Introduction
A skin flap is a common autograft procedure, which
has been widely used in surgical practice. Skin flaps are often
used to repair wounds, as well as in the field of plastic and
reconstructive surgery (1).
However, the clinical application of skin flaps for tissue repair
and organ reconstruction is often accompanied by
ischemia-reperfusion injury. Ischemia results in tissue reperfusion
injury with the restoration of blood flow, which may lead to
partial or complete skin flap necrosis, and a decrease in the
success rate of the surgical procedure. Therefore, reducing skin
flap ischemia-reperfusion injury is of important clinical
significance. Numerous compounds have been demonstrated to reduce
ischemia-reperfusion injury; however, clinical trials have yet to
be successful due to toxic side-effects (2). Therefore, the development of novel
drugs and the identification of novel mechanisms for the treatment
of skin flap ischemia are required.
Endoplasmic reticulum (ER) stress is defined as an
accumulation of unfolded or misfolded proteins in the ER, a
subcellular organelle predominantly involved in protein folding
(3–6). The ER is also responsible for
regulating protein synthesis, protein folding and trafficking, and
intracellular calcium levels (7,8).
Accumulation of unfolded or misfolded proteins in the ER may result
in initiation of the unfolded protein response (UPR) or ER stress.
Activation of the UPR may increase the expression levels of
CCAAT/enhancer-binding protein-homologous protein (CHOP) and
glucose-regulated protein 78 (GRP78). CHOP, which is considered a
marker of ER stress, is an apoptotic transcription factor that is
induced in response to ER stress (9). In addition, GRP78 is an ER chaperone,
which stably interacts with unfolded or misfolded proteins;
therefore, upregulation of GRP78 expression is commonly considered
a marker of ER stress (3,10). It has previously been demonstrated
that ER stress mediates cell apoptosis by generating endogenous
reactive oxygen species or disturbing mitochondrial Ca2+
homeostasis, leading to the activation of caspase-3, which is a
regulator of caspase-dependent apoptosis (11). Therefore, targeting the ER may
provide a therapeutic approach for blocking the pathological
progression induced by skin flap ischemia.
4-phenylbutyrate (4-PBA) has been demonstrated to
contribute to the treatment of spinal muscular atrophy by altering
gene expression patterns (12,13).
Furthermore, 4-PBA is able to inhibit disease progression and
neuroinflammation in multiple sclerosis (14). Numerous studies have described the
use of 4-PBA as a chemical chaperone that reverses the
mislocalization and/or aggregation of proteins associated with
human disease (15–17). In addition, 4-PBA reduces the
levels of mutant or dislocated proteins retained in the ER under
conditions associated with cystic fibrosis and liver injury
(18). Despite the fact that skin
flaps have been shown to cause ER dysfunction, it remains unclear
whether 4-PBA is able to protect against
ischemia-reperfusion-induced damage, and regulate the protein
expression of ER stress markers.
The present study used in vivo experimental
systems to examine the effects of ER stress, as well as the
underlying molecular mechanisms, on ischemia-reperfusion in rat
skin flaps. The effects of 4-PBA may provide a novel therapeutic
strategy for the treatment of skin flap ischemia-reperfusion
injury.
Materials and methods
Induction of ischemia-reperfusion and
4-PBA treatment
Care of the laboratory animals and animal
experimentation were performed in accordance with animal ethics
guidelines and approved protocols. All animal studies were approved
by the Animal Ethics Committee of the Xiaoshan Traditional Chinese
Medical Hospital (Hangzhou, China). A total of 75 healthy male
Wistar rats (age, 6 weeks; weight, 300–350 g; Shanghai SLAC
Laboratory Animal Co., Ltd., Shanghai, China) were randomly divided
into three groups: The control group; the ischemia-reperfusion and
4-PBA administration group; and the ischemia-reperfusion and saline
administration group, which served as a negative control (NC). Each
group comprised 25 rats. The rats were maintained at room
temperature, in a 12-h light/dark cycle with access to food and
water ad libitum. Preparation of a skin flap was performed
in all of the rats, as follows: The rats were anesthetized with 3%
sodium pentobarbital (40 mg/kg; Sigma-Aldrich, St. Louis, MO, USA)
by intraperitoneal injection, their limbs were subsequently fixed,
and the fur on their abdomens was sheared. The skin of the abdomen
was sterilized with 75% alcohol. A right lower abdominal island
skin flap (6×3 cm) was created, according to the method described
by Shimamatsu and Wanless (19).
