Introduction
Recent studies regarding type 2 diabetes mellitus
(DM) have demonstrated that DM is an independent risk factor that
can lead to diabetic-associated cognitive decline (DACD), and even
dementia (1–3). However, the exact mechanism of type 2
DACD is currently unclear, and there are various factors, such as
glucose metabolism, vascular structural function abnormalities,
diabetic complications, and aging that may be involved in its
pathogenesis. In addition, an overlap phenomenon exists between
these various risk factors (4–6).
DACD dysfunction includes increased inducible nitric
oxide synthase (iNOS)/nitric oxide (NO) expression, oxidative
stress and the production of various cytokines (7). iNOS is a key enzyme that regulates
the generation of endothelial-derived NO via the phosphoinositide
3-kinase (PI3K)/Akt signaling pathway. The PI3K/Akt signaling
pathway has an important role in the regulation of cellular
metabolism, growth, migration and proliferation. Zhu et al
(8) previously demonstrated that
ganoderma atrum polysaccharide was able to increase the aortic
relaxation of diabetic rats via activation of the PI3K/Akt signal
pathway. In addition, Jia et al (9) reported that fish oil augmented
learning impairments of diabetic rats through upregulating the
PI3K/AKT pathway (9).
The process of oxidative stress is inseparable from
protein glycosylation. The high blood sugar levels associated with
DM lead to the increased formation of advanced glycation
end-products (AGEs), which accumulate in the blood vessel walls and
interfere with endogenous NO synthesis and vasodilatation (10,11).
Low-density lipoprotein (LDL) oxidative modification weakens the
recognition by the receptor (12),
reducing LDL clearance and resulting in elevated LDL levels
(13). Oxidative stress has an
important role in the activation and acceleration of
arteriosclerosis. Furthermore, the chemical structure, and cell and
tissue effects of AGEs accelerate aging-related alterations;
previous studies have demonstrated that oxidative stress and the
formation of AGEs may lead to DACD (14–16).
Astaxanthin (AST) is an oxygen-containing derivative
of carotenoids, which can effectively quench activated oxygen, and
has high nutritional and medicinal value (17). In the 1930s, AST was separated from
shrimp and crab shells; however, the physiological function of AST
did not gather attention until the 1980s (18). Since then, numerous studies have
demonstrated that AST is able to inhibit tumorigenesis, enhance
immune function, and prevent a wide range of physiological
conditions, such as cardiovascular disease (19). AST is therefore considered to
possess broad application prospects in animal studies and clinical
trials (20). A recent study
demonstrated that AST could attenuate diabetes through its effects
on oxidative stress and inflammation (21). However, despite the promising
evidence regarding the effects of AST on diabetes, little is
currently known regarding its effects on the amelioration of DACD
in diabetic rats. Therefore, the present study aimed to investigate
whether AST was able to attenuate pathological changes on DACD, and
to explore the underlying molecular signaling pathway of this
beneficial effect.
Materials and methods
Drugs and chemicals
AST (purity ≥97%; Fig.
1) and streptozotocin (STZ) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Glucose radioimmunoassay kit was purchased
from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Bicinchoninic acid (BCA) protein assay kit, the glutathione
peroxidase (GPx) and superoxide dismutase (SOD) activity assay
kits, and the glutathione (GSH) and malondialdehyde (MDA) content
assay kits were purchased from BestBio (Shanghai, China). An iNOS
assay kit (cat. no. E-CL-R0358c; Wuhan Elabscience Biological
Technology Co., Ltd., Wuhan, China), and caspase-3 and caspase-9
activity assay kits (cat. no. E-EL-R0160c; Wuhan Elabscience
Biological Technology Co., Ltd; cat. no. C1158, Beyotime Institute
of Biotechnology (Haimen, China) were also purchased.
Experimental animals
The present study was conducted in accordance with
the Principles of Laboratory Animal Care, and the Guide for the
Care and Use of Laboratory Animals at Dalian Medical University
(Dalian, China). Sprague-Dawley (SD) rats (n=40; age, 8 weeks;
weight, 200–230 g) were purchased from the Comparative Medicine
Center, Dalian Medical University and approved by the ethics
committee of The First Affiliated Hospital of Dalian Medical
University. All of the rats were allowed to acclimatize in an
animal room (temperature, 22–25°C; humidity, 55–60%; 12 h
light/dark cycle), and were provided access to chow and water ad
libitum.
