mice of the Al and Al-CpG
groups exhibited a markedly lower T cell-proliferative response to
HBsAg, with average peak stimulation indices of 2.3 and 2.7,
respectively (Fig. 6E). The
failure of all the mice in the various groups to respond to the
negative protein BSA (even at 30 µg/ml) indicated that the T
cell-proliferative responses were specific for HBsAg.
Discussion
Vaccines consisting of subunit or protein antigens
are often poorly immunogenic, compared with traditional vaccines
(24), and, therefore, require
approaches to improve their immunogenicity. Strategies, which can
enhance the immunogenicity predominantly focus on two factors, the
antigen and the adjuvant. For the antigen, previous studies have
demonstrated that using overlapping peptides (prS1 or prS2) with
mammalian cell-produced HBsAg can overcome immune
non-responsiveness (25). However,
the expense of mammalian-produced antigens limits its clinical
application. Another study demonstrated that prS1 or prS2 peptide
vaccines evoked a potent T cell-mediated immune response when
supplied with more antigen (26).
Owing to the use of a high doses of antigen, this approach is
limited by the reactivity, leading to more side effects. However,
in the present study, when the HBsAg dose was decreased between 1
and 0.1 mg, the Al-CpG-HH2 complex maintained its effects of
earlier seroconversion and higher anti-HBs titer, compared with the
Al or Al-CpG treatments (Figs. 4
and 5). In particular, combination
of the Al-CpG-HH2 complex with 0.1 mg of HBsAg induced a more
marked anti-HBs immune response, compared with that induced by Al
with 1 mg of HBsAg in the experimental period, after the first 3
weeks (Fig. 5A).
The investigation of novel adjuvants, essential
components of vaccines, has become a crucial method in improving
the immunogenicity of antigens. Alum, the only adjuvant approved by
the Food and Drug Administration, has become the standard adjuvant
for use in humans, and been used in a large number of immunization
regimens (27). Alum is
traditionally considered to form a long-lasting depot for antigens
to promote their uptake by antigen-presenting cells (APCs),
resulting in the induction of a Th2 immune response with fewer
cytotoxic T lymphocyes (28). The
present study confirmed that alum evoked a Th2 immune response,
with predominant IL-4 production being detected using ELIspot
(Fig. 6C) and poor T-cell
proliferation activity (Fig.
6E).
Human host defense peptides are key in linking
innate and adoptive immunity (29), therefore, they are typically used
as vaccine adjuvants. Examples include cathelicidin (LL-37), human
neutrophil peptide (HNP)1–3 and human β-defensin (30–33).
The immunomodulatory activities of these peptides include the
chemoattraction of macrophages and T lymphocytes, and the induction
of dendritic cell maturation (34). Such peptides are often long or
complex, limiting their synthesis and use in manufacturing.
IDR-HH2, a short novel host defense peptide synthetic mimic, was
designed and investigated by Kindrachuk et al (11), who suggested that IDR-HH2 is a
potential immune modulating peptide. In the present study, it was
first demonstrated that IDR-HH2 was chemotactic for monocytes
(THP-1) and macrophages (RAW264.7) in addition to neutrophils
(Fig. 1A–C). These innate immune
cells migrated to the injection site of vaccine immunization, in
addition to phagocytizing and presenting antigens to APCs through
the secretion of various cytokines, enhancing the innate immune
response.
The novel CpG ODN adjuvant induces an
immunomodulatory cascade that involves B and T cells, natural
killer cells and APCs (15,35).
CpG ODN induces a Th1-type immune response, producing IL-12 and
IFN-γ (36). Preclinical and
ongoing investigations indicate that CpG ODN is effective and safe
for use as an adjuvant in various vaccines (37). The combination of alum and CpG ODN
used as an adjuvant for HBV vaccines evoked a Th1 immune response
in one study (38). Another study
found that alum-CpG in an HBV vaccine induced a mixed Th1/Th2 (Th0)
response in young mice (39). In
the present study, Al-CpG produced a mixed Th1-type immune response
to HBsAg, as demonstrated by a higher IgG2a titer (Fig. 6A and B) and the predominant
production of IFN-γ, according to the ELIspot assay (Fig. 6C). The adjuvant combination induced
more marked humoral- and cell-mediated immune responses, compared
with those evoked by alum alone, as indicted by the higher anti-HBs
titer and increased production of IFN-γ. Previous studies have
suggested the use of CpG-HH2 complex as a potential vaccine
adjuvant due to its ability to upregulate immature DCs and enhance
antibody titers, and its low associated cytoxicity (11). Consistent with these findings, the
present study demonstrated that the peptide IDR-HH2 exerted
effective immunomodulatory activity when used as in complex with
CpG-HH2, but not alone.
