Introduction
Primary liver cancer is the fifth most common type
of cancer worldwide and the third most common cause of
cancer-related mortality (1,2).
Hepatocellular carcinoma (HCC) accounts for 85–90% of primary liver
cancers (2). Progress has been
made in detecting and treating localized disease, however the
5-year survival of patients with liver cancer was ~15% in the USA
in 2002–2008, ~12% in Europe in 2000–2007, and 5% in low-income
countries in 2002 (3). Thus,
establishing an appropriate animal model is critical for
understanding the molecular, cellular and pathophysiological
mechanisms of HCC, and is essential for the development of novel
therapeutic strategies.
The constant evolution of model design and
technological development allows for numerous experimental models
of HCC to be developed including spontaneous, induced,
transplantable, and genetically engineered models (4). Currently, the most commonly employed
models of HCC are transplantable ones including subcutaneous and
orthotopic transplantation in nude mice (4). However, they present a difficulty in
distinguishing the difference in position and morphology between
the tumor cells and the stroma. The lack of information regarding
the interaction between tumor and host is largely due to the
absence of suitable models that allow visualization and precise
investigation of the tumor-host interaction in vivo
(5). The introduction of
fluorescent protein visualization to this field allows for the
labeling of host and tumor cells with different color fluorescence
proteins, thus enabling investigation of the tumor microenvironment
(TME). Previous studies have reported a dual-color fluorescence
tracing transplantation model in various tumor types (5–9).
However, to the best of our knowledge, a dual-color fluorescence
tracing orthotopic transplantation model of HCC has not been
established. Therefore, the current study aimed to establish a
dual-color fluorescence tracing orthotopic transplantation model of
HCC, based on green fluorescence protein (GFP)-expressing nude mice
and red fluorescence protein (RFP)-expressing hepatoma cells.
Materials and methods
Cell culture
The HepG2 human hepatoma cell line and Hepa1-6 mice
hepatoma cell line (Type Culture Collection of the Chinese Academy
of Sciences, Shanghai, China) were cultured in Dulbecco's modified
Eagle's medium (HyClone; GE Healthcare Life Sciences, Beijing,
China) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
atmosphere of 5% CO2 at 37°C.
Red fluorescence labeling of HCC cell
lines
According to the manufacturer's instructions, HepG2
and Hepa1-6 HCC cell lines were transfected with RFP gene using a
lentivirus-mediated gene transfection kit (pLenO-RIP; Shanghai
Innovation Biotechnology Co., Ltd., Shanghai, China). HepG2 and
Hepa1-6 cells were then respectively cultured in growth medium to
30–50% confluence at the time of transduction (1×105
cells/well in 24-well plates). Then the cells were incubated with
the RFP-lentivirus at a multiplicity of infection of 10 for HepG2
cells and 5 for Hepa1-6 cells. After 72 h the positive transduction
rate was visualized using fluorescence microscopy. The cells were
then passaged at a ratio of 1:3 in a selective medium that
contained 10 µg/ml puromycin (Sigma-Aldrich, St Louis, MO,
USA). Cell clones with high RFP-expression were selected in 96-well
plates. They were amplified and transferred by conventional culture
methods. The cells that underwent successful transduction and
screening were termed Hepa1-6-RFP and HepG2-RFP, respectively.
Adherent cells were digested in cell suspension and were
subsequently assessed by flow cytometry (Beckman Coulter, Miami,
FL, USA) to analyze the RFP-positive cells
Animals
NC-C57BL6J-GFP nude mice (Department of
Neurosurgery, Second Affiliated Hospital of Suzhou University,
Suzhou, China) (10) were housed
in microisolator cages with sterile bedding (NASA 1000), water and
food was provided ad libitum. The mice were maintained in an
environment of 24–26°C, in a humidity of 50–60% with 12 h
light/dark cycles. As previously described (10), GFP-expressing nude mice were
obtained by crossing non-transgenic NC athymic nude mice with the
GFP transgenic C57BL6J mice. The present study was performed in
strict accordance to the recommendations of the Guide for the Care
and Use of Laboratory Animals of the National Institutes of Health
(publication no. 85–23, revised 1985). The animal use protocol was
reviewed and approved by the Institutional Animal Care and Use
Committee of Soochow University (Suzhou, China).