Establishment of the model and
measurement of DM
After 1 week acclimatization, each rat was injected
intraperitoneally (i.p.) with 65 mg/kg STZ (pH 4.4), in order to
induce DM. A total of 48 h after STZ injection, fasting blood
glucose levels were determined using a radioimmunoassay kit
(Nanjing Jiancheng Bioengineering Institute). Rats with fasting
blood glucose levels >250 mg/dl were considered diabetic, and
were used for further experimentation.
Experimental groups
A total of 40 rats were randomly divided into five
groups: (i) The control (Con) group (n=8), which consisted of rats
treated with i.p. isotonic NaCl; (ii) the DM group (n=8), which
consisted of diabetic rats treated with i.p. saline; (iii) the AST
(50) group (n=8), which consisted of diabetic rats treated with
i.p. 50 mg/kg AST; (iv) the AST (100) group (n=8), which consisted
of diabetic rats treated with i.p.100 mg/kg AST; and (v) the
AST+LY294002 (AST+LY; Sigma-Aldrich) group (n=8), which consisted
of diabetic rats treated with i.p. 50 mg/kg AST and 0.25
µg/100 g LY294002.
Morris water maze test
Following a 14-day treatment with AST, cognitive
function was evaluated using the Morris water maze test, as
described previously (22). Each
rat was trained five times a day at 20 min intervals. The test was
performed blindly every day for 5 days. Swimming was video tracked
(ANY-maze; Stoelting Co., Wood Dale, IL, USA), and latency, path
length, swim speed, and cumulative distance from the platform was
recorded. Mean swim latency for all of the rats was evaluated on
each day. After a training trial, the mean time spent in the
correct quadrant containing the platform, and the mean number of
times the mice crossed the former platform position during 60 sec
was estimated.
Western blotting of phosphorylated
(p)-Akt and Akt
To determine protein concentrations, blood was
obtained from every rat by retro-orbital blood collection under
anesthetization (pentobarbital sodium, 30 mg/kg, P11011; Merck
KGaA, Darmstadt, Germany) and 50 mg brain tissue from the cerebral
cortex/hippocampus was removed, which was incubated with 100
µl tissue lysis buffer on ice for 30 min and centrifuged at
20,000 × g for 15 min at 4°C. Protein concentration was then
determined using the BCA protein assay kit. For each sample, 50
µg of protein was separated by SDS-PAGE (Beyotime Institute
of Biotechnology) and transferred to a nitrocellulose membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was
then blocked with 5% non-fat milk in PBS for 3 h, and washed in
Tris-buffered saline containing Tween-20 (Beyotime Institute of
Biotechnology) overnight at 4°C. Protein bands were detected using
a BCIP-NBT kit (Promega Corporation, Madison, WI, USA). The
antibodies used were as follows: Monoclonal rabbit anti-mouse
anti-p-Akt (1:800 dilution; cat. no. sc-293094; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), monoclonal rabbit anti-mouse
anti-Akt (1:1,000 dilution; cat. no. sc-7126; Santa Cruz
Biotechnology, Inc.) and anti-β-actin (1:500 dilution; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) at 4°C overnight. After
incubation with the primary antibodies, the membrane was incubated
with horseradish peroxidase-conjugated anti-mouse IgG (ZF-0312;
1:5,000; Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) for 1 h at room temperature, followed by incubation with ECL
Super Signal® West Pico Chemiluminescent Substrate
(Pierce Biotechnology, Inc., Rockford, IL, USA).
Measurement of GPx, GSH, SOD and MDA
activities
Approximately 5–10 mg brain tissue was added to 100
µl tissue lysis buffer (Beyotime Institute of
Biotechnology). The tissue pellet was homogenized on ice, and the
mixture was incubated for 5–10 min. The supernatant was obtained
following centrifugation at 16,000–20,000 × g for 15 min at 4°C.
The activities of GPx, GSH, SOD and MDA were detected using kits,
according to the manufacturer's instructions. Serum GPx activity
was detected by measuring the absorbance at 340 nm using a plate
reader (Wallac VICTOR 1420; PerkinElmer, Inc., Waltham, MA, USA).
The levels of GPx and SOD were expressed in U/mg. The levels of
serum GSH and MDA levels were expressed in µg/g and nmol/mg,
respectively.