The present study used the CpG-HH2 complex as an
adjuvant in a commercial HBV vaccine. The results indicated that
the CpG-HH2 complex significantly increased the secretion of
chemokines and cytokines, including MCP-1, TNF-α and IFN-γ in
vitro (Fig. 3). Its adjuvant
effects with the HBV vaccine were then assessed in vivo, and
the combination of the CpG-HH2 complex with alum resulted in
earlier seroconversion and higher anti-HBs titers, compared with
those in the control groups (Figs.
4 and 5). Notably, the
combination induced marked anti-HBs immune responses when the dose
of HBsAg was decreased between 1 and 0.1 µg, and
immunization of this complex with a low dose of HBsAg (0.1
µg) elicited higher antibody titers, compared with those
evoked by alum with a high dose (1 µg) of HBsAg (Fig. 5A). These findings suggested that
the Al-CpG-HH2 complex may enable a reduction in the levels of
antigen in the HBV vaccine, without compromising its adjuvant
effect, resulting in a decrease in the cost and risk associated
with administration of HBV vaccines.
Previous studies have indicated that hyporesponsive
and unresponsive individuals fail to respond to HBV vaccines,
primarily due to a defect in the production of primary
HBsAg-specific T cells or functional defective B cells
(40–44). In the present study, the antibody isotypes and
ELIspot assay suggested that the Al-CpG-HH2 complex induced a
balanced Th1/Th2 immune response. The cell-medicated immune
response induced by the Al-CpG-HH2 complex and HBsAg may be key in
clearing HBV infections. Furthermore, the T-cell proliferation
indicated the potential application of this adjuvant to overcome
non-responsiveness to HBsAg.
In conclusion, the present study demonstrated that
Al-CpG-HH2 may be used as a novel adjuvant for HBV vaccines, as it
enhanced humoral immunity and cell-mediated immune responses in
C57/BL mice. Therefore, this complex may reduce the dose of HBsAg
in HBV vaccines, resulting in a decrease in manufacturing costs and
increases in vaccine safety, with applications in unresponsive
individuals, including those with perinatal infection. According to
the results presented, it is suggested that the combination of the
CpG-HH2 complex with alum may be a novel and effective
adjuvant.
Acknowledgments
The study was supported by the National Major
Scientific and Technological Special Project for 'Significant New
Drugs Development' (grant. no. 2013ZX09102030) and the Foundation
for Sichuan Distinguished Young Academic and Technology Leaders
(grant. no. 2012JQ0014).
References
|
1
|
Reed SG, Orr MT and Fox CB: Key roles of
adjuvants in modern vaccines. Nat Med. 19:1597–1608. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mutwiri G, Gerdts V, van Drunen Littel-van
den Hurk S, Auray G, Eng N, Garlapati S, Babiuk LA and Potter A:
Combination adjuvants: The next generation of adjuvants? Expert Rev
Vaccines. 10:95–107. 2011. View Article : Google Scholar
|
|
3
|
Aebig JA, Mullen GE, Dobrescu G, Rausch K,
Lambert L, Ajose-Popoola O, Long CA, Saul A and Miles AP:
Formulation of vaccines containing CpG oligonucleotides and alum. J
Immunol Methods. 323:139–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kovacs-Nolan J, Latimer L, Landi A,
Jenssen H, Hancock RE, Babiuk LA and van Drunen Littel-van den Hurk
S: The novel adjuvant combination of CpG ODN, indolicidin and
polyphos-phazene induces potent antibody- and cell-mediated immune
responses in mice. Vaccine. 27:2055–2064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao D, Li H, Jiang Z, Xu C, Cheng Q, Yang
Z, Cao G and Zhang L: Synthetic innate defence regulator peptide
enhances in vivo immunostimulatory effects of CpG-ODN in newborn
piglets. Vaccine. 28:6006–6013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weeratna RD, Brazolot Millan CL, McCluskie
MJ and Davis HL: CpG ODN can re-direct the Th bias of established
Th2 immune responses in adult and young mice. FEMS Immunol Med
Microbiol. 32:65–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thaker HD, Som A, Ayaz F, Lui D, Pan W,
Scott RW, Anguita J and Tew GN: Synthetic mimics of antimicrobial
peptides with immunomodulatory responses. J Am Chem Soc.
134:11088–11091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hancock RE and Sahl HG: Antimicrobial and
host-defense peptides as new anti-infective therapeutic strategies.