In the control group, no other treatments were performed. In the
4-PBA and NC groups, the femoral artery located at the proximal end
of the superficial epigastric artery was occluded using a vascular
clamp. An OPMI 1 FR pro surgical microscope (Carl Zeiss AG,
Oberkochen, Germany) was used to confirm the femoral artery and
superficial epigastric artery were completely occluded. The flap
was then sutured and the vascular clamp was removed after 8 h.
4-PBA was prepared by titrating equimolar amounts of
4-phenylbutyric acid (Wako Pure Chemicals Co., Ltd., Tokyo, Japan)
and sodium hydroxide to pH 7.4. 4-PBA was administered
intragastrically at a volume of 2 ml/kg 24 h and 30 min prior to
the surgical procedure in the 4-PBA group. Saline was administered
in the NC group. In the NC and 4-PBA groups, a 1×0.5 cm skin flap
tissue sample was removed at the time the skin flap was created (0
h), and 1, 6, 12 and 24 h following ischemia-reperfusion. In the
control group, a 1×0.5 cm skin flap tissue sample was removed at
the time the skin flap was created (0 h), and 1, 6, 12 and 24 h
following the surgical procedure.
Histology
The skin flap samples of the rats were removed and
fixed with 10% (v/v) neutral-buffered formalin. The tissue samples
were then dehydrated and embedded in paraffin. For histological
examination, 4 µm sections of the fixed embedded tissue
samples were cut using a Leica 2165 rotary microtome (Leica
Microsystem GmbH, Wetzlar, Germany). The sections were placed on
glass slides, deparaffinized, and stained sequentially with
hematoxylin and eosin (Richard-Allan Scientific Co., Kalamazoo, MI,
USA). The stained tissue sections were analyzed using a light
microscope (Axio Imager M1; Carl Zeiss AG) at x100
magnification.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
TUNEL staining was performed using the Roche In
Situ Cell Death Detection kit for the detection of programmed
cell death (Roche Applied Science, Pleasanton, CA, USA) (20). The tissue sections were then
examined by microscopy (CX41RF; Olympus Corporation, Tokyo, Japan)
and the number of TUNEL-positive cells was counted using National
Institutes of Health (NIH) Image software version 1.61 (NIH,
Bethesda, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the rat skin flap tissue
samples using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), as previously described
(21) and stored at −80°C. cDNA
was reverse transcribed from RNA using a cDNA synthesis kit (Thermo
Fisher Scientific, Inc.). The cDNA synthesis condi tions were as
follows: 37°C for 60 min, followed by 85°C for 5 min and 4°C for 5
min. A DyNAmo Flash SYBR® Green qPCR kit (Finnzymes Oy,
Espoo, Finland) was used, according to the manufacturer's protocol,
the thermal cycler used was an ABI 7500 (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in order to detect the mRNA expression
levels of CHOP and GRP78. The gene expression was calculated using
the 2−ΔΔCq method (22). The following primers were used:
CHOP, forward 5′-GGAGAAGGAGCAGGAGAATG-3′, reverse
5′-GAGACAGACAGGAGGTGATG-3′; GRP78, forward
5′-TAATCAGCCCACCGTAAC-3′, reverse 5′-GTTTCCTGTCCCTTTGTC-3′; and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward.
5′-GTCGGTGTGAACGGATTTG-3′, and reverse 5′-TCCCATTCTCAGCCTTGAC-3′
(Sangon Biotech Co., Ltd., Shanghai, China). Relative
quantification of the signals was performed by normalizing the gene
signals with those of GAPDH. The PCR cycling condi tions were as
follows: 95°C for 10 min; followed by 40 cycles at 95°C for 15 sec
and 60°C for 45 sec; and a final extension step of 95°C for 15 sec,
60°C for 1 min, 95°C for 15 sec and 60°C for 15 sec.