Measurement of iNOS activity
Approximately 5–10 mg brain tissue was added to 100
µl tissue lysis buffer. The tissue pellet was homogenized on
ice, and the mixture was incubated for 5–10 min. The supernatant
was obtained following centrifugation at 16,000–20,000 × g for 15
min at 4°C. iNOS activity was measured using an iNOS assay kit in
accordance with the manufacturer's instructions. The absorbance was
measured at a wavelength of 530 nm to determine iNOS activity.
Measurement of caspase-3 and caspase-9
activity
Approximately 5–10 mg brain tissue was added to 100
µl tissue lysis buffer. The tissue pellet was homogenized on
ice, and the mixture was incubated for 5–10 min. The supernatant
was obtained following centrifugation at 16,000–20,000 × g for 15
min at 4°C. Caspase-3 and caspase-9 activity was measured using
activity assay kits, according to the manufacturer's instructions;
the samples were incubated at 37°C for 2 h. Absorbance was measured
at a wavelength of 405 nm to determine caspase-3 and caspase-9
activity.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical significance was analyzed using one-way analysis of
variance followed by Dunnett's test. SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA) was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of AST on body weight and blood
glucose levels
The present study determined whether AST could
influence the body weight and blood glucose levels of diabetic
rats. The body weight of the DM group was successfully reduced, as
compared with the control group (Fig.
2A). In addition, the blood glucose levels of the DM group were
notably increased, as compared with the control group (Fig. 2B). Following a 14-day treatment
with AST, the body weight and blood glucose levels were markedly
augmented and reduced, as compared with the control group (Fig. 2A and B). However, the body weight
and blood glucose levels of the AST+LY group were similar to the DM
group (Fig. 2A and B).
Effects of AST on cognitive function
The escape latency of the DM rats was markedly
increased, as compared with the control rats (Fig. 3A). Following a 14-day treatment
with AST, the escape latency of the DM rats was significantly
reduced, as compared with the DM group (Fig. 3A).
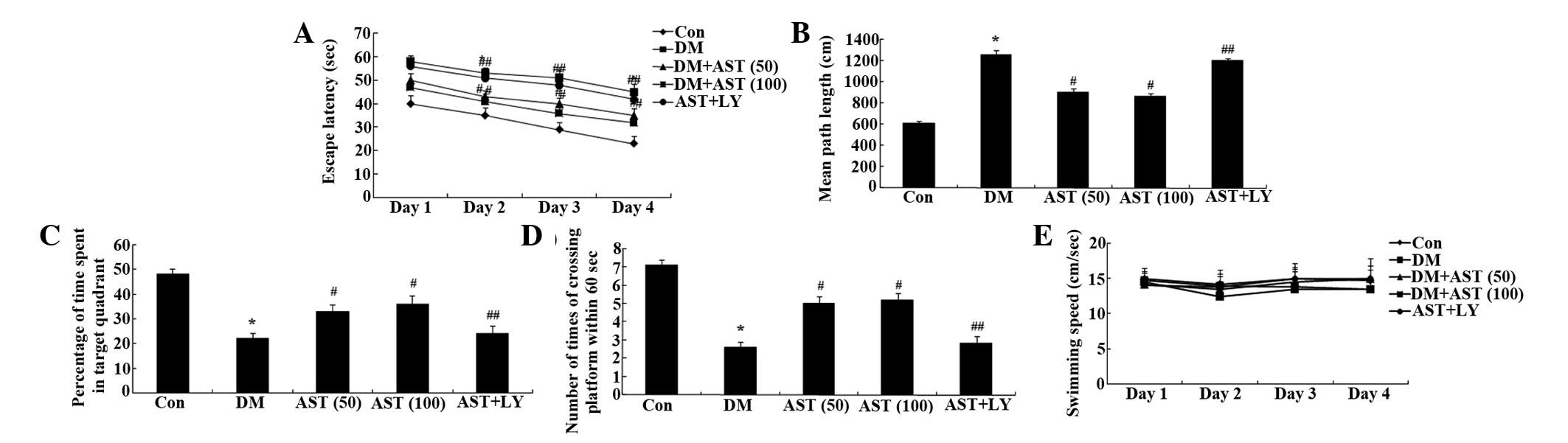 | Figure 3Effects of AST on diabetes-induced
cognitive deficit. Effects of AST on the (A) escape latency, (B)
mean path length, (C) mean percentage of time spent in the target
quadrant, (D) the number of times the platform was crossed, and (E)
swimming speed in rats. *P<0.01, compared with the
Con group; #P<0.01, compared with the DM group;
##P>0.05, compared with the DM group. AST,
astaxanthin; Con, control group; DM, diabetes group; AST (50), AST
(50 mg/kg)-treated group; AST (100), AST (100 mg/kg)-treated group;
AST+LY, AST (50 mg/kg) and LY294002 (0.25 µg/100 g)-treated
group. |
Mean path length was markedly increased in the DM
rats, as compared with the control rats, whereas the mean path
length of the AST-treated rats was significantly decreased, as
compared with the DM rats (Fig.