Establishment of the dual-color
orthotopic transplantation tumor model
A total of 20 NC-C57BL6J-GFP nude mice (age, 6
weeks; body weight, 20 g) were inoculated with 1×106 HCC
cells. They were divided into two groups: Group I (n=10) were
injected with HepG2-RFP cells and group II (n=10) were injected
with Hepa1-6-RFP cells. All the surgical procedures were performed
under general anesthesia using intraperitoneal injection of 10%
chloral hydrate (200 mg/kg; Sigma-Aldrich). Once anesthetized, the
mice were fixed on an experimental board in a supine position. A
2-mm transverse incision was made below the xiphoid, following
sterilization of the area with 70% alcohol, which was perpendicular
to the median line and was 1–1.5 cm long. The right liver lobes
were carefully pulled out of the abdominal cavity with a sterile
cotton swab. Tumor cells were resuspended in phosphate-buffered
saline. The red fluorescent cell suspension (50 µl;
1×106 cells) was injected into the left liver lobes at a
depth of 3.5 mm over 15 min using a 50 µl Hamilton syringe
(Anhui Zhenghua Biological Instrument Equipment Co., Ltd., Huaibei,
China). Following the injection, a small piece of sterile gauze was
placed on the injection site, and light pressure was applied for 1
min to prevent bleeding and spilling. The skin was then sterilized
with 70% alcohol and the wound sutured with a Plus 5–0 suture line.
The mean duration of surgery was 30 min. Postsurgery, mice were
treated with 0.1 mg ketoprofen (Sigma-Aldrich) for pain control and
were observed continuously for signs of pain or distress
(hypoactivity, restlessness, vocalization, hiding, lack of
grooming, abnormal posture, tremor or respiratory distress) until
they recovered from anesthesia and for the next 48 h. The living
conditions of the mice were inspected daily.
Whole-body fluorescence imaging
Following the implantation of tumor cells, the mice
were anesthetized via intraperitoneal injection of 10% chloral
hydrate (200 mg/kg), on week 3, 5 and 7 of the current study in
order to perform whole-body fluorescent imaging using the in
vivo fluorescence imaging system (Kodak, Rochester, NY, USA).
The GFP excitation and emission wavelengths were 470 and 535 nm,
respectively. The RFP excitation and emission wavelengths were 553
and 574 nm, respectively. Once imaging was complete, each animal
was removed from the imaging stage, placed on a heated platform in
its original cage and allowed to recover. Subsequent to full
recovery from the anesthesia, the animals were returned to the IVC
isolation device.
Histological evaluation and
subculturing
When tumor-bearing mice appeared distressed (as
determined by cachexia, loss of appetite, hypoactivity, lack of
grooming or abnormal posture), they were sacrificed by cervical
dislocation and an autopsy was conducted. A heart perfusion was
performed with 5–10 ml of 4% paraformaldehyde. The whole liver was
harvested, frozen and sectioned at a thickness of 5 µm using
conventional culture methods. Samples were then stained with
hematoxylin and eosin (Beyotime Institute of Biotechnology,
Shanghai, China) using routine histopathological procedures or
observed under a fluorescent microscope (Carl Zeiss, Oberkochen,
Germany). Cell nuclei were stained blue using
4,6-diamidino-2-phenylindole (Nanjing KeyGen Biotech Co., Ltd,
Nanjing, China).
Ascites were obtained from the tumor-bearing mice,
were centrifuged and the sediment was cultured in culture medium,
containing 10% FBS. The tumor nodules was harvested and washed with
phosphate-buffered saline, containing penicillin and streptomycin,
three times. The tumor tissue was subsequently minced with fine
scissors into small fragments, and cultured in culture medium,
containing 10% FBS. The medium was replaced with fresh culture
medium on the third day. Normal cell culture conditions were
maintained for the subsequent days.