Western blotting
The skin flaps were homogenized and total cell
lysates were extracted using radioimmunoprecipitation assay buffer
(JRDUN Biotechnology, Co., Ltd., Shanghai, China), containing 50
mmol/l Tris-HCl (pH 8.8), 150 mmol/l NaCl, 1% Triton X-100, 0.1%
SDS and 1% deoxycholic acid sodium. Total protein concentration in
each tissue sample was measured using a Lowry protein assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equivalent
quantities (50 µg) of protein lysates were separated by 10
or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred to nitrocellulose membranes (Sigma-Aldrich),
followed by blocking in fat-free milk overnight at 4°C. The
membranes were incubated with primary antibodies for 2 h with
gentle agitation at room temperature, or overnight at 4°C, and then
washed three times with Tris-buffered saline with Tween 20
(Amresco, LLC, Solon, OH, USA). The following primary antibodies
were used and diluted as per the manufacturer's recommendations:
Rabbit monoclonal anti-GRP78 (1:1,000; Abcam, Cambridge, MA, USA;
cat. no. ab108613), mouse monoclonal anti-CHOP (1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA; cat. no. 2895) and
rabbit monoclonal anti-GAPDH (1:1,500; Cell Signaling Technology
Inc.; cat. no. 5174) for 2 h at 4°C. The membranes were then
incubated for 1 h at 37°C with either anti-mouse or anti-rabbit
horseradish peroxidase-conjugated immunoglobulin G secondary
antibodies (1:1,000; Dako North America, Inc., Carpinteria, CA,
USA; cat. nos. A0208 and A0216). Chemiluminescence detection was
conducted using Western Lightning Chemiluminescence Reagent Plus
(PerkinElmer, Inc., Waltham, MA, USA) and signals were quantified
by densitometry (Quantity One software, version 4.62; Bio-Rad
Laboratories, Inc.).
Immunofluorescence staining for CHOP and
GRP78