3B). Furthermore, there was a marked reduction in the time
spent in the target quadrant for the DM rats, whereas the time
spent in the target quadrant for the AST rats was significantly
increased, as compared with the DM rats (Fig. 3C). Notably, the number of times the
rats crossed the former platform location was also decreased for
the DM rats, as compared with the control group, whereas the number
of times the rats crossed the former platform location was
significantly increased for the AST rats, as compared with the DM
rats (Fig. 3D). There was no
significant difference in swimming speed between the various groups
(Fig. 3E). Notably, the cognitive
function of the AST+LY group was similar to that of the DM group
(Fig. 3).
Effects of AST on diabetes-induced
changes in p-Akt and Akt expression
To assess the impact of AST on the expression levels
of Akt proteins in the brain cells of rats, the expression levels
of Akt proteins were detected in the cerebral cortex and
hippocampus. Treatment with AST (50 and 100 mg/kg) significantly
activated the expression of p-Akt and Akt in the cerebral cortex
and hippocampus of diabetic rats, as compared with the DM group
(Fig. 4). The protein expression
levels of p-Akt and Akt in the AST+LY group were similar to in the
DM group (Fig. 4).
Effects of AST on diabetes-induced
changes in GPx, GSH, SOD and MDA
To clarify the anti-oxidative effects of AST on
diabetic rats, the levels of GPx, GSH, SOD and MDA were detected.
Following a 14-day treatment, the levels of GPx, GSH and SOD in the
cerebral cortex and hippocampus of the DM group were markedly
decreased, as compared with the control group (Fig. 5A–C). However, the levels of GPx,
GSH and SOD in the cerebral cortex and hippocampus of the diabetic
rats were markedly increased following a 14-day treatment with AST,
as compared with the DM group (Fig.
5A–C). Furthermore, the GPx, GSH and SOD levels in the AST+LY
group were similar to those in the DM group (Fig. 5A–C).
 | Figure 5Effects of AST on diabetes-induced
changes in GPx, SOD, GSH and MDA levels. Effects of AST on the
levels of (A) GPx, (B) GSH and (C) SOD, and (D) the MDA content in
rats. *P<0.01, compared with the Con group;
#P<0.01, compared with the DM group;
##P>0.05, compared with the DM group. AST,
astaxanthin; GSH, glutathione; GPx, glutathione peroxidase; SOD,
superoxide dismutase; MDA, malondialdehyde; Con, control group; DM,
diabetes group; AST (50), AST (50 mg/kg)-treated group; AST (100),
AST (100 mg/kg)-treated group; AST+LY, AST (50 mg/kg) and LY294002
(0.25 µg/100 g)-treated group. |
Simultaneously, the MDA content in the cerebral
cortex and hippocampus of the rats with DM was significantly
increased, as compared with the control group (Fig. 5D). However, the MDA content in the
cerebral cortex and hippocampus of the DM rats was significantly
decreased following a 14-day treatment with AST, as compared with
the DM group (Fig. 5D).
Furthermore, the MDA content of the AST+LY group was similar to
that in the DM group (Fig.
5D).
Effects of AST on iNOS activity
To investigate whether AST exerted protective
effects via reducing NOS production, the activity of iNOS was
detected. iNOS activity in the cerebral cortex and hippocampus of
the DM group was increased, as compared with the control group
(Fig. 6). However, iNOS activity
in the cerebral cortex and hippocampus of diabetic rats was
decreased following a 14-day treatment with AST, as compared with
the DM group (Fig. 6).
Furthermore, the iNOS activity of the AST+LY group was similar to
the DM group (Fig. 6).
 | Figure 6Effects of AST on iNOS activity.