Results
Red fluorescence labeling of HCC cell
lines
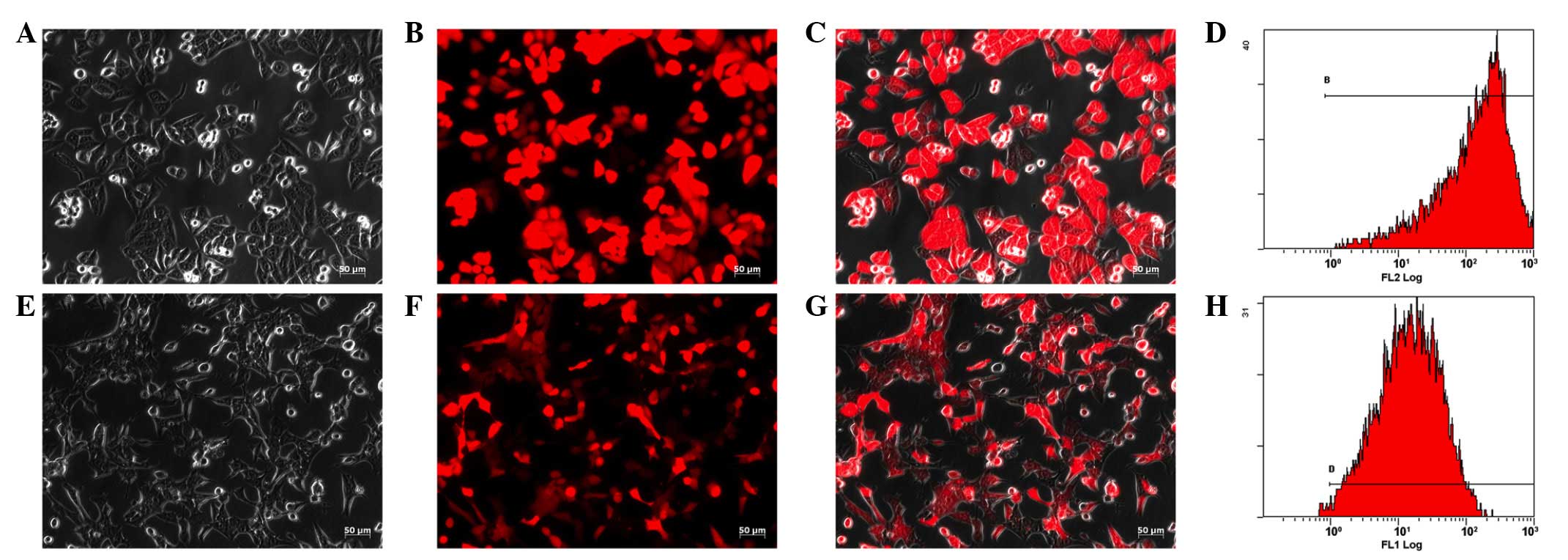
Lentivirus-mediated RFP gene transfection of HepG2
and Hepa1-6 cells achieved excellent results as ~100% of tumor
cells expressed RFP under a fluorescence microscope (Fig. 1). Due to the stable integration of
the RFP gene in the target cell genome, the expression of RFP in
the trans-fected tumor cells was maintained. Flow cytometric
analysis identified that >98% of the cells expressed RFP
(Fig. 1). The cells exhibited no
change in morphology and proliferation following transfection. The
HepG2-RFP and Hepa1-6-RFP cells were maintained for >1 year, and
their expression of the RFP gene remained stable.
Dual-fluorescence imaging in vivo
tumor-bearing mice
All mice were alive following the cell
transplantation. Fig. 2
demonstrated the dual-fluorescence imaging in the living
tumor-bearing mice. RFP signals were used to identify the growth of
transplanted tumors in the mice. The intensity of the fluorescence
signal was associated with the size of the tumor. The signal
intensity steadily increased from week 3 to week 7.