Paraffin-embedded skin flap tissue sections were
deparaffinized and hydrated for histological assessment. For
antigen retrieval, the tissues were put into the sodium citrate
solution (JRDUN Biotechnology, Co., Ltd.) and heated using a
microwave oven at 92–98°C for 10–30 min. Following antigen
retrieval, the tissue sections were permeabilized with 0.1% Triton
X-100 in phosphate-buffered saline (PBS). Following blocking with
2% bovine serum albumin (Sigma-Aldrich, Shanghai, China) in PBS for
1 h, the tissue sections were incubated with the anti-GRP78 and
anti-CHOP antibodies, prior to incubation with the corresponding
fluorescein isothiocyanate-conjugated secondary antibody. The
nuclei were then stained with 4′,6-diamidino-2-phenylindole. The
fluorescence signal was measured by CX41RF microscope at
magnification ×200.
Statistical analysis
The data are presented as the mean ± standard
deviation. A paired, two-tailed Student's t-test was used to
analyze the significance of statistical differences between the
groups. Statistical analyses were performed using GraphPad Prism
software, version 5 (GraphPad Software, Inc., La Jolla, CA, USA)
and P<0.05 was considered to indicate a statistically
significant difference.
Results
4-PBA attenuates ischemia-reperfusion
injury in the skin flap of the rats
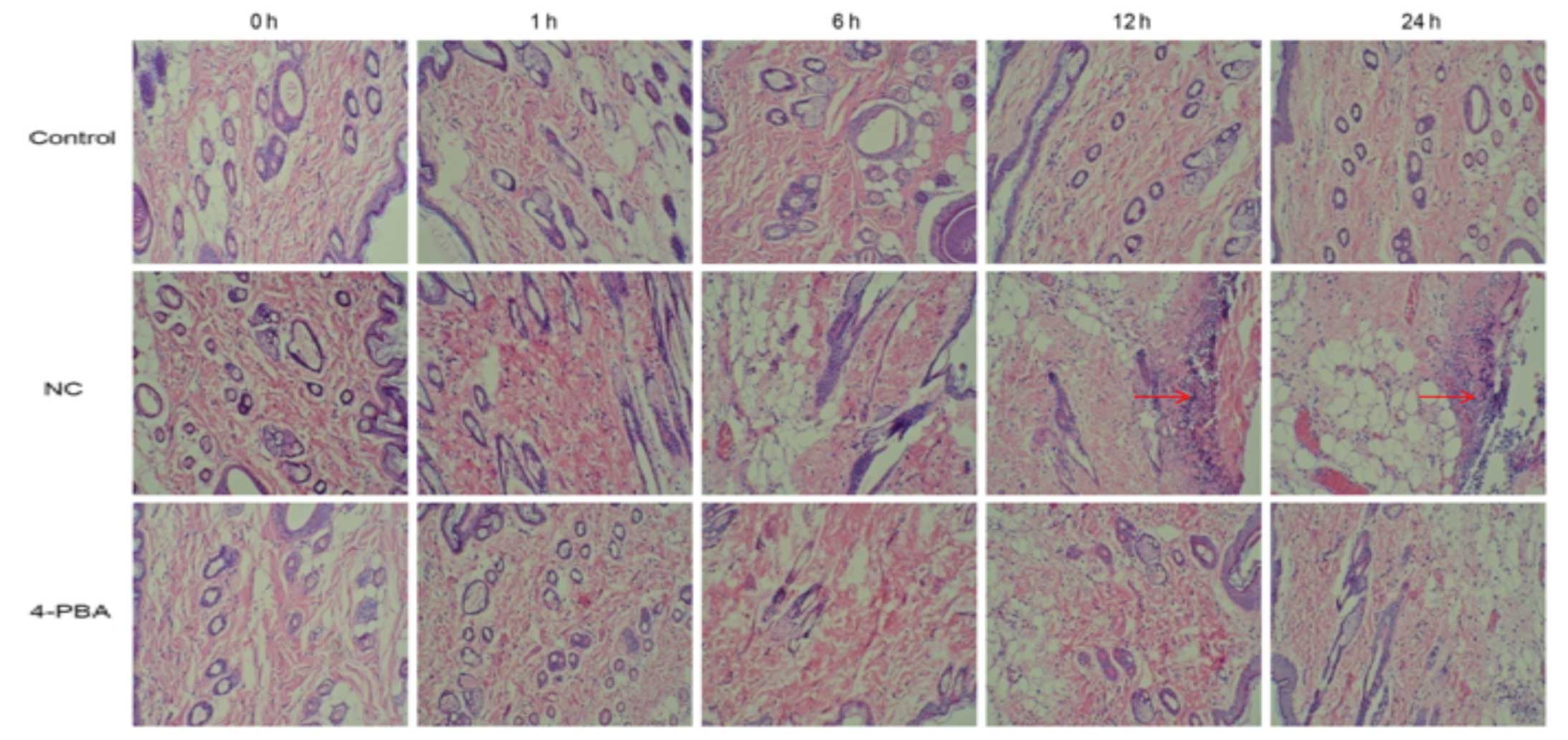
Histological analyses detected significant swelling
and increased numbers of inflammatory cells in the tissue space of
the NC group at 6 h, and the injury increased in severity 24 h
following ischemia-reperfusion. Conversely, the morphological
structure of the control group was normal, and no such changes were
observed. The presence of abnormal cells was markedly increased in
the NC group, as compared with in the 4-PBA group, in which damaged
and inflammatory cells, and swelling in the tissue sections were