Effects of AST on diabetes-induced alterations in iNOS activity.
*P<0.01, compared with the Con group;
#P<0.01, compared with the DM group;
##P>0.05, compared with the DM group. AST,
astaxanthin; iNOS, inducible nitric oxide synthase; Con, control
group; DM, diabetes group; AST (50), AST (50 mg/kg)-treated group;
AST (100), AST (100 mg/kg)-treated group; AST+LY, AST (50 mg/kg)
and LY294002 (0.25 µg/100 g)-treated group. |
Effects of AST on caspase-3 and caspase-9
activity
To evaluate the effects of AST on cellular apoptosis
in diabetic rats, caspase-3 and caspase-9 activity was measured in
the cerebral cortex and hippocampus. Caspase-3 and caspase-9
activity in the cerebral cortex and hippocampus of the DM group was
markedly increased, as compared with the control group (Fig. 7A and B). However, treatment with
AST (50 and 100 mg/kg) significantly reduced caspase-3 and
caspase-9 activity in the cerebral cortex and hippocampus of
diabetic rats following a 14-day treatment, as compared with the DM
group (Fig. 7A and B).
Furthermore, caspase-3 and caspase-9 activity was similar in the
AST+LY group, as compared with the DM group (Fig. 7A and B).
Discussion
Type 2 (T2) DM is a major cause of morbidity and
mortality worldwide, and the prevalence of T2DM is increasing
(23). The greater proportion of
this is in Asia, Africa and South America. The biological
mechanisms linking T2DM with impaired DACD remain unclear.
Individuals with T2DM are at an enhanced risk for stroke, which is
a risk factor for cognitive decline, and may also contribute to
microvascular alterations and cerebral ischemia (24,25).
The present study demonstrated that AST was able to markedly
augment body weight and reduce the blood glucose levels of DM rats.
Naito et al (26)
previously reported that AST reduced blood glucose levels and
increased body weight in a rodent model of T2DM. However, the
effects of AST on the body weight and blood glucose levels of rats
with DM were reversed by LY294002. Furthermore, the cognitive
function of DM rats was improved following treatment with AST,
whereas treatment with the Akt inhibitor LY294002 reduced the
cognitive function of DM rats.
A recent study demonstrated that activation of the
PI3K/Akt pathway serves as a pro-survival signal in the protection
of nerve cells, and has an important role in hypoxic-ischemic
neuronal damage (27). The
PI3K/Akt pathway controls cell survival by regulating apoptosis and
autophagy in the nervous system (28). Activation of PI3K and Akt can
promote endothelial cell survival, reduce nerve damage, reduce
inflammatory cell death, and block neuronal damage (29,30).
Insulin-like growth factor, hypothermia and ischemic
preconditioning also have a protective role in the brain via the
PI3K/Akt signaling pathway (31).
In the present study, in the cerebral cortex and hippocampus, the
protein expression levels of p-Akt and Akt were significantly
upregulated by treatment with AST in the rats with DM. However,
treatment with AST (50 mg/kg) with LY294002 (0.25 mg/kg) was able
to weaken the expression levels of p-Akt and Akt in rats with DM.
Li et al (32) reported
that AST decreased oxidative stress of ARPE-19 cells via activation
of PI3K/Akt. In addition, treatment with AST increased cell
apoptosis in a hamster model of oral cancer via inactivation of
extracellular signal-regulated kinases/mitogen-activated protein
kinases and PI3K/Akt pathways (33). Furthermore, Zhang et al
(34) reported that the PI3K/Akt
inhibitor, LY294002, was able to partially reverse the
neuroprotection of AST in the early period after subarachnoid
hemorrhage, by downregulating AST-induced activation of Akt.
Numerous studies have focused on the association
between oxidative stress and DACD. Free radicals are highly
reactive, and can exert strong oxidative effects that damage
biological macromolecules and numerous cellular components, and
have significant toxic effects on nerve cells that can lead to
lipofuscin deposition, increasing age spots, and vacuolar
degeneration (35). In the present
study, AST markedly increased GPx, GSH and SOD levels, and
decreased MDA content in the cerebral cortex and hippocampus of
diabetic rats. Notably, LY294002 effectively reduced the protective
effects of AST on the oxidative stress of rats with DM. Wu et
al (36) demonstrated that AST
was able to alleviate brain aging in rats through repairing the
activities of GPx and SOD, increasing GSH content, and reducing MDA
content. Augusti et al (37) indicated that AST may reduce
oxidative stress in rabbits with atherosclerosis.
