Introduction
Patients suffering from depression present with
dysfunctions in brain morphology and activity (1,2). Due
to its relatively high lifetime prevalence and substantial
associated disability, depression is considered a worldwide problem
in humans (1,2). According to statistics, depression
affects almost 15–25% of the world's population, and is expected to
become the second largest global burden by 2020 (3). According to the monoamine hypothesis,
depression is interrelated with the metabolic turnover of dopamine
(DA) in the hippocampus, and/or serotonin (5-hydroxytryptamine;
5-HT) in the prefrontal cortex and striatum in rat brains (4). In addition, serotonin transporter,
dopamine transporter (DAT) and tyrosine hydroxylase (TH) are
important in antidepressants (5).
The four types of antidepressant drugs, selective serotonin
re-uptake inhibitors (SSRIs), tricyclic antidepressants,
serotonin-noradrenergic re-uptake inhibitors and monoamine oxidase
inhibitors, are commonly used in clinical treatment (6,7).
However, each of these drugs exhibit various adverse effects, with
high relapse rates and a long onset of therapeutic action (8). Due to the limited using of existing
antidepressant drugs, the development of novel alternative
therapies is in high demand (9).
Herbal medicine have become a valuable reservoir for
novel drugs due their limited side effects (9). The use of Traditional Chinese
medicine has been confirmed to be an effective alterative in
treating depression. Acanthopanax senticosus possesses
antidepressant-like effects and has beneficial effects in patients
with depression (10). Studies
have suggested that Areca catechu fruit extract exerts
antidepressant activities in an animal model (11). Wuling capsule, produced by Wuling
submerged fermentation mycelium, has also been used as an
antidepressant agent for clinical use in China (12,13).
Marasmius androsaceus, a well-known medical fungus,
possesses antihypertensive, analgesic and antioxidant properties
(14,15). In China, 'An-Luo Tong', which is
produced from the fermented mycelium of Marasmius
androsaceus, has been used as a painkiller for ~10 years
(16). However, the regulatory
effects of Marasmius androsaceus by-products following its
submerged fermentation on depressant-like effects in mice remain to
be elucidated.
Exopolysaccharide (EPS) is produced from
microorganism secretion and can be readily separated from bacteria.
Compared with plant polysaccharides, EPS has the advantage of
efficient production duration, convenient extraction and the
absence of geographical restrictions (17). Investigations on the various types
of biological activity of EPS have revealed antioxidant effects
(18) and enhanced immunity
(19). The EPS produced by
Cordycept militaris has an antihyperglycemic effect in
diabetic mice induced by streptozotocoin injection (20), and EPS separated from Morchella
conica markedly prolongs the life-span of fruit flies (21).
Therefore, the present study hypothesized that The
EPS produced during Marasmius androsaceus submerge
fermentation may possess antidepressant effects. To test this
hypothesis, the present study aimed to investigate the associated
biological activities of Marasmius androsaceus EPS (MEPS)
using a mouse model. The effects of MEPS were analyzed by
performing a mouse forced swimming test (FST) and tail suspension
test (TST), and changes in the concentrations of monoamine
neurotransmitters, including DA, norepinephrine (NE), 5-HT and
5-hydroxyindoleacetic acid (5-HIAA) in the hypothalamus were
detected. To further analyze its underlying mechanism, the
expression levels of DAT and TH in the hypothalamus were detected
using western blot analysis. The resulting data may provide
experimental evidence supporting the clinical use of Marasmius
androsaceus exopolysaccharide as an effective agent against
depression.
Materials and methods
Submerged fermentation of Marasmius
androsaceus. Marasmius androsaceus
(CCTCC M2013175; China Center for Type Culture
Collection, Wuhan, China) was cultured in a 100 liter
fully-automatic fermentor (BaoXing Bioscience Company, Shanghai,
China) using a defined liquid medium, containing 20 g/l sucrose, 10
g/l peptone, 10 g/l yeast extract powder, 1 g/l
MgSO4•7H2O, 1 g/l
KH2PO4•3H2O, and 0.1 g/l vitamin
B1. The fermentation conditions were as follows: Initial
pH 6.5; rotation speed, 300 rpm; culture duration, 6 days; culture
temperature, 26°C; inoculum volume, 5%; ventilation volume, 200
l/h; inoculum age, 4 days; loading volume, 70/100 liters. All
chemical reagents used in submerged fermentation were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Sample preparation
The fermentation products were filtered using 120
mesh sifters and centrifuged at 4,500 × g for 10 min. Using Sevag
reagent (chloroform: n-butyl alcohol, 4:1), the existing proteins
were extracted (22). A four-fold
volume anhydrous ethanol was added for 12 h at 4°C to precipitate
the crude EPS. Following 30 min centrifugation at 4,500 × g, the
precipitate was washed with anhydrous ethanol and acetone
(Sigma-Aldrich) three times. Following dialyzing, the crude EPS was
lyophilized using a vacuum freeze dryer (GENESIS SQ 25ES; SP
Industries Inc., Warminster, PA, USA) with primary drying at 60 mT
vacuum and a shelf temperature set at −25°C for 10 h. Prior to
animal administration, the Marasmius androsaceus EPS (MEPS)
and fluoxetine hydrochloride capsules (Shanghai Zhongxi
Pharmaceutical Group Co., Ltd, Shanghai, China) were dissolved in
physiological saline to 1 mg/ml.
Animals and animal care
The experimental protocol used in the present study
was approved by the ethics committee of the School of Life
Sciences, Jilin University (Changchun, China). KunMing (KM) mice
(6-week-old; 20–22 g; 1:1 male:female ratio) were housed in clear
plastic cages and maintained in a 12 h light/dark cycle (lights on
7:00–19:00 h) at 23±1°C, with water and food available ad
libitum. At 8 hours prior to the experiment, the animals were
deprived of food, but had free access to water. All experiments
were performed in a quiet room, and each animal was assessed only
once.