rarely observed (Fig. 1).
4-PBA inhibits cell apoptosis in the skin
flap
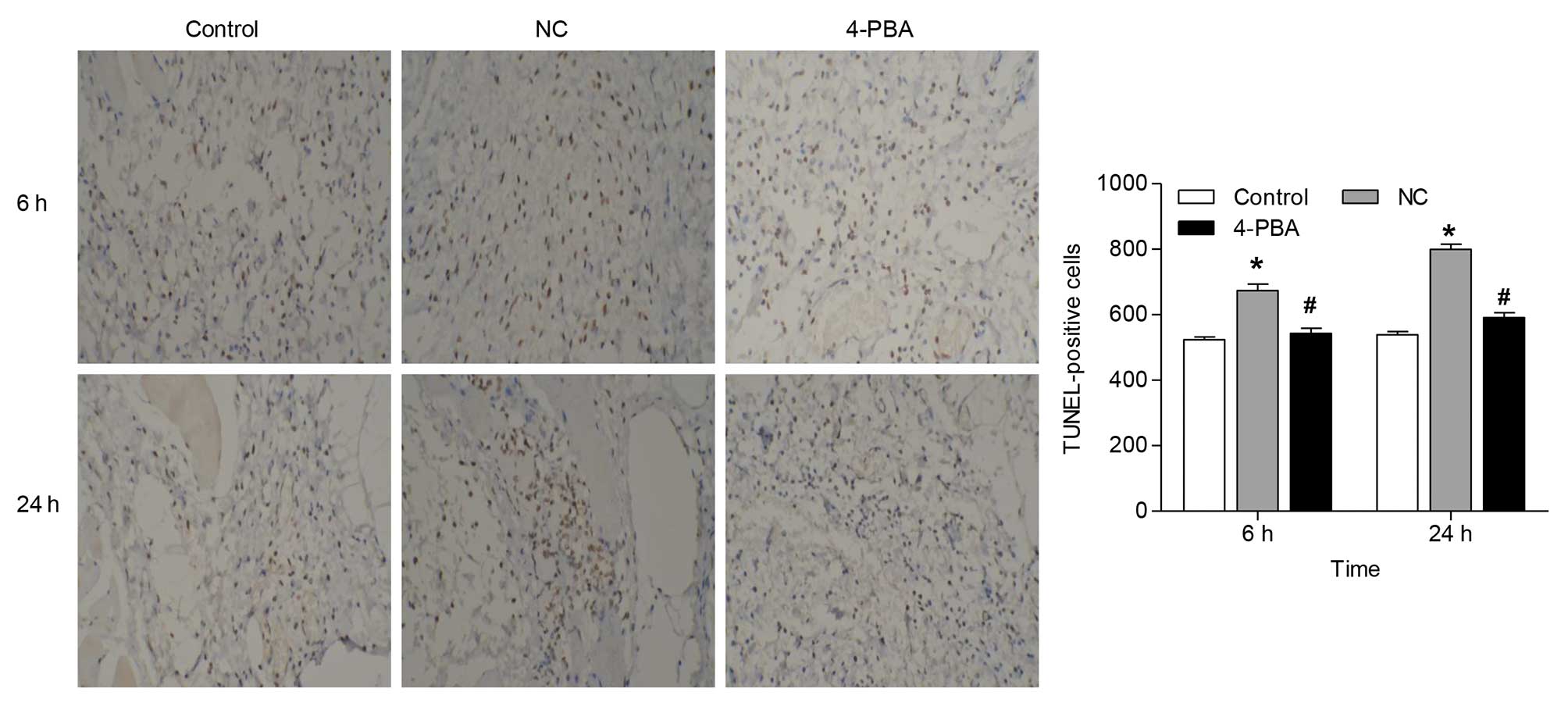
To determine whether 4-PBA was associated with
antiapoptotic activity, apoptosis was evaluated using a TUNEL
assay. The results demonstrated an increase in the number of
TUNEL-positive cells to 28.6 and 48.4%, 6 and 24 h following
ischemia-reperfusion injury, respectively. Treatment with 4-PBA
significantly decreased the number of TUNEL-positive cells in the
skin flap by ~19.4 and 26.1%, as compared with administration of
saline, at 6 and 24 h following ischemia-reperfusion (Fig. 2). Furthermore, the number of
TUNEL-positive cells in the NC group increased in a time-dependent
manner, with a statistically significant increase 6 h following
ischemia-reperfusion.
4-PBA suppresses the mRNA expression of
CHOP and GRP78 in the skin flap
To evaluate whether ER stress was involved in
ischemia-reperfusion injury in the skin flap, the mRNA expression
levels of CHOP and GRP78 were quantified in the skin flap tissue
samples of the rats. The mRNA expression levels of CHOP were
increased by ~61.1, 88.9 and 161% at 6, 12, and 24 h following
ischemia-reperfusion, respectively, as compared with the control
group at 14, 20 and 32 h following skin flap creation (Fig. 3A). The mRNA expression levels of
GRP78 were increased by ~34.0, 64.7 and 126% at 6, 12, and 24 h
following ischemia-reperfusion, respectively, as compared with the
control group at 6, 12 and 24 h following skin flap creation
(Fig. 3B).
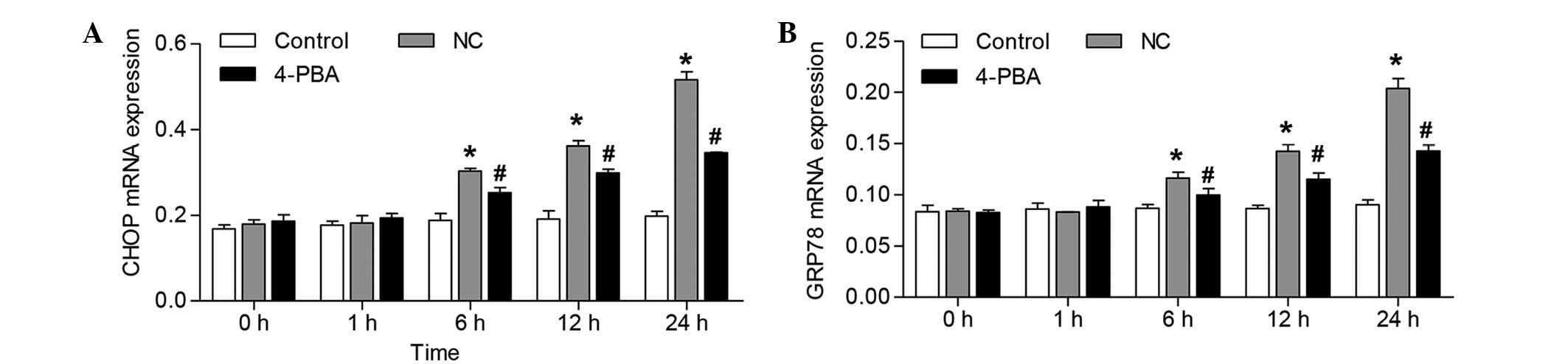 | Figure 3Effects of 4-PBA on the mRNA
expression levels of CHOP and GRP78 in the skin flap tissue samples
of rats following ischemia-reperfusion. Reverse
transcription-quantitative polymerase chain reaction analysis of
(A) CHOP and (B) GRP78 in the skin flap tissue samples of the rats.