Nat Biotechnol. 24:1551–1557. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li M, Shi H, Mu Y, Luo Z, Zhang H, Wan Y,
Zhang D, Lu L, Men K, Tian Y, et al: Effective inhibition of
melanoma tumorigenesis and growth via a new complex vaccine based
on NY-ESO-1-alum-polysaccharide-HH2. Mol Cancer. 13:1792014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu X, Wang Z, Li X, Fan Y, He G, Wan Y, Yu
C, Tang J, Li M, Zhang X, et al: In vitro and in vivo activities of
antimicrobial peptides developed using an amino acid-based activity
prediction method. Antimicrob Agents Chemother. 58:5342–5349. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kindrachuk J, Jenssen H, Elliott M,
Townsend R, Nijnik A, Lee SF, Gerdts V, Babiuk LA, Halperin SA and
Hancock RE: A novel vaccine adjuvant comprised of a synthetic
innate defence regulator peptide and CpG oligonucleotide links
innate and adaptive immunity. Vaccine. 27:4662–4671. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niyonsaba F, Madera L, Afacan N, Okumura
K, Ogawa H and Hancock RE: The innate defense regulator peptides
IDR-HH2, IDR-1002 and IDR-1018 modulate human neutrophil functions.
J Leukoc Biol. 94:159–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klinman DM: CpG DNA as a vaccine adjuvant.
Expert Rev Vaccines. 2:305–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nierkens S, den Brok MH, Garcia Z, Togher
S, Wagenaars J, Wassink M, Boon L, Ruers TJ, Figdor CG,
Schoenberger SP, et al: Immune adjuvant efficacy of CpG
oligonucleotide in cancer treatment is founded specifically upon
TLR9 function in plasma-cytoid dendritic cells. Cancer Res.
71:6428–6437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooper CL, Davis HL, Morris ML, Efler SM,
Adhami MA, Krieg AM, Cameron DW and Heathcote J: CPG 7909, an
immu-nostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant
to Engerix-B HBV vaccine in healthy adults: A double-blind phase
I/II study. J Clin Immunol. 24:693–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee CC, Avalos AM and Ploegh HL: Accessory
molecules for Toll-like receptors and their function. Nat Rev
Immunol. 12:168–179. 2012.PubMed/NCBI
|
|
17
|
Mendez S, Tabbara K, Belkaid Y, Bertholet
S, Verthelyi D, Klinman D, Seder RA and Sacks DL: Coinjection with
CpG-containing immunostimulatory oligodeoxynucleotides reduces the
pathogenicity of a live vaccine against cutaneous Leishmaniasis but
maintains its potency and durability. Infect Immun. 71:5121–5129.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brown TH, David J, Acosta-Ramirez E, Moore
JM, Lee S, Zhong G, Hancock RE, Xing Z, Halperin SA and Wang J:
Comparison of immune responses and protective efficacy of
intranasal prime-boost immunization regimens using adenovirus-based
and CpG/HH2 adjuvanted-subunit vaccines against genital Chlamydia
muridarum infection. Vaccine. 30:350–360. 2012. View Article : Google Scholar
|
|
19
|
Kumar S, Jones TR, Oakley MS, Zheng H,
Kuppusamy SP, Taye A, Krieg AM, Stowers AW, Kaslow DC and Hoffman
SL: CpG oligodeoxynucleotide and Montanide ISA 51 adjuvant
combination enhanced the protective efficacy of a subunit malaria
vaccine. Infect Immun. 72:949–957. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chow YH, Chiang BL, Lee YL, Chi WK, Lin
WC, Chen YT and Tao MH: Development of Th1 and Th2 populations and
the nature of immune responses to hepatitis B virus DNA vaccines
can be modulated by codelivery of various cytokine genes. J
Immunol. 160:1320–1329. 1998.PubMed/NCBI
|
|
21
|
Scott MG, Dullaghan E, Mookherjee N,
Glavas N, Waldbrook M, Thompson A, Wang A, Lee K, Doria S, Hamill
P, et al: An anti-infective peptide that selectively modulates the
innate immune response. Nat Biotechnol. 25:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishida T, Oyama T, Carbone DP and
Gabrilovich DI: Defective function of Langerhans cells in
tumor-bearing animals is the result of defective maturation from
hemopoietic progenitors. J Immunol. 161:4842–4851. 1998.PubMed/NCBI
|
|
23
|
Mackay CR: Chemokines: Immunology's high
impact factors. Nat Immunol. 2:95–101. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Billeskov R, Elvang TT, Andersen PL and
Dietrich J: The HyVac4 subunit vaccine efficiently boosts BCG-
primed anti- mycobac-terial protective immunity. PLoS One.
7:e399092012. View Article : Google Scholar
|
|
25
|
Milich DR, Thornton GB, Neurath AR, Kent
SB, Michel ML, Tiollais P and Chisari FV: Enhanced immunogenicity
of the pre-S region of hepatitis B surface antigen. Science.