Acute toxicity assessment
The KM mice (6-week-old; 20–22 g; n=20/dosing group)
were treated with MEPS at different concentrations (0.1, 1.0, 2.0,
4.0 and 6.0 g/kg) via gavage for a period of 7 days. In a separate
group, the mice were treated with equal volumes of normal saline,
which served as a control group. The body weights of the mice were
measured prior to administration and on day 7. The animal were
maintained under surveillance every day to record any adverse
symptoms.
FST
Following MEPS treatment, an FST was used, in a
similar manner to that described in a previous study with minor
modifications (23). Briefly, the
mice (n=10/group) were orally administered with distilled water
(vehicle), MEPS (10, 50 or 250 mg/kg) or fluoxetine (10 mg/kg) for
1 week, respectively. At 30 min following the final administration,
the FST was performed. The mice were placed in an open cylindrical
container (30×18 cm) with a 15 cm depth, which did not permit the
animal failed to touch the bottom of the cylinder. The water placed
in the container was at a temperature of 24±1°C. The duration of
immobility was defined as the duration spent by the mouse floating
in the water without struggling, and making only small movements
necessary to maintain its head above the water. By using a
stopwatch, the total duration of immobility was recorded in the
final 5 min of a total duration of 6 min. A decrease in the
duration of immobility was considered to be a measurement of
antidepressant activity.
TST
The TST was performed in a quiet experimental room,
according to previous report (4).
The mice (n=10/group) were orally administered with either
distilled water, MEPS (10, 50 or 250 mg/kg) or fluoxetine (10
mg/kg) for 1 week, respectively. At 30 min following the final
administration, the TST was performed. Each mouse was suspended by
its tail to a horizontal wooden bar, which was located in a yellow
plastic box (40×40×40 cm), ~30 cm above the bottom. The mouse was
secured to the bar by adhesive tape, which was placed 1 cm from the
tip of tail so that its head was ~20 cm above the floor. The trial
was performed for 6 min, during which two observers scored the
latency of the first immobility episode and the total duration of
immobility using a stopwatch in a blinded-manner. The mouse was
considered immobile only when it hung passively and completely
motionless. Mice that climbed upwards grasping with its tail were
eliminated from further analysis.
Behavioral assessment
With the purpose of excluding sedative or motor
abnormality, the spontaneous locomotor activity was assessed
(24). A clear acrylic chamber
(11×11×15 cm), equipped with 12 infrared sensors for antomatic
recording, was used. The mice (n=10/group) were orally administered
with either distilled water, MEPS (10, 50 or 250 mg/kg) or
fluoxetine (10 mg/kg) for 1 week, respectively. At 30 min following
the final administration, each mouse was initially placed in the
center of the testing chamber. Following a 2-min period of
adaptation, behavioral data Data associated with horizontal and
vertical movements were collected and recorded for 5 min.
To examine the coordination and balance of mouse
movement, a rotation test was performed. The mice (n=10/group) were
orally administered with either distilled water, MEPS (10, 50 or
250 mg/kg) or fluoxetine (10 mg/kg) for 1 week, respectively. At 30
min following the last administration, the mice were placed on a
fatigue turning device (ZB-200; Chengdu Taimeng Software, Co.,
Ltd., Chengdu, China). The rotation speed was maintained at 20 rpm.
Prior to assessment, each mouse was trained three times and during
the subsequent assessment a stopwatch was used to record the total
duration each mouse spent on the rod prior to falling.
5-hydroxytryptophan (5-HTP)-induced
head-twitch assessment
A 5-HTP-induced head-twitch assessment (25) was used to investigate whether
serotonergic mechanisms were involved in the MEPS-mediated
antidepressant-like effects. The mice (n=10/group) were orally
administered with either distilled water, MEPS (10, 50 or 250
mg/kg) or fluoxetine (10 mg/kg) for 1 week, respectively. At 30 min
following the last administration, 5-HTP (100 mg/kg) was
intraperitoneally administered to mice. Following administration,
the mice were immediately placed into cages, and the cumulative
number of head twitches during a 15 min period was recorded by two
observers in a blinded-manner.
Reserpine-induced hypothermia
assessment
The mice in each group, with the exception of the
vehicle-treated control group, were injected intraperitoneally with
4.0 mg/kg reserpine 1 h following the 7-day MEPS (10, 50 or 250
mg/kg) and fluoxetine (10 mg/kg) treatments. After 2 h, the rectal
temperatures of the mice were determined. At the end of experiment,
the mice were sacrificed by administration of 20 mg/kg
pentobarbital, and the hypothalamus was collected. Tissue samples
(n=8) were homogenized with phosphate-buffered saline (PBS).
Following centrifugation at 10,000 × g for 15 min at 4°C, the level
of monoamine neurotransmitters, including DA, NE, 5-HT and 5-HIAA,
were detected using mouse ELISA kits for DA (cat. no. H170), 5-HT
(cat. no. H104), NE (cat. no. H096) and 5-HIAA (cat. no. H104),
from NanJing Biotechnology Co., Ltd. (NanJing, China). Briefly, 50
µl of the horseradish peroxidase (HRP)-linked target
solution and 50 µl samples were added to the antibody-coated
assay plate, which was incubated at room temperature for 1 h on a
horizontal orbital plate shaker. The plate contents were then
discarded and the wells were washed three times with 200
µl/well of 1X wash buffer. The solution was discarded, and
100 µl tetramethylbenzidine substrate was added to each
well, and incubated for 10 min at room temperature. Following the
addition of 100 µl STOP solution, the absorbance was
measured at 450 nm within 30 min.