Expression levels were detected 0, 1, 6, 12, and 24 h following
ischemia-reperfusion. The error bars represent the mean ± standard
deviation from five rats per group. *P<0.05, vs. the
control group; #P<0.05, vs. the rats treated with
saline (NC). NC, negative control; 4-PBA, 4-phenylbutyrate; CHOP,
CCAAT/enhancer-binding protein-homologous protein; GRP78,
glucose-regulated protein 78. |
The increases in CHOP and GRP78 mRNA expression
levels were significantly reduced following administration of 4-PBA
from 6–24 h (~16.8, 17.4 and 32.9% reduction in CHOP expression
levels, and ~14.2, 19.2 and 29.9% reduction in GRP78 expression
levels), as compared with the NC group (Fig. 3).
4-PBA inhibits the upregulation of CHOP
and GRP78 protein expression in skin flap tissue samples
The protein expression levels of CHOP and GRP78 were
examined by western blot analysis. The results demonstrated that
the protein expression levels of CHOP and GRP78 were similar to the
mRNA expression levels, and were significantly increased 24 h
following ischemia-reperfusion (Fig.
4A). Pretreatment with 4-PBA significantly inhibited the
increases in protein expression levels of CHOP and GRP78 in the
skin flap tissue samples at 6 and 24 h following
ischemia-reperfusion.
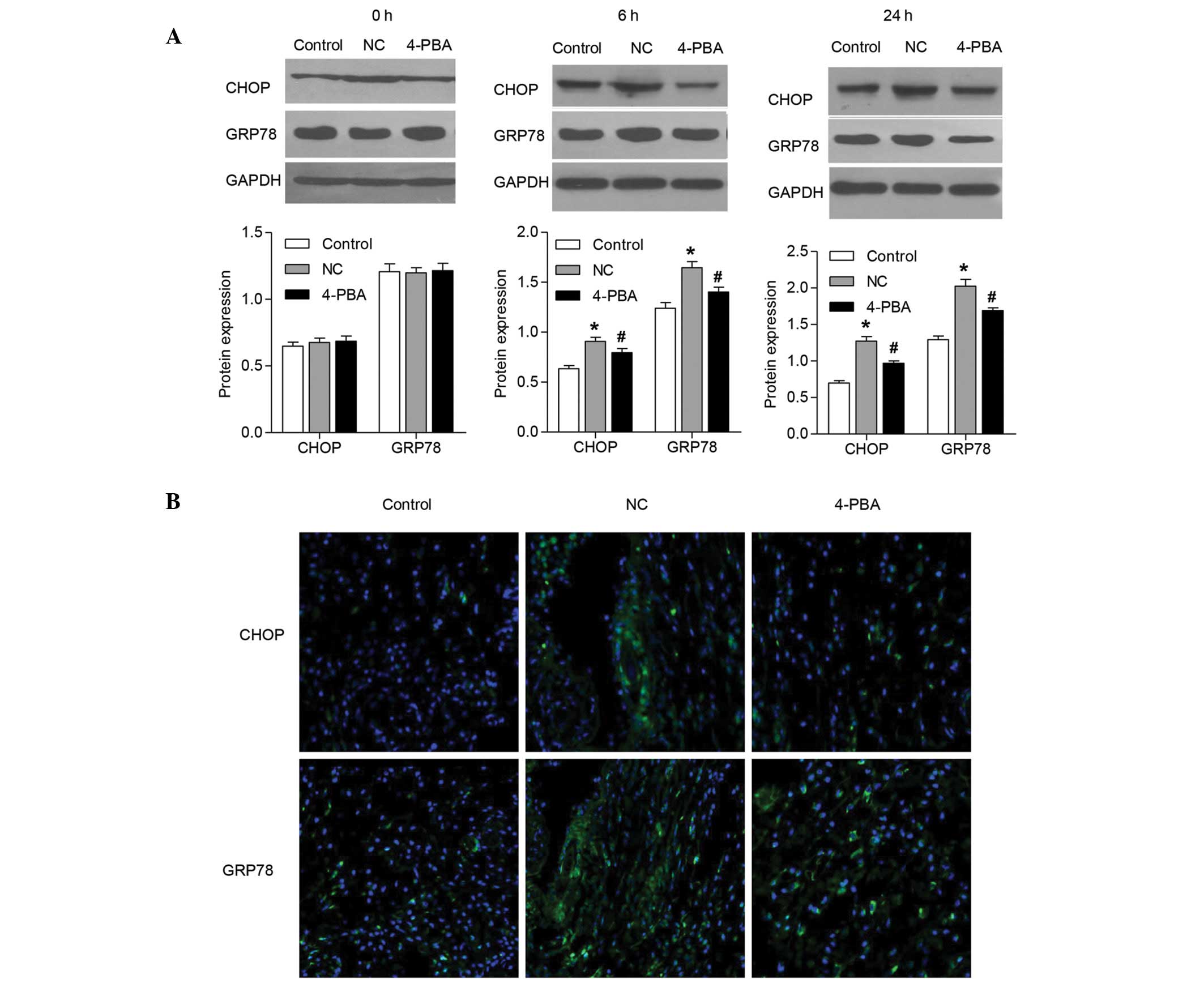 | Figure 4Effects of 4-PBA on the protein
expression of CHOP and GRP78 in rat skin flaps following
ischemia-reperfusion. CHOP and GRP78 protein expression levels were
detected in the skin flap tissue samples (A) 6 and 24 h following
ischemia-reperfusion by western blotting, and (B) 24 h following
ischemia-reperfusion by immunofluorescence staining. Error bars
represent the mean ± standard deviation from five rats/group.
*P<0.05, vs. the control group;
#P<0.05, vs. the rats treated with saline (NC).
Green, CHOP and GRP78 staining; blue, nuclei staining with
4′,6-diamidino-2-phenylindole. NC, negative control; 4-PBA,
4-phenylbutyrate; CHOP, CCAAT/enhancer-binding protein-homologous
protein; GRP78, glucose-regulated protein 78; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
To further investigate the hypothesis that CHOP and
GRP78 expression levels were increased in the rat skin flap tissue