The present study demonstrated that in the cerebral
cortex and hippocampus, iNOS activity was decreased following
treatment with AST; however, the effects of AST on iNOS activity in
DM rats were reduced by LY294002. Choi et al (38) previously suggested that AST was
able to inhibit the production of inflammatory mediators by
blocking iNOS activation. In addition, AST may inhibit NO
production by suppressing nuclear factor-κB activation (39). Simultaneously, in the present study
AST protected brain cells and reduced the cell apoptosis of DM rats
by suppressing caspase-3 and caspase-9 activity. Song et al
(40) demonstrated that AST
inhibited apoptosis of alveolar epithelial cells via inhibition of
cytochrome c release, and caspase-9 and caspase-3 activation
(40). In addition, AST has been
shown to protect against
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/1-methyl-4-phenylpyridinium-induced
mitochondrial dysfunction via inhibition of the activation of
caspase-3 (22).
In conclusion, the present study demonstrated that
treatment with AST may reduce type 2 DACD in rats via activation of
PI3K/Akt and downregulation of the oxidative stress pathway. These
results suggest that AST may protect against cognitive decline in
rats with T2DM.
References
|
1
|
Iwanami J, Mogi M, Tsukuda K, Jing F,
Ohshima K, Wang XL, Nakaoka H, Kan-no H, Chisaka T, Bai HY, et al:
Possible synergistic effect of direct angiotensin II type 2
receptor stimulation by compound 21 with memantine on prevention of
cognitive decline in type 2 diabetic mice. Eur J Pharmacol.
724:9–15. 2014. View Article : Google Scholar
|
|
2
|
Hishikawa N, Yamashita T, Deguchi K, Wada
J, Shikata K, Makino H and Abe K: Cognitive and affective functions
in diabetic patients associated with diabetes-related factors,
white matter abnormality and aging. Eur J Neurol. 22:313–321. 2015.
View Article : Google Scholar
|
|
3
|
Wang YB, Wang S, Bai R, Du JL, Xing Q, Ba
Y, Yang Y, Zhang XY, Shi CH and Yao JJ: Efficacy of switching from
premixed insulin to insulin glargine regimen in Type 2 diabetes
mellitus patients with different islet functions. Mol Med Rep.
10:1096–1102. 2014.PubMed/NCBI
|
|
4
|
Lian G, Yue X, Xianxiang Z, Yong L,
Weijuan L and Bing C: Insulinization: A promising strategy for the
treatment of type 2 diabetes mellitus. Exp Ther Med. 6:1300–1306.
2013.PubMed/NCBI
|
|
5
|
Mossello E, Ballini E, Boncinelli M,
Monami M, Lonetto G, Mello AM, Tarantini F, Baldasseroni S,
Mannucci E and Marchionni N: Glucagon-like peptide-1, diabetes, and
cognitive decline: Possible pathophysiological links and
therapeutic opportunities. Exp Diabetes Res. 2011:2816742011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marder TJ, Flores VL, Bolo NR, Hoogenboom
WS, Simonson DC, Jacobson AM, Foote SE, Shenton ME, Sperling RA and
Musen G: Task-induced brain activity patterns in type 2 diabetes: A
potential biomarker for cognitive decline. Diabetes. 63:3112–3119.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu B, Kuang L and Liu J: Bariatric
surgery relieves type 2 diabetes and modulates inflammatory factors
and coronary endothelium eNOS/iNOS expression in db/db mice. Can J
Physiol Pharmacol. 92:70–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu KX, Nie SP, Li C, Gong D and Xie MY:
Ganoderma atrum polysaccharide improves aortic relaxation in
diabetic rats via PI3K/Akt pathway. Carbohydr Polym. 103:520–527.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia D, Heng LJ, Yang RH and Gao GD: Fish
oil improves learning impairments of diabetic rats by blocking
PI3K/AKT/nuclear factor-κB-mediated inflammatory pathways.