Oncobiological characteristics of nude
mice
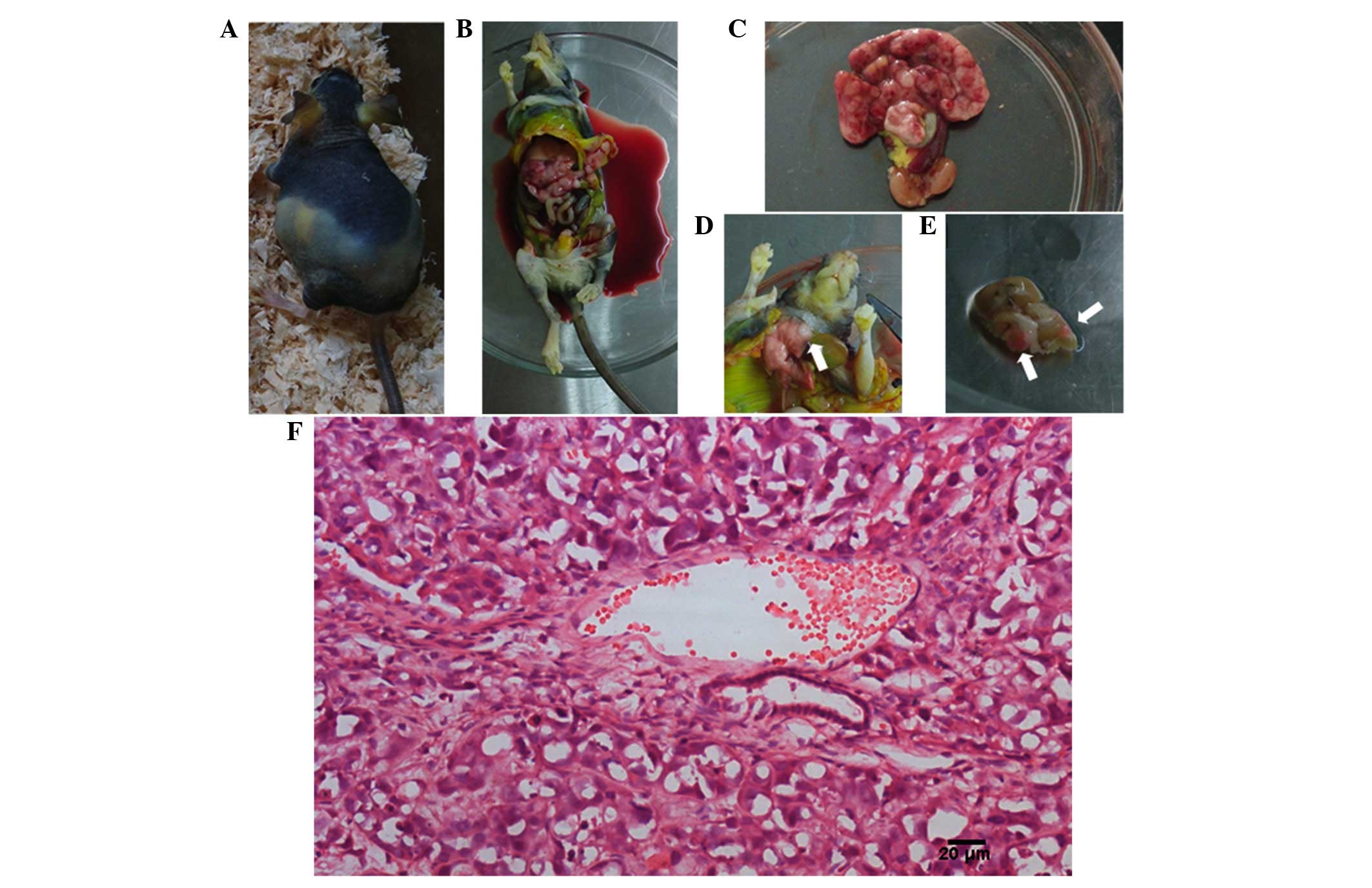
When the tumor-bearing mice appeared distressed,
they were sacrificed and underwent autopsy. The results are
displayed in Table I and Fig. 3. The median duration of survival of
HepG2-RFP tumor-bearing mice and Hepa1-6-RFP tumor-bearing mice
were 9 and 5 weeks, respectively. The rates of spontaneous
metastasis of HepG2-RFP tumor-bearing mice and Hepa1-6-RFP
tumor-bearing mice reached 100% each in the liver, 0 and 20% in the
lung, 70 and 80% in the abdominal wall, 80 and 90% in the
peritoneum, and 10 and 0 in the brain, respectively. A total of 70
and 90% of HepG2-RFP tumor-bearing mice and Hepa1-6-RFP
tumor-bearing mice, respectively, exhibited bloody ascites.
 | Table IOncobiological characteristics of
tumor-bearing mice. |
Table I
Oncobiological characteristics of
tumor-bearing mice.