Western blot analysis
Sections of the collected hypothalamus samples were
homogenized in radioimmunoprecipitation assay buffer
(Sigma-Aldrich) containing 1% protease inhibitor cocktail
(Sigma-Aldrich) (26). Protein
concentrations were determined using the Bradford method (27), and 30 µg of the proteins
were separated on an 10% SDS-PAGE gel and transferred
electrophoretically onto nitrocellulose membranes (0.45 mm; Bio
Basic, Inc., Markham, ON, Canada). The transferred membranes were
blocked with 5% bovine serum albumin for 3 h at room temperature,
followed by three washes with PBS. The membranes were then
incubated with the following primary antibodies at 4°C overnight,
at a dilution of 1:500: DAT (rabbit anti-rat monoclonal; cat. no.
sc-32258; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), TH
(rabbit anti-rat monoclonal; cat. no. sc-14007 Santa Cruz
Biotechnology, Inc.) and GAPDH (rabbit anti-rat monoclonal; cat.
no. ABS16 (Merck Millipore, Darmstadt, Germany), followed by
treatment with horseradish peroxidase-conjugated secondary
antibodies (mouse anti-rabbit monoclonal; cat. no. sc-2357; Santa
Cruz Biotechnology, Inc.) for 4 h at 4°C. Chemiluminescence was
detected using ECL detection kits (GE Healthcare Life Sciences;
Chalfont, UK). The intensity of the bands were quantified by
scanning densitometry using Quantity One-4.5.0 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All values are expressed as the mean ± standard
error of the mean One-way analysis of variance was use to detect
statistical significance, followed by post-hoc multiple comparisons
(Dunn's test). SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Acute toxicity assessment of MEPS
Following 7 days of administration with MEPS (0.1,
1.0, 2.0, 4.0 and 6.0 g/kg) via gavage, none of the mice in any
groups succumbed to mortality. The mice maintained a suitable state
of growth, and few adverse effects were noted. In addition, no
significant differences in body weight were observed among the
groups (Table I). These data
suggested that, at the doses selected in the present study, MEPS
was deemed a safe agent for further investigation.
 | Table IAnalysis of body weights following
treatment with MEPS to assess acute toxicity. |
Table I
Analysis of body weights following
treatment with MEPS to assess acute toxicity.
| Body weights (g) at
different concentrations of MEPS (g/kg)
|
|---|
| Day | 0 | 0.1 | 1.0 | 2.0 | 4.0 | 6.0 |
|---|
| 1 | 23.5±1.0 | 23.8±0.9 | 23.6±1.1 | 23.4±0.8 | 22.3±0.9 | 23.2±1.6 |
| 2 | 24.6±1.8 | 24.5±1.3 | 24.7±1.2 | 24.3±1.7 | 25.0±0.8 | 24.4±1.7 |
| 3 | 25.2±2.3 | 24.7±1.8 | 25.5±1.7 | 26.0±1.4 | 25.1±1.3 | 24.5±1.7 |
| 4 | 26.1±1.9 | 26.4±1.9 | 25.7±1.7 | 26.1±1.3 | 25.2±1.3 | 25.1±1.8 |
| 5 | 27.1±1.6 | 27.1±2.3 | 26.9±2.2 | 27.3±1.6 | 26.2±1.3 | 25.6±1.9 |
| 6 | 27.9±2.1 | 28.1±2.3 | 27.6±2.4 | 27.8±1.5 | 27.3±1.7 | 26.1±1.9 |
| 7 | 28.3±2.3 | 28.4±1.7 | 27.9±2.1 | 28.3±1.7 | 27.8±1.6 | 26.6±1.6 |
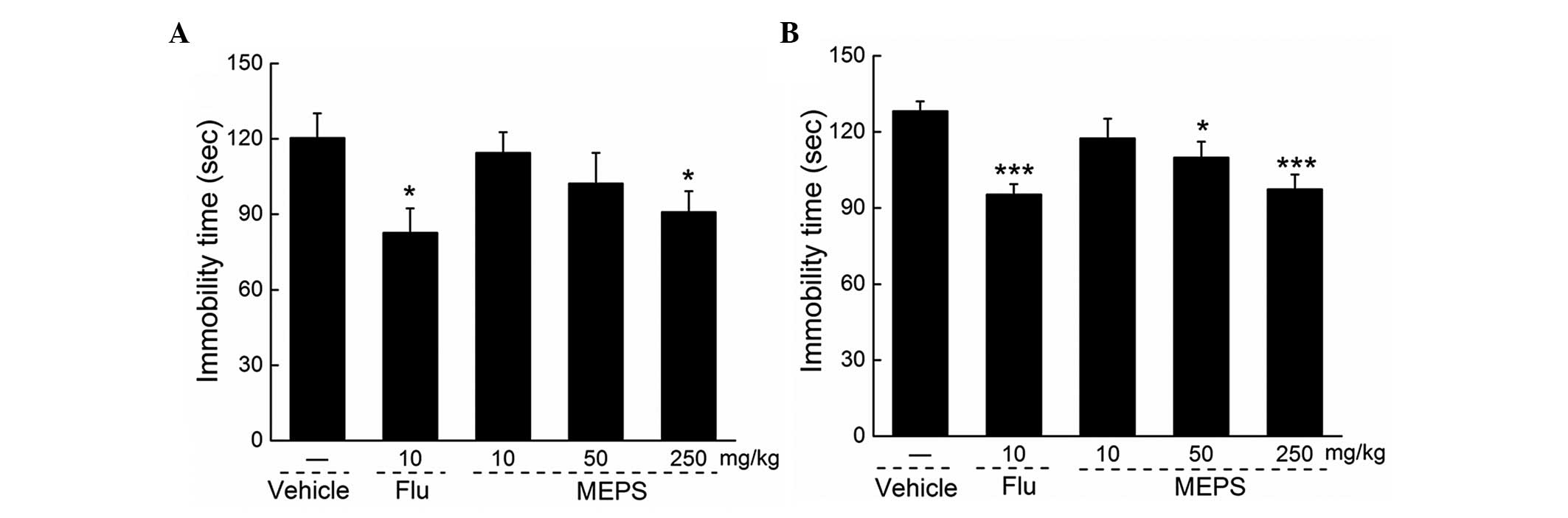
Effects of MEPS on immobility duration in
the FST and TST
Compared with the vehicle-treated mice, intrastral
(i.g.) MEPS (250 mg/kg) and fluoxetine (10 mg/kg) administration
significantly reduced immobility duration in the FST (P<0.05;
Fig. 1A). Similarly, MEPS (50 and
250 mg/kg and fluoxetine (10 mg/kg) treatment resulted in
reductions in the duration of immobility in the TST (P<0.05;
Fig. 1B).