samples following ischemia-reperfusion injury, these proteins were
visualized by immunofluorescence staining (Fig. 4B). Fluorescence microscopy revealed
that the expression of CHOP and GRP78 were markedly increased at 24
h following ischemia-reperfusion, as compared with the control
group. However, following pretreatment with 4-PBA, the increases in
CHOP and GRP78 protein expression were significantly inhibited at
24 h in the skin flap tissue samples following
ischemia-reperfusion.
Discussion
To the best of our knowledge, the results of the
present study demonstrated for the first time that the intragastric
administration of 4-PBA at therapeutic doses protected rat skin
flaps against ischemia-reperfusion injury. The protective effects
of 4-PBA were further demonstrated by an inhibition of ER stress,
and a decrease in the number of apoptotic cells. Notably,
administration of 4-PBA was effective not only prior to but also
following ischemia-reperfusion injury.
Ischemia-reperfusion injury is a severe limitation
in the survival of tissues involved in reconstructive microsurgery,
and skeletal muscles and skin flaps are particularly susceptible
(23–26). 4-PBA is a potent ER stress
inhibitor and numerous studies have reported the effect of 4-PBA on
ischemia-reperfusion injury (27,28).
Consistent with these observations, the results of the present
study demonstrated that pretreatment with 4-PBA markedly attenuated
ischemia-reperfusion injury in the skin flap of rats, and damaged
and inflammatory cells and tissue swelling were rarely observed. In
addition, the data demonstrated that 4-PBA attenuated ER
stress-mediated cell apoptosis in the rat skin flaps following
ischemia-reperfusion injury. Therefore, targeting the ER-mediated
apoptotic signaling pathway may be an effective strategy for the
treatment of cellular injury.
ER stress is involved in the pathogenesis of various
cardiovascular diseases, and promotes disease progression (29). CHOP is a downstream component of ER
stress at the convergence of the endoplasmic reticulum-to-nucleus
signaling 1, protein kinase RNA-like endoplasmic reticulum kinase
and activating transcription factor-6 signaling pathways (30–33).