Neuroscience. 258:228–237. 2014. View Article : Google Scholar
|
|
10
|
Lin X, Chen X, Ye J, Li Q, Zhou J, Wu X,
Huang Y, Li X, Shi Y, Li S, Li L and Cai H: Association between
endogenous secretory receptor for advanced glycation-end products
(esRAGE) and carotid intima-media thickness in type 2 diabetes. Exp
Clin Endocrinol Diabetes. 122:277–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Si X, Li P, Zhang Y, Zhang Y, Lv W and Qi
D: Renoprotective effects of olmesartan medoxomil on diabetic
nephropathy in streptozotocin-induced diabetes in rats. Biomed Rep.
2:24–28. 2014.PubMed/NCBI
|
|
12
|
Pietzsch J, Laube M, Bechmann N, Pietzsch
FJ and Kniess T: Protective effects of 2,3-diaryl-substituted
indole-based cyclooxygenase-2 inhibitors on oxidative modification
of human low density lipoproteins in vitro. Clin Hemorheol
Microcirc. Dec 29–2014.Epub ahead of print. PubMed/NCBI
|
|
13
|
Price TO, Eranki V, Banks WA, Ercal N and
Shah GN: Topiramate treatment protects blood-brain barrier
pericytes from hyperglycemia-induced oxidative damage in diabetic
mice. Endocrinology. 153:362–372. 2012. View Article : Google Scholar :
|
|
14
|
Liu Y, Tian X, Gou L, Sun L, Ling X and
Yin X: Luteolin attenuates diabetes-associated cognitive decline in
rats. Brain Res Bull. 94:23–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stinghen AE, Massy ZA, Vlassara H, Striker
GE and Boullier A: Uremic Toxicity of Advanced Glycation End
Products in CKD. J Am Soc Nephrol. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao L, Stolzenberg-Solomon R, Zimmerman
TP, Duan Z, Chen L, Kahle L, Risch A, Subar AF, Cross AJ,
Hollenbeck A, et al: Dietary consumption of advanced glycation end
products and pancreatic cancer in the prospective NIH-AARP Diet and
Health Study. Am J Clin Nutr. 101:126–134. 2015. View Article : Google Scholar
|
|
17
|
Chang CS, Chang CL and Lai GH: Reactive
oxygen species scavenging activities in a chemiluminescence model
and neuroprotection in rat pheochromocytoma cells by astaxanthin,
beta-carotene, and canthaxanthin. Kaohsiung J Med Sci. 29:412–421.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Visser H, van Ooyen AJ and Verdoes JC:
Metabolic engineering of the astaxanthin-biosynthetic pathway of
Xanthophyllomyces dendrorhous. FEMS Yeast Res. 4:221–231. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu H, Niu H, Shao A, Wu C, Dixon BJ, Zhang
J, Yang S and Wang Y: Astaxanthin as a Potential Neuroprotective
Agent for Neurological Diseases. Mar Drugs. 13:5750–5766. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rao AR, Sindhuja HN, Dharmesh SM, Sankar
KU, Sarada R and Ravishankar GA: Effective inhibition of skin
cancer, tyrosinase, and antioxidative properties by astaxanthin and
astaxanthin esters from the green alga Haematococcus pluvialis. J
Agric Food Chem. 61:3842–3851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan KC, Pen PJ and Yin MC:
Anticoagulatory and antiinflammatory effects of astaxanthin in
diabetic rats. J Food Sci. 77:H76–H80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee DH, Kim CS and Lee YJ: Astaxanthin
protects against MPTP/MPP+-induced mitochondrial
dysfunction and ROS production in vivo and in vitro. Food Chem
Toxicol. 49:271–280. 2011. View Article : Google Scholar
|
|
23
|
Sun K, Chang X, Yin L, Li J, Zhou T, Zhang
C and Chen X: Expression and DNA methylation status of microRNA-375
in patients with type 2 diabetes mellitus. Mol Med Rep. 9:967–972.
2014.
|
|
24
|
Petty MA and Lo EH: Junctional complexes
of the blood-brain barrier. Permeability changes in
neuroinflammation. 68:311–323. 2002.
|
|
25
|
Biessels GJ, Deary IJ and Ryan CM:
Cognition and diabetes: A lifespan perspective. Lancet Neurol.