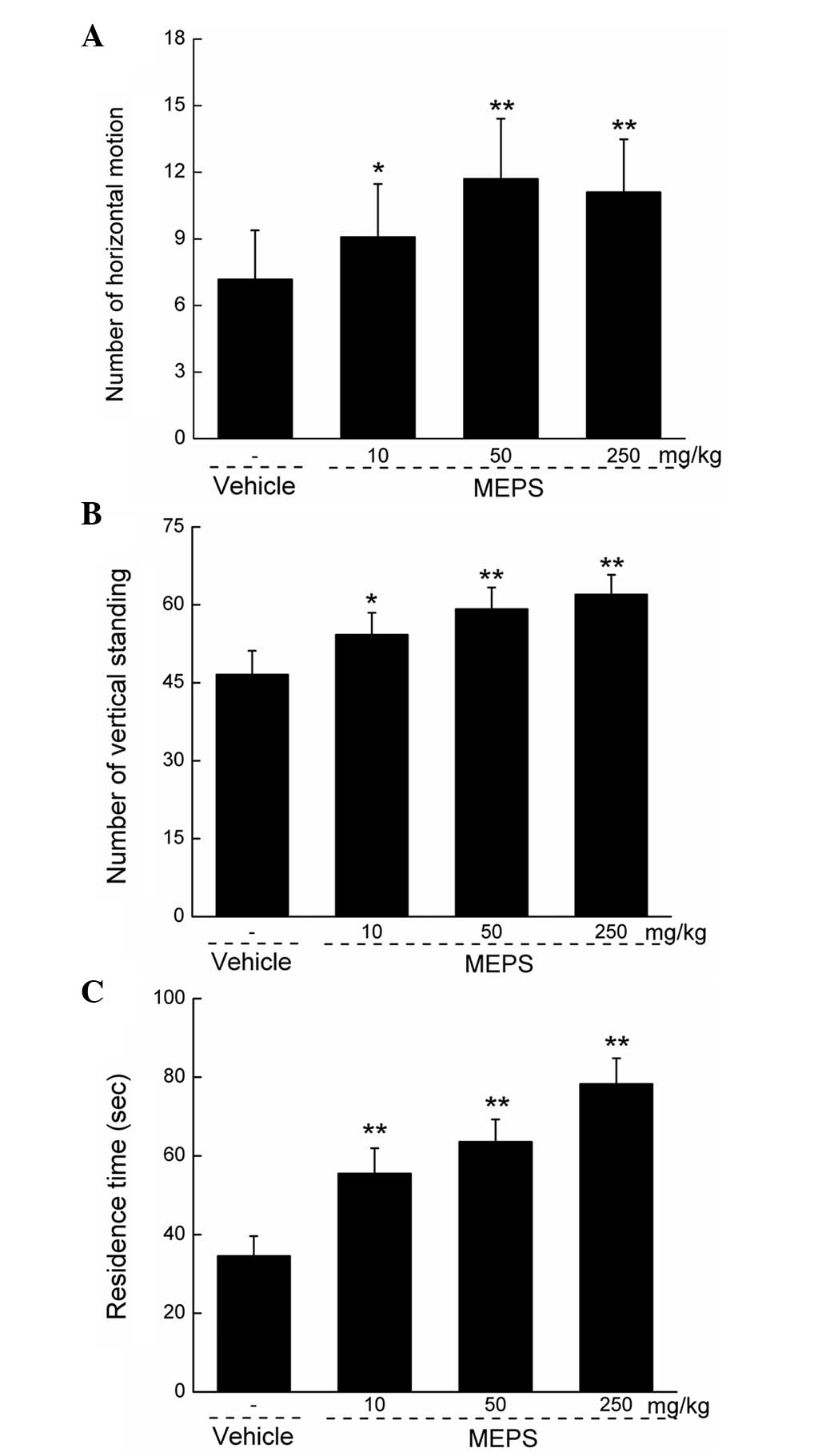
Effects of MEPS on animal behavior
Compared with the vehicle-treated group, the
administration of MEPS at all the selected doses significantly
increased locomotor ability, not only horizontally (P<0.05;
Fig. 2A), but also vertically
(P<0.05; Fig. 2B). In addition,
MEPS enhanced mouse coordination and balance, which was indicated
by the increase in duration of retention on the Rota-Rod
(P<0.01; Fig. 2C).