| Oncobiological
characteristic | HepG2-RFP cell
line | Hepa1-6-RFP cell
line |
|---|
| Median duration of
survival (weeks) | 9 | 5 |
| Orthotopic
tumorigenesis | 100% (10/10) | 100% (10/10) |
| Intrahepatic
metastases | 100% (10/10) | 100% (10/10) |
| Pulmonary
metastases | 0% (0/10) | 20% (2/10) |
| Abdominal wall
invasion | 70% (7/10) | 80% (8/10) |
| Peritoneal
seeding | 80% (8/10) | 90% (9/10) |
| Bloody ascites | 70% (7/10) | 90% (9/10) |
| Brain metastases | 10% (1/10) | 0% (0/10) |
Interactions between tumor cells and host
cells
In non-fluorescent tracing solid tumor models, it is
often difficult to identify the origin of tumor stroma, and to
distinguish between the tumor cells and the stroma. In this
dual-color tumor model, transplanted RFP-HCC cells and their
descendant cells inside the tumor parenchyma were clearly
distinguished from the green host tissue. Mergence was defined as
interactions between tumor cells and host stroma during
tumorigenesis (Fig. 4A–E). The
overlapping distribution of tumor cells and host cells was the
elemental form of tumor tissue remodeling. In this dual-color
xenograft tumor model, apart from RFP and GFP cells, small numbers
of orange cells (merging of red and green cells) were also
identified (Fig 4A and E). They
were considered as hybrids of tumor cells and host cells. To verify
the presence of fused cells, ascites and tumor nodules from
tumor-bearing mice were cultured according to the method of a
previous study (11). These
results also identified fused cells in the ascites and tumor
nodules of cultured cells (Fig
4F–H).
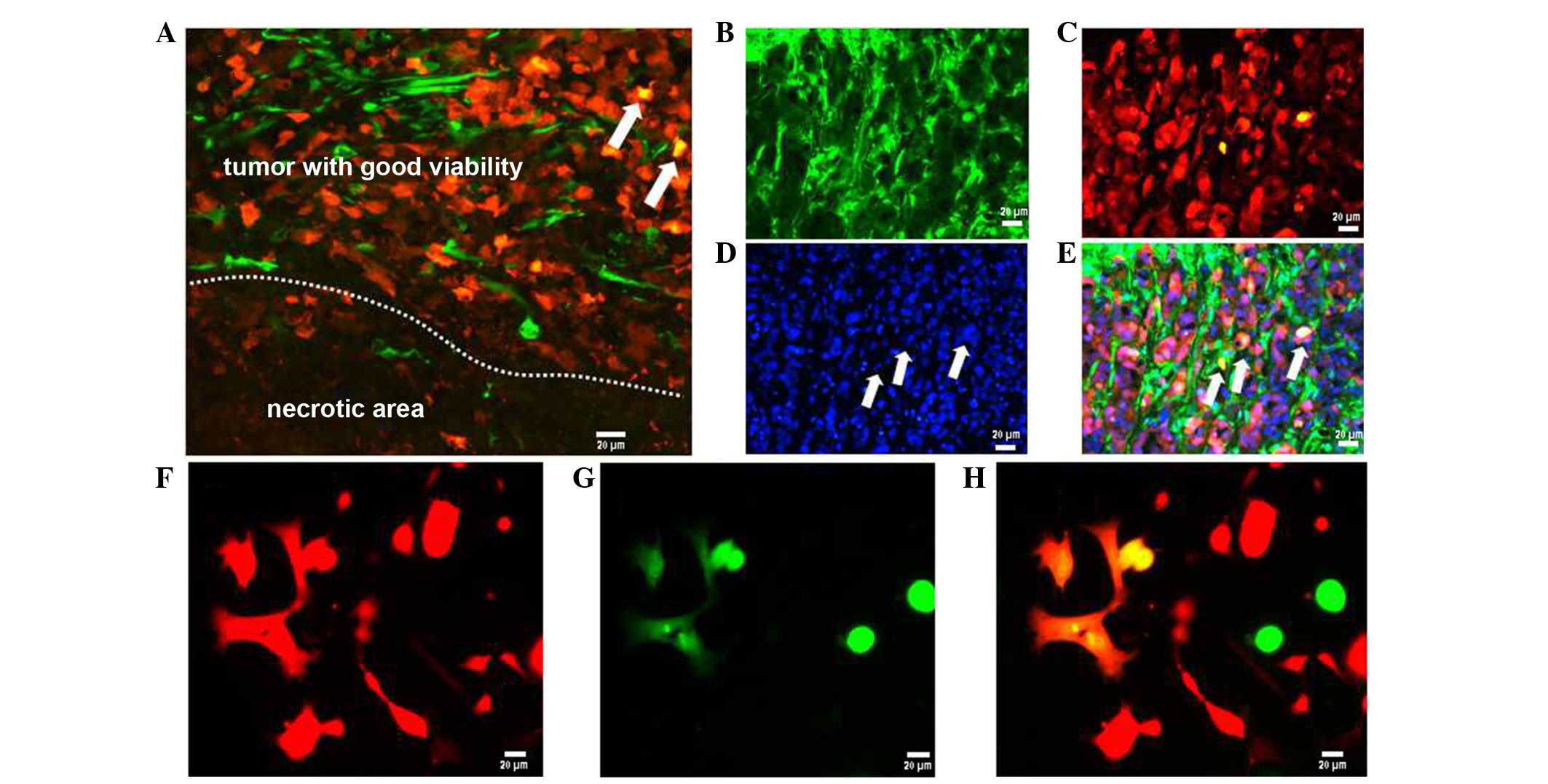 | Figure 4Interweaving distribution and fusion
between the tumor cells and host cells (scale bar, 20 µm).