7:184–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naito Y, Uchiyama K, Aoi W, Hasegawa G,
Nakamura N, Yoshida N, Maoka T, Takahashi J and Yoshikawa T:
Prevention of diabetic nephropathy by treatment with astaxanthin in
diabetic db/db mice. Biofactors. 20:49–59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bulhak AA, Jung C, Ostenson CG, Lundberg
JO, Sjöquist PO and Pernow J: PPAR-alpha activation protects the
type 2 diabetic myocardium against ischemia-reperfusion injury:
Involvement of the PI3-Kinase/Akt and NO pathway. Am J Physiol
Heart Circ Physiol. 296:H719–H727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
You JJ, Yang CH, Yang CM and Chen MS:
Cyr61 induces the expression of monocyte chemoattractant protein-1
via the integrin αvβ3, FAK, PI3K/Akt and NF-κB pathways in retinal
vascular endothelial cells. Cell Signal. 26:133–140. 2014.
View Article : Google Scholar
|
|
29
|
Cho YR, Lim JH, Kim MY, Kim TW, Hong BY,
Kim YS, Chang YS, Kim HW and Park CW: Therapeutic effects of
fenofibrate on diabetic peripheral neuropathy by improving
endothelial and neural survival in db/db mice. PLoS One.
9:e832042014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malemud CJ: The PI3K/Akt/PTEN/mTOR
pathway: a fruitful target for inducing cell death in rheumatoid
arthritis? Future Med Chem. 7:1137–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang T, Mao X, Li H, Qiao S, Xu A, Wang J,
Lei S, Liu Z, Ng KF, Wong GT, et al: N-Acetylcysteine and
allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via
adiponectin and attenuated myocardial postischemic injury in
diabetes. Free Radic Biol Med. 63:291–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y,
Hou D and Zhang X: Astaxanthin protects ARPE-19 cells from
oxidative stress via upregulation of Nrf2-regulated phase II
enzymes through activation of PI3K/Akt. Mol Vis. 19:1656–1666.
2013.PubMed/NCBI
|
|
33
|
Kavitha K, Kowshik J, Kishore TK, Baba AB
and Nagini S: Astaxanthin inhibits NF-κB and Wnt/β-catenin
signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to
induce intrinsic apoptosis in a hamster model of oral cancer.
Biochim Biophys Acta. 1830.4433–4444. 2013.
|
|
34
|
Zhang XS, Zhang X, Wu Q, Li W, Zhang QR,
Wang CX, Zhou XM, Li H, Shi JX and Zhou ML: Astaxanthin alleviates
early brain injury following subarachnoid hemorrhage in rats:
Possible involvement of Akt/bad signaling. Mar Drugs. 12:4291–4310.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu YW, Zhu X, Yang QQ, Lu Q, Wang JY, Li
HP, Wei YQ, Yin JL and Yin XX: Suppression of methylglyoxal
hyperactivity by mangiferin can prevent diabetes-associated
cognitive decline in rats. Psychopharmacology (Berl). 228:585–594.
2013. View Article : Google Scholar
|
|
36
|
Wu W, Wang X, Xiang Q, Meng X, Peng Y, Du
N, Liu Z, Sun Q, Wang C and Liu X: Astaxanthin alleviates brain
aging in rats by attenuating oxidative stress and increasing BDNF
levels. Food Funct. 5:158–166. 2014. View Article : Google Scholar
|
|
37
|
Augusti PR, Conterato GM, Somacal S,
Sobieski R, Quatrin A, Maurer L, Rocha MP, Denardin IT and
Emanuelli T: Astaxanthin reduces oxidative stress, but not aortic
damage in atherosclerotic rabbits. J Cardiovasc Pharmacol Ther.
14:314–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi SK, Park YS, Choi DK and Chang HI:
Effects of astaxanthin on the production of NO and the expression
of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J
Microbiol Biotechnol. 18:1990–1996. 2008.
|
|
39
|
Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ,
Ha KS, Han JA, Yim SV, Chang K, Kwon YG, et al: Astaxanthin
inhibits nitric oxide production and inflammatory gene expression
by suppressing I (kappa)B kinase-dependent NF-kappaB activation.
Mol Cells. 16:97–105. 2003.PubMed/NCBI
|
|
40
|
Song X, Wang B, Lin S, Jing L, Mao C, Xu
P, Lv C, Liu W and Zuo J: Astaxanthin inhibits apoptosis in
alveolar epithelial cells type II in vivo and in vitro through the
ROS-dependent mitochondrial signalling pathway. J Cell Mol Med.
18:2198–2212. 2014. View Article : Google Scholar : PubMed/NCBI
|





