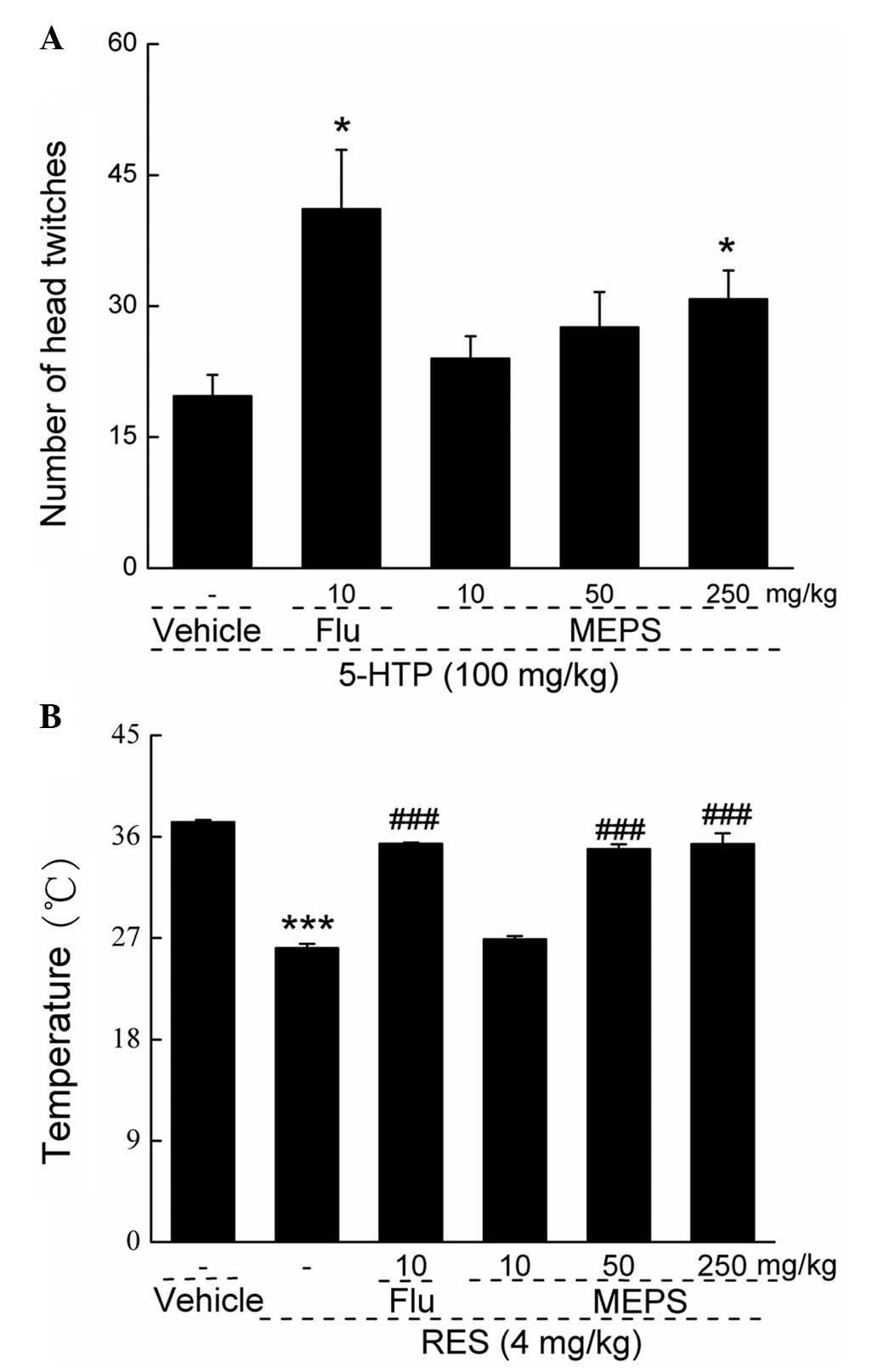
Effects of MEPS in the 5-HTP-induced
head-twitch assessment
To investigate the possible involvement of
serotonergic mechanisms in MEPS-mediated antidepressant-like
effects, a 5-HTP-induced head-twitch assessment was performed. MEPS
(250 mg/kg) and fluoxetine (10 mg/kg) increased the number of head
twitches by ~56.27 and 108.63%, respectively, compared with the
mice treated with 5-HTP (Sigma-Aldrich) alone (P<0.05; Fig. 3A). The administration of MEPS at
lower doses of 10 and 50 mg/kg increased the numbers of head
twitches by 21.88 and 39.92%, respectively.
Effects of EPS on reserpine-induced
hypothermia
The administration of 4.0 mg/kg reserpine
significantly reduced rectal temperatures in the mouse model
(P<0.001; Fig. 3B). The
administration of MEPS at doses of 50 and 250 mg/kg, and of
fluoxetine (10 mg/kg) significantly enhanced reserpine-reduced
rectal temperature (P<0.001; Fig.
3B), with an increment magnitude of 33.7% in the 50 mg/kg MEPS
group, 35.5% in the 250 mg/kg MEPS group and 35.6% in the
fluoxetine group, compared with the group treated with reserpine
alone.
Effects of MEPS on neurotransmitters in
reserpine-induced hypothermia
The levels of neurotransmitters in the hypothalamic
tissues were detected in the reserpine-induced hypothermia mouse
model. Reserpine at dose of 4.0 mg/kg significantly reduced the
levels of 5-HT, DA and NE, compared with the vehicle group. By
contrast, a significant increase in the concentration of 5-HIAA was
observed (P<0.05; Fig. 4).
Similar to the effects of fluoxetine (10 mg/kg) treatment, the
administration of MEPS at a dose of 250 mg/kg markedly elevated the
levels of 5-HT and DA, and reduced the increased levels of 5-HIAA,
compared with the reserpine-only group (P<0.05; Fig. 4A–C). Treatment with MEPS (250
mg/kg) for 7 days reversed the suppressive effect of reserpine on
the level of NE (P<0.05; Fig.
4D).
 | Figure 4Effects of MEPS on neurotransmitters
in reserpine-induced hypothermia. Following administration of MEPS
(10, 50 and 250 mg/kg.) or fluoxetine (10 mg/kg) for 7 days, 4.0
mg/kg reserpine was injected intraperitoneally. The levels of (A)
5-HT, (B) 5-HIAA, (C) DA and (D) NE in the mouse hypothalamus were
measured. Data are expressed as the mean ± standard error of the
mean (n=8) and were analyzed using one-way analysis of variance.