In tumor parenchyma, GFP-expressing host cells were observed. (A)
RFP-expressing tumor tissue was readily identified in the area
where the tumor tissue maintains good viability; however, only the
remnants of GFP-expressing tissue can be visualized in the necrotic
area. (B) Frozen sections were observed under green fluorescence
microscope, (C) red fluorescence microscope, (D) blue fluorescence
microscope. (E) In the merged image, orange cells (product of the
merging of red and green cells) were identified (arrows) in the
tumor parenchyma. (F) In the ascites and tumor nodules of cultured
cells, the left six fused cells were observed under red
fluorescence microscope, (G) green fluorescence microscope, and (H)
merged image. GFP, green fluorescence protein. |
Tumor cell invasion and migration at the
single-cell level
The metastases spawned by carcinomas are formed
following the completion of a complex succession of cell biological
events collectively termed the invasion-metastasis cascade
(12). The exit of tumor cells
from their primary sites of growth is the first step in metastatic
progression. In the present study, using the frozen tissue
sections, the invasion and migration of the tumor cells at the
single-cell level was be observed (Fig. 5). It is clear in Fig. 5D that three fluorescent cells have
exited the primary tumor site. The initial step of metastasis is
the exit of single cells from the primary site.
Discussion
A well-defined liver cancer model mimicking human
liver cancer is essential to reliably reflect the progression of
human disease, for basic studies on tumor biology and experimental
therapeutic purposes (4). The aim
of these models is to provide suitable conditions for the viability
of the tumor samples so that they may grow and establish an
interaction with the host that resembles the situation observed in
the donor. The most commonly employed models for liver cancer are
xenograft models including subcutaneous and orthotopic transplant,
each having advantages and limitations (4). Subcutaneous xenograft models provide
an easy and simple means to implant tumor cells or tissues into the
study animal and to monitor tumor size. However, tumor progression
in humans is a complicated process in which the interaction of
neoplastic cells and the surrounding tumor environment is important
(13). A major disadvantage of
subcutaneous transplant models is the lack of interaction between
the host and the tumor, thus rendering them unable to mimic the
TME. By contrast, orthotopic animal models provide highly valuable
clinical information including the rate of tumor growth, the
therapeutic effect of tested materials, and the in vivo
tumor cell behavior as the tumor is located within the targeted
organ (14). Thus, orthotopic
animal models of HCC have many advantages, including providing
highly valuable clinical information on the rate of tumor growth,
therapeutic effect of certain materials and the ability to assess
the behaviors of tumors in vivo. Differences among these
orthotopic animal models were due to the type of the
transplantation sample (tumor cell suspension in culture or fresh
tumor pieces from surgery). The tumor cell injection animal model
is more relevant for clinical practice. However, one drawback of
the tumor cell injection model is that it is hard to control tumor
cell leakage during injection. In the present study, the cancer
cells were directly inoculated into the liver parenchyma. Despite
the measures taken to avoid leakage of cancer cells, the
possibility of leakage into the abdominal cavity was not completely
eliminated. Therefore, analyses were complicated by the fact that
apparent metastasis particularly peritoneal seeding may be due to
leakiness. Nevertheless, pulmonary and brain metastases observed in
the current study were certainly due to spontaneous metastasis
in vivo.