228:1195–1199. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liljeqvist S and Stahl S: Production of
recombinant subunit vaccines: Protein immunogens, live delivery
systems and nucleic acid vaccines. J Biotechnol. 73:1–33. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flach TL, Ng G, Hari A, Desrosiers MD,
Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, et al:
Alum interaction with dendritic cell membrane lipids is essential
for its adjuvanticity. Nat Med. 17:479–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fritz JH, Brunner S, Birnstiel ML, Buschle
M, Gabain Av, Mattner F and Zauner W: The artificial antimicrobial
peptide KLKLLLLLKLK induces predominantly a TH2-type immune
response to co-injected antigens. Vaccine. 22:3274–3284. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang W, Seo J, Willingham SB, Czyzewski
AM, Gonzalgo ML, Weissman IL and Barron AE: Learning from
host-defense peptides: Cationic, amphipathic peptoids with potent
anticancer activity. PLoS One. 9:e903972014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren SX, Cheng AS, To KF, Tong JH, Li MS,
Shen J, Wong CC, Zhang L, Chan RL, Wang XJ, et al: Host immune
defense peptide LL-37 activates caspase-independent apoptosis and
suppresses colon cancer. Cancer Res. 72:6512–6523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hurtado P and Peh CA: LL-37 promotes rapid
sensing of CpG oligodeoxynucleotides by B lymphocytes and
plasmacytoid dendritic cells. J Immunol. 184:1425–1435. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soehnlein O, Kai-Larsen Y, Frithiof R,
Sorensen OE, Kenne E, Scharffetter-Kochanek K, Eriksson EE, Herwald
H, Agerberth B and Lindbom L: Neutrophil primary granule proteins
HBP and HNP1-3 boost bacterial phagocytosis by human and murine
macrophages. J Clin Invest. 118:3491–3502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hilchie AL, Wuerth K and Hancock RE:
Immune modulation by multifaceted cationic host defense
(antimicrobial) peptides. Nat Chem Biol. 9:761–768. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harder J, Bartels J, Christophers E and
Schröder JM: A peptide antibiotic from human skin. Nature.
387:8611997. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bode C, Zhao G, Steinhagen F, Kinjo T and
Klinman DM: CpG DNA as a vaccine adjuvant. Expert Rev Vaccines.
10:499–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klinman DM, Yi AK, Beaucage SL, Conover J
and Krieg AM: CpG motifs present in bacteria DNA rapidly induce
lymphocytes to secrete interleukin 6, interleukin 12 and interferon
gamma. Proc Natl Acad Sci USA. 93:2879–2883. 1996. View Article : Google Scholar
|
|
37
|
Klinman DM: Immunotherapeutic uses of CpG
oligodeoxy-nucleotides. Nat Rev Immunol. 4:249–258. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weeratna RD, McCluskie MJ, Xu Y and Davis
HL: CpG DNA induces stronger immune responses with less toxicity
than other adjuvants. Vaccine. 18:1755–1762. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brazolot Millan CL, Weeratna R, Krieg AM,
Siegrist CA and Davis HL: CpG DNA can induce strong Th1 humoral and
cell-mediated immune responses against hepatitis B surface antigen
in young mice. Proc Natl Acad Sci USA. 95:15553–15558. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chedid MG, Deulofeut H, Yunis DE,
Lara-Marquez ML, Salazar M, Deulofeut R, Awdeh Z, Alper CA and
Yunis EJ: Defect in Th1-like cells of nonresponders to hepatitis B
vaccine. Hum Immunol. 58:42–51. 1997. View Article : Google Scholar
|
|
41
|
Larsen CE, Xu J, Lee S, Dubey DP, Uko G,
Yunis EJ and Alper CA: Complex cytokine responses to hepatitis B
surface antigen and tetanus toxoid in responders, nonresponders and
subjects naive to hepatitis B surface antigen. Vaccine.
18:3021–3030. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goncalves L, Albarran B, Salmen S, Borges
L, Fields H, Montes H, Soyano A, Diaz Y and Berrueta L: The
nonresponse to hepatitis B vaccination is associated with impaired
lymphocyte activation. Virology. 326:20–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chiou SS, Yamauchi K, Nakanishi T and
Obata H: Nature of immunological non-responsiveness to hepatitis B
vaccine in healthy individuals. Immunology. 64:545–550.
1988.PubMed/NCBI
|
|
44
|
Shokrgozar MA and Shokri F: Enumeration of
hepatitis B surface antigen- specific B lymphocytes in responder
and non- responder normal individuals vaccinated with recombinant
hepatitis B surface antigen. Immunology. 104:75–79. 2001.
View Article : Google Scholar : PubMed/NCBI
|