#P<0.05, vs. vehicle; *P<0.05 and
**P<0.01, vs. RES alone. MEPS, Marasmius
exopolysaccharide; Flu, fluoxetine; RES, reserpine; 5-HT,
5-hydroxytryptamine; 5-HIAA 5-hydroxy-indoleacetic acid; DA,
dopamine; NE, norepinephrine. |
Effects of MEPS on the expression levels
of DAT and TH in the hypothalamus
The expression levels of DAT and TH in the
hypothalamus in the reserpine-induced hypothermia mouse model were
also determined. Reserpine (4.0 mg/kg) injection resulted in a
37.57% reduction in the expression of TH in the hypothalamus,
compared with the vehicle-treated group (P<0.05; Fig. 5), however, no significant effect
was detected in the level of DAT. Fluoxetine restored the abnormal
expression of TH and increased the level of DAT (P<0.05;
Fig. 5). Similarly, MEPS at doses
between 10 and 250 mg/kg significantly suppressed the levels of
DAT, between 76.90±3.88 and 50.43±5.51% (P<0.05; Fig. 5), compared with the reserpine-only
group. MEPS treatment at 50 and 250 mg/kg for 7 days resulted in a
43.56 and 57.61% increase in the levels of TH, respectively
(P<0.05; Fig. 5).
 | Figure 5Effects of MEPS on the expression
levels of DAT and TH in the hypothalamus. Following administration
of MEPS (10, 50 and 250 mg/kg.) or fluoxetine (10 mg/kg) for 7
days, 4.0 mg/kg reserpine was injected intraperitoneally. The
hypothalamus of the mice in each group were collected. Changes in
the levels of TH and DAT in the hypothalamus were determined using
western blot analysis. Quantifiied data were normalized by GAPDH,
respectively. Data are expressed as the mean ± standard error of
the mean (n=8) and were analyzed using one-way analysis of
variance. #P<0.05, vs vehicle; *P<0.05,
**P<0.01 and ***P<0.001, vs. RES alone.
MEPS, Marasmius exopolysaccharide; Flu, fluoxetine; RES,
reserpine; DAT, dopamine transporter; TH, tyrosine hydroxylase. |
Discussion
The safety of natural products has been an area of
concern in the medical community and public (28). In the acute toxicity investigation
performed in the present study, the highest non-toxic dose of MEPS
selected for treatment of the male and female mice was >20-fold
higher than its effective dose. Based on the results of the
preliminary safety investigation, which revealed no significant
adverse effects, the antidepressant-like effects of MEPS were
assessed. Animal models of depression, including behavioral despair
models (FST and TST) and drug-induced models (5-HTP-induced
head-twitch assessment and antagonism of reserpine-induced
hypothermia) are important in the scientific screening and
evaluation of antidepressants (29,30).
MEPS enhanced mouse locomotor activity following a 7-day
administration period. In addition, 250 mg/kg MEPS treatment
resulted in a reduction on the duration of immobility in the FST
and TST, suggesting its antidepressant activities. The
5-HTP-induced head-twitch and reserpine-induced hypothermia
assessments were also used to confirm its effects.
As reported previously, SSRIs, including fluoxetine
and paroxetine, can reduce immobility and increase swimming
duration without affecting climbing (31). By contrast, selective NE or DA
reuptake inhibitors, including desipramine or maprotiline, reduce
immobility and increase climbing, without altering swimming
(32). In the FST and TST
performed in the present study, no swimming or climbing durations
were recorded, therefore, additional experiments are required to
determine whether MEPS regulates the 5-HT system, the dopaminergic
system or both.
5-HT receptor agonists induce a characteristic
head-twitch response, the frequency of which is dose-dependent
(33). Due to it is ease of
quantification, this response provides an attractive animal model
for the investigation of transmitter interactions with 5-HTergic
mechanisms (34). 5-HTP is one
type of amino acid, which acts as a precursor of 5-HT (35). In the 5-HTP-induced head-twitch
assessment performed in the present study, MEPS (250 mg/kg)
significantly increased the number of head twitches (P<0.05).
Furthermore, similar to the effects of fluoxetine, MEPS
administration enhanced the concentration of 5-HT in the
hypothalamus. However, the present study did not determine the
changes in the expression levels of 5-HT receptors in the mouse
model. A previous clinical study suggested that platelet 5-HT
content may serve as a supplementary biomarker for the function of
uptake-associated mediators, including 5-HT1A receptors,
in drug-free depressed patients (36). Numerous studies involving animal
models have confirmed that an increase in 5-HT1A
autoreceptor density in the dorsal raphe reduces and delays the
therapeutic response to SSRIs (37,38).
However, based on the results of the present study, it is difficult
to conclude whether the MEPS-mediated antidepressant activity was
associated with its regulation of the 5-HT system.
Reserpine is involved in the consumption of NE and
5-HT; 5-HIAA is the metabolic end product of 5-HT, and DA is the
premise compound of NE (30).
Based on these considerations, the levels of 5-HT, 5-HIAA, DA and
NE in the hypothalamus were determined in the reserpine-induced
hypothermia mouse model in the present study. Unlike fluoxetine,
MEPS not only normalized the levels of 5-HT, 5-HIAA and DA; but it
also reduced the hyperexpression of NE. These data suggested that
the dopaminergic system may be involved in its antidepressant-like
effect. To further confirm this hypothesis, the expression levels
of DAT and TH in the mouse brain were analyzed. Selective
noradrenaline re-uptake inhibitors and DAT inhibitors are important
clinical antidepressants (39).
The inhibition of DAT is considered a major mechanism underlying
the therapeutic benefits of antihyperactivity medications, smoking
cessation and antidepressants (40,41).
In the present study, MEPS markedly suppressed the expression of
DAT in the hypothalamus of the resperpine-treated group, indicating
that MEPS exerted a similar effect as the DAT inhibitor. In
addition, TH is reported to be an enzyme responsible for the
catalysis of amino acid L-tyrosine into dihydroxyphenylalanine
(42), which is the premise
compound of DA, therefore, TH exerts a rate-limiting role for DA
synthesis (43,44). In the present study, MEPS
dose-dependently enhanced the expression of TH in the hypothalamus,
which was consistent with the increase in the level of DA. Studies
reporting results, which are consistent with the monoamine
hypothesis have indicated that the majority of patients with
depression have a deficit in brain DA and/or DA metabolites
(45), and by increasing the
expression levels of DA receptors or DA, the antidepressant
enhances DA function (46).
Taken together, the results of the present study
demonstrated that EPS produced during Marasmius androsaceus
submerge fermentation exerts antidepressant-like effects, confirmed
through use of a FWT, TST, 5-HTP-induced head-twitch assessment and
reserpine-induced hypothermia assessment. MEPS regulated the
concentration of neurotransmitters in the mouse hypothalamus. In
addition, following MEPS administration for 7 days, the expression
of DAT was suppressed; whereas the level of TH was enhanced. These
results provide experimental evidence supporting the potential
clinical use of MEPS as an effective agent against depression.