Postoperative evaluation of orthotopic tumors is
also a challenge. The most commonly used imaging modalities for
orthotopic tumors are ultrasound, computed tomography (CT),
magnetic resonance imaging (MRI), and
18F-fluorodeoxyglucose-positron emission tomography (PET)/CT.
However, there are drawbacks associated with each method.
Ultrasonic examination is inexpensive, however analysis of the data
requires a skilled operator (15).
CT/MRI/PET-CT scanning is noninvasive, however the cost is high and
it is difficult to perform (16).
The use of fluorescent proteins for imaging is revolutionizing
in vivo biology. With the use of an in vivo
fluorescence imaging system, the transplanted subcutaneous tumors
may be measured non-invasively and tumor growth continuously
monitored in real time. As a result of the deep position of
orthotopic transplantation HCC, it is difficult to identify and
measure this at an early stage. Fluorescence technology was
introduced for this reason and according to the fluorescent signal
intensity, tumor size can be compared.
The progression of a tumor is a multistep process.
The TME is critical for malignancy, which is in part the product of
the interaction between different cancer types and their host
cells. The development of solid tumors is due to the remodeling
processes of tumor cells against host tissue. However, the
mechanism of this remodeling process and the relevant dynamic
changes remain unknown. The dual-fluorescent xenograft HCC model
offers a platform to directly monitor multiple interactions between
RFP-labeled human tumor cells and GFP-labeled murine host cells. It
was determined that dynamic interweaving always exists in the
remodeling process of tumor and host tissues. Well grown tumor
sites are normally located at those sites where tumor cells and
host cells are interweaving distribution. However, in the necrotic
area, the host cells have a small proportion. Overall, the TME is
provided by the host, however, it is also transformed constantly by
tumor cells.
Cell fusion is not only a common physiological
phenomenon, but also an essential mechanism in tumorigenesis and
progression. Previous studies have provided evidence that hybrid
cells exhibit altered properties including increased metastatic
ability and enhanced resistance to apoptosis as well as an enhanced
drug resistance compared with the parental tumor cells (17–19).
A recent study confirmed that fusion occurs between bone
marrow-derived cells and tumor cells in human cancer (20). Cell fusion was identified in cell
culture and animal studies in various tumors including melanoma,
intestinal tumors, and breast carcinoma (18,20,21).
However, it is rarely reported in HCC in vivo. The present
dual-fluorescent xenograft HCC model offered an easy and direct
method to confirm the presence of fused cells via the expression of
RFP and GFP. The fused cells observed in the present study, may be
derived from the fusion of the RFP and GFP parental cells,
originating from the fusion of tumor cells with normal host cells.
However the function of fused cells in the development of HCC
requires further investigation. As demonstrated by Fig. 5D, cell‚ cells which was marked with
the second and third arrows were also fusion cells. Further
research is required to determine whether the fused cells increase
the malignancy of tumors including invasion and migration in
HCC.
Metastasis represents the end product of a multistep
cellular process termed the invasion metastasis cascade, which
involves dissemination of cancer cells to anatomically distant
organ sites and their subsequent adaptation to foreign tissue
microenvironments (12). The
present study confirmed that the first step in metastasis of HCC is
the exit of single cells from the primary site. At a cellular
level, the majority of types of carcinomas may invade as cohesive
multicellular units through a process termed 'collective invasion'.
Alternatively, individual tumor cells may invade via two distinct
pathways: The protease-, stress fiber-, and integrin-dependent
'mesenchymal invasion' pathway or the protease-, stress fiber-, and
integrin-independent, Rho/ROCK-dependent 'amoeboid invasion'
pathway (22). However, it is
largely unknown which pathway takes place during HCC invasion, and
further investigation is required.