Acknowledgments
This study was supported by the National Science and
Technology Support Program of P.R. China (grant no. 2012BAL29B05),
the Natural Science Foundation of China (grant no. 81402955) and
the Science and Technology Key Project of Jilin Province (grant no.
20130201006ZY).
References
|
1
|
Réus GZ, Vieira FG, Abelaira HM, Michels
M, Tomaz DB, dos Santos MA, Carlessi AS, Neotti MV, Matias BI, Luz
JR, et al: MAPK signaling correlates with the antidepressant
effects of ketamine. J Psychiatr Res. 55:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kessler RC and Wang PS: The descriptive
epidemiology of commonly occurring mental disorders in the United
States. Ann Rev Public Health. 29:115–129. 2008. View Article : Google Scholar
|
|
3
|
Manji HK, Drevets WC and Charney DS: The
cellular neurobiology of depression. Nat Med. 7:541–547. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steru L, Chermat R, Thierry B and Simon P:
The tail suspension test: A new method for screening
antidepressants in mice. Psychopharmacology (Berl). 85:367–370.
1985. View Article : Google Scholar
|
|
5
|
Komiya M, Takeuchi T and Harada E: Lemon
oil vapor causes an anti-stress effect via modulating the 5-HT and
DA activities in mice. Behav Brain Res. 172:240–249. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Z, Ji W, Qu R, Wang M, Yang W, Zhan Z,
Fu Q and Ma S: Metabonomic study on the antidepressant-like effects
of banxia houpu decoction and its action mechanism. Evid Based
Complement Alternat Med. 2013:2137392013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang A, Hao H, Zheng X, Liang Y, Xie Y,
Xie T, Dai C, Zhao Q, Wu X, Xie L and Wang G: Peripheral
anti-inflammatory effects explain the ginsenosides paradox between
poor brain distribution and anti-depression efficacy. J
Neuroinflammation. 8:1002011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kennedy SH: A review of antidepressant
treatments today. Eur Neuropsychopharm. 16(Suppl): S619–S623. 2006.
View Article : Google Scholar
|
|
9
|
Novak M and Vetvicka V: Beta-glucans,
history and the present: Immunomodulatory aspects and mechanisms of
action. J Immunotoxicol. 5:47–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin L, Wu F, Li X, Li H, Du C, Jiang Q,
You J, Li S and Xu Y: Anti-depressant effects of aqueous extract
from Acanthopanax senticosus in mice. Phytother Res. 27:1829–1833.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dar A, Khatoon S, Rahman G and
Atta-Ur-Rahman: Anti-depressant activities of areca catechu fruit
extract. Phytomedicine. 4:41–45. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng L, Zhang X, Kang DY, Liu XT and Hong
Q: Effectiveness and safety of wuling capsule for post stroke
depression: A systematic review. Complement Ther Med. 22:549–566.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XJ, Li J, Zou QD and Jin L: Wuling
capsule for climacteric patients with depression and anxiety state:
A randomized, positive parallel controlled trial. Zhong Xi Yi Jie
He Xue Bao. 7:1042–1046. 2009.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Liang QM, Li TT, Zhi R, Zhang N
and Lu J: Study on extraction and anti-oxidation of Marasmius
androsaceus mycelium polysaccharides. Food Sci Technol. 12:80–83.
2006.
|
|
15
|
Ye WF, Yang XT, Chen Y and Li QL:
Long-time analgesic effect of Marasmius androsaceus in rats. Zhong
yao yao li yu lin chuang. 18:19–21. 2002.In Chinese.
|
|
16
|
Gao Yang YX-l and Xu Duo-Duo: Glycopeptide
physicochemical properties and analgesic effect of Marasmius
androsaceus. Changchun Zhong yi xue yuan xue bao. 777–778. 2013.In
Chinese.
|
|
17
|
Kim SW, Hwang HJ, Xu CP, Sung JM, Choi JW
and Yun JW: Optimization of submerged culture process for the
production of mycelial biomass and exo-polysaccharides by Cordyceps
militaris C738. J Appl Microbiol. 94:120–126. 2003. View Article : Google Scholar
|
|
18
|
Chen H, Yan M, Zhu J and Xu X: Enhancement
of exo-polysaccharide production and antioxidant activity in
submerged cultures of Inonotus obliquus by lignocellulose
decomposition. J Ind Microbiol Biotechnol. 38:291–298. 2011.
View Article : Google Scholar
|
|
19
|
Shih IL: Microbial exo-polysaccharides for
biomedical applications. Mini Rev Med Chem. 10:1345–1355. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Na-Xin YG-W and An Li-Guo:
Anti-hyperglycemic activities of exopolysaccharides from Cordyceps
militaris in diabetic mice induced by multiple low-dose
streptozotocoin. Shipin Kexue (Beijing). 288–295. 2013.In
Chinese.
|
|
21
|
Lan Ying PZ-F, Zhang Song and Yang
Xiao-Bing: The Influence of exopolysaccharide extract of Morchella
conica on fruit Fly's life-span. Zhong guo shi yong jun bian ji bu.
43–45. 2010.In Chinese.
|
|
22
|
Yan H, Zhu D, Xu D, Wu J and Bian X: A
study on Cordyceps militaris polysaccharide purification,
composition and activity analysis. Afr J Biotechnol. 7:4004–4009.
2008.
|
|
23
|
Porsolt RD, Bertin A and Jalfre M:
Behavioral despair in mice: A primary screening test for
antidepressants. Arch Int Pharmacodyn Ther. 229:327–336.