In conclusion, a GFP/RFP dual-color tumor model is
useful for visualizing early-stage tumors and screening therapeutic
agents for HCC. The TME and the connectivity of tumor cells should
be investigated due to the direct interactions of tumor cells with
host tissue cells during the tumor remodeling process.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 32:2557–2576. 2007. View Article : Google Scholar
|
|
3
|
Bosetti C, Turati F and La Vecchia C:
Hepatocellular carcinoma epidemiology. Best Pract Res Clin
Gastroenterol. 28:753–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu L, Tang ZY and Li Y: Experimental
models of hepato-cellular carcinoma: Developments and evolution. J
Cancer Res Clin Oncol. 135:969–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang M, Li L, Jiang P, Moossa AR, Penman S
and Hoffman RM: Dual-color fluorescence imaging distinguishes tumor
cells from induced host angiogenic vessels and stromal cells. Proc
Natl Acad Sci USA. 100:14259–14262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayashi K, Yamauchi K, Yamamoto N,
Tsuchiya H, Tomita K, Amoh Y, Hoffman RM and Bouvet M: Dual-color
imaging of angiogenesis and its inhibition in bone and soft tissue
sarcoma. J Surg Res. 140:165–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bouvet M and Hoffman RM: In vivo imaging
of pancreatic cancer with fluorescent proteins in mouse models.
Methods Mol Biol. 872:51–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto N, Tsuchiya H and Hoffman RM:
Tumor imaging with multicolor fluorescent protein expression. Int J
Clin Oncol. 16:84–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffman RM: Transgenic nude mice
ubiquitously expressing fluorescent proteins for color-coded
imaging of the tumor micro-environment. Methods Mol Biol.
1194:353–365. 2014. View Article : Google Scholar
|
|
10
|
Dong J, Zhang Q, Huang Q, Chen H, Shen Y,
Fei X, Zhang T, Diao Y, Wu Z, Qin Z, et al: Glioma stem cells
involved in tumor tissue remodeling in a xenograft model. J
Neurosurg. 113:249–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang A, Dai X, Cui B, Fei X, Chen Y, Zhang
J, Zhang Q, Zhao Y, Wang Z, Chen H, et al: Experimental research of
host macrophage canceration induced by glioma stem progenitor
cells. Mol Med Rep. 11:2435–2442. 2015.
|
|
12
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heindryckx F, Colle I and Van Vlierberghe
H: Experimental mouse models for hepatocellular carcinoma research.
Int J Exp Pathol. 90:367–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walters DM, Stokes JB, Adair SJ, Stelow
EB, Borgman CA, Lowrey BT, Xin W, Blais EM, Lee JK, Papin JA, et
al: Clinical, molecular and genetic validation of a murine
orthotopic xenograft model of pancreatic adenocarcinoma using fresh
human specimens. PLoS One. 8:e770652013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao GJ, Xu LX, Chu ES, Zhang N, Shen JY,
Damirin A and Li XX: Establishment of an orthotopic transplantation
tumor model of hepatocellular carcinoma in mice. World J
Gastroenterol. 18:7087–7092. 2012. View Article : Google Scholar
|
|
16
|
Abou-Elkacem L, Gremse F, Barth S, Hoffman
RM, Kiessling F and Lederle W: Comparison of µ CT, MRI and optical
reflectance imaging for assessing the growth of GFP/RFP-expressing
tumors. Anticancer Res. 31:2907–2913. 2011.PubMed/NCBI
|
|
17
|
Berndt B, Zänker KS and Dittmar T: Cell
fusion is a potent inducer of aneuploidy and drug resistance in
tumor cell/normal cell hybrids. Crit Rev Oncog. 18:97–113. 2013.
View Article : Google Scholar
|
|
18
|
Dittmar T, Schwitalla S, Seidel J,
Haverkampf S, Reith G, Meyer-Staeckling S, Brandt BH, Niggemann B
and Zänker KS: Characterization of hybrid cells derived from
spontaneous fusion events between breast epithelial cells
exhibiting stem-like characteristics and breast cancer cells. Clin
Exp Metastasis. 28:75–90. 2011. View Article : Google Scholar
|
|
19
|
Pawelek JM: Fusion of bone marrow-derived
cells with cancer cells: Metastasis as a secondary disease in
cancer. Chin J Cancer. 33:133–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lazova R, Laberge GS, Duvall E, Spoelstra
N, Klump V, Sznol M, Cooper D, Spritz RA, Chang JT and Pawelek JM:
A melanoma brain metastasis with a donor-patient hybrid genome
following bone marrow transplantation: First evidence for fusion in
human cancer. PLoS One. 8:e667312013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Powell AE, Anderson EC, Davies PS, Silk
AD, Pelz C, Impey S and Wong MH: Fusion between intestinal
epithelial cells and macrophages in a cancer context results in
nuclear reprogramming. Cancer Res. 71:1497–1505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|