1977.PubMed/NCBI
|
|
24
|
Pesarico AP, Sampaio TB, Stangherlin EC,
Mantovani AC, Zeni G and Nogueira CW: The antidepressant-like
effect of 7-fluoro-1,3-diphenylisoquinoline-1-amine in the mouse
forced swimming test is mediated by serotonergic and dopaminergic
systems. Prog Neuropsychopharmacol Biol Psychiatry. 54:179–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiang H, Liu Y, Zhang B, Huang J, Li Y,
Yang B, Huang Z, Xiang F and Zhang H: The antidepressant effects
and mechanism of action of total saponins from the caudexes and
leaves of Panax notoginseng in animal models of depression.
Phytomedicine. 18:731–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang CL, Chik SC, Li JC, Cheung BK and Lau
AS: Identification of the bioactive constituent and its mechanisms
of action in mediating the anti-inflammatory effects of black
cohosh and related Cimicifuga species on human primary blood
macrophages. J Med Chem. 52:6707–6715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noble JE and Bailey MJ: Quantitation of
protein. Methods Enzymol. 463:73–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao L, Liu G, Kang J, Niu M, Wang Z, Wang
H, Ma J and Wang X: Paclitaxel nanosuspensions coated with P-gp
inhibitory surfactants: I. Acute toxicity and pharmacokinetics
studies. Colloids Surf B Biointerfaces. 111:277–281. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Willner P: The validity of animal models
of depression. Psychopharmacology (Berl). 83:1–16. 1984. View Article : Google Scholar
|
|
30
|
Gao S, Cui YL, Yu CQ, Wang QS and Zhang Y:
Tetrandrine exerts antidepressant-like effects in animal models:
Role of brain-derived neurotrophic factor. Behav Brain Res.
238:79–85. 2013. View Article : Google Scholar
|
|
31
|
Cryan JF and Lucki I: Antidepressant-like
behavioral effects mediated by 5-Hydroxytryptamine (2C) receptors.
J Pharmacol Exp Ther. 295:1120–1126. 2000.PubMed/NCBI
|
|
32
|
Page ME, Detke MJ, Dalvi A, Kirby LG and
Lucki I: Serotonergic mediation of the effects of fluoxetine, but
not desipramine, in the rat forced swimming test.
Psychopharmacology (Berl). 147:162–167. 1999. View Article : Google Scholar
|
|
33
|
Handley SL and Singh L: Modulation of
5-hydroxytryptamine-induced head-twitch response by drugs acting at
GABA and related receptors. Br J Pharmacol. 86:297–303. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peroutka SJ, Lebovitz RM and Snyder SH:
Two distinct central serotonin receptors with different
physiological functions. Science. 212:827–829. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukuda K: 5-HTP hypothesis of
schizophrenia. Med Hypotheses. 82:20–23. 2014. View Article : Google Scholar
|
|
36
|
Zhang ZJ, Wang D, Man SC, Ng R, McAlonan
GM, Wong HK, Wong W, Lee J and Tan QR: Platelet 5-HT (1A) receptor
correlates with major depressive disorder in drug-free patients.
Prog Neuropsychopharmacol Biol Psychiatry. 53:74–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gardier AM, Malagié I, Trillat AC, Jacquot
C and Artigas F: Role of 5-HT1A autoreceptors in the mechanism of
action of serotoninergic antidepressant drugs: Recent findings from
in vivo microdialysis studies. Fundam Clin Pharmacol. 10:16–27.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rainer Q, Nguyen HT, Quesseveur G, Gardier
AM, David DJ and Guiard BP: Functional status of somatodendritic
serotonin 1A autoreceptor after long-term treatment with fluoxetine
in a mouse model of anxiety/depression based on repeated
corticosterone administration. Mol Pharmacol. 81:106–112. 2012.
View Article : Google Scholar
|
|
39
|
Haenisch B and Bönisch H: Depression and
antidepressants: Insights from knockout of dopamine, serotonin or
noradrenaline re-uptake transporters. Pharmacol Ther. 129:352–368.
2011. View Article : Google Scholar
|
|
40
|
Tashkin DP, Rabinoff M, Noble EP, Ritchie
TL, Simmons MS and Connett J: Association of dopamine-related gene
alleles, smoking behavior and decline in FEV1 in subjects with
COPD: findings from the lung health study. COPD. 9:620–628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Madras BK, Fahey MA, Miller GM, De La
Garza R, Goulet M, Spealman RD, Meltzer PC, George SR, O'Dowd BF,
Bonab AA, et al: Non-amine-based dopamine transporter (reuptake)
inhibitors retain properties of amine-based progenitors. Eur J
Pharmacol. 479:41–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cunha MP, Pazini FL, Oliveira Á, Bettio
LE, Rosa JM, Machado DG and Rodrigues AL: The activation of
α1-adrenoceptors is implicated in the antidepressant-like effect of
creatine in the tail suspension test. Prog Neuropsychopharmacology
Biol Psychiatry. 44:39–50. 2013. View Article : Google Scholar
|
|
43
|
Rovin ML, Boss-Williams KA, Alisch RS,
Ritchie JC, Weinshenker D, West CH and Weiss JM: Influence of
chronic administration of antidepressant drugs on mRNA for galanin,
galanin receptors and tyrosine hydroxylase in catecholaminergic and
serotonergic cell-body regions in rat brain. Neuropeptides.
46:81–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagatsu T, Levitt M and Udenfriend S:
Tyrosine Hydroxylase. The initial step in norepinephrine
biosynthesis. J Biol Chem. 239:2910–2917. 1964.PubMed/NCBI
|
|
45
|
Lambert G, Johansson M, Agren H and
Friberg P: Reduced brain norepinephrine and dopamine release in
treatment-refractory depressive illness: Evidence in support of the
catecholamine hypothesis of mood disorders. Arch Gen Psychiatry.
57:787–793. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ainsworth K, Smith SE, Zetterstrom TS, Pei
Q, Franklin M and Sharp T: Effect of antidepressant drugs on
dopamine D1 and D2 receptor expression and dopamine release in the
nucleus accumbens of the rat. Psychopharmacology (Berl).
140:470–477. 1998. View Article : Google Scholar
|