In the early stages of ER stress, GRP78 expression is upregulated,
and in prolonged ER stress over-expression of CHOP is observed. In
the present study, the mRNA and protein expression levels of CHOP
and GRP78 were significantly increased in a time-dependent manner 6
h following ischemia-reperfusion injury in the skin flap tissue
samples of the rats. These findings suggested that CHOP and GRP78
are two important targets for therapeutic intervention, which may
allow the inhibition of ischemia-reperfusion injury progression in
the skin flap of rats.
Previous studies have suggested that 4-PBA is
implicated in the inhibition of ER stress-mediated ischemic injury
via the transcriptional regulation of CHOP and GRP78 (27,33,34).
Concordant with these observations, the results of the present
study demonstrated that pretreatment with 4-PBA significantly
reduced the upregulation of CHOP and GRP78 in the skin flaps of the
rats following ischemia-reperfusion. These alterations in CHOP and
GRP78 expression levels were further supported by
immunofluorescence staining. These results suggested that ER stress
is important for the induction and maintenance of
ischemia-reperfusion injury, and 4-PBA attenuates ER stress in this
pathological condition.
In conclusion, the results of the present study
suggested that 4-PBA protects against skin flap
ischemia-reperfusion injury, and the mechanism underlying this
process involves the inhibition of ER stress-mediated apoptosis and
expression of ER stress markers, CHOP and GRP78. In addition, the
study presented evidence that targeting the ER may provide a
therapeutic approach for blocking the apoptotic process induced by
skin flap ischemia. Finally, we proposed that the therapeutic
potential of 4-PBA may extend to other ER stress-associated
diseases.
Acknowledgments
The present study was supported by the Scientific
Research Project of the Hangzhou Department of Technology (grant
no. 20130733Q44).
References
|
1
|
Yao ST: Vascular implantation into skin
flap: Experimental study and clinical application: A Preliminary
report. Plast Reconstr Surg. 68:404–409. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manson PN, Anthenelli RM, Im MJ, Bulkley
GB and Hoopes JE: The role of oxygen-free radicals in ischemic
tissue injury in island skin flaps. Ann Surg. 198:87–90. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hosoi T and Ozawa K: Endoplasmic reticulum
stress in disease: Mechanisms and therapeutic opportunities. Clin
Sci (Lond). 118:19–29. 2009. View Article : Google Scholar
|
|
5
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anelli T and Sitia R: Protein quality
control in the early secretory pathway. EMBO J. 27:315–327. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim I, Shu CW, Xu W, Shiau CW, Grant D,
Vasile S, Cosford ND and Reed JC: Chemical biology investigation of
cell death pathways activated by endoplasmic reticulum stress
reveals cytoprotective modulators of ASK1. J Biol Chem.
284:1593–1603. 2009. View Article : Google Scholar :
|
|
9
|
Endo M, Mori M, Akira S and Gotoh T: C/EBP
homologous protein (CHOP) is crucial for the induction of
caspase-11 and the pathogenesis of lipopolysaccharide-induced
inflammation. J Immunol. 176:6245–6253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Wang JJ, Yu Q, Wang M and Zhang SX:
Endoplasmic reticulum stress is implicated in retinal inflammation
and diabetic retinopathy. FEBS Lett. 583:1521–1527. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leem J and Koh EH: Interaction between
mitochondria and the endoplasmic reticulum: Implications for the
pathogenesis of type 2 diabetes mellitus. Exp Diabetes Res.
2012:2429842012. View Article : Google Scholar
|
|
12
|
Kang HL, Benzer S and Min KT: Life
extension in Drosophila by feeding a drug. Proc Natl Acad Sci USA.
99:838–843. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andreassi C, Angelozzi C, Tiziano FD,
Vitali T, De Vincenzi E, Boninsegna A, Villanova M, Bertini E, Pini
A, Neri G and Brahe C: Phenylbutyrate increases SMN expression in
vitro: Relevance for treatment of spinal muscular atrophy. Eur J
Hum Genet. 12:59–65. 2004. View Article : Google Scholar
|
|
14
|
Dasgupta S, Zhou Y, Jana M, Banik NL and
Pahan K: Sodium phenylacetate inhibits adoptive transfer of
experimental allergic encephalomyelitis in SJL/J mice at multiple
steps. J Immunol. 170:3874–3882. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burrows JA, Willis LK and Perlmutter DH:
Chemical chaperones mediate increased secretion of mutant
alpha1-antitrypsin (alpha1-AT) Z: A potential pharmacological
strategy for prevention of liver injury and emphysema in alpha 1-AT
deficiency. Proc Natl Acad Sci USA. 97:1796–1801. 2000. View Article : Google Scholar
|
|
16
|
Rubenstein RC and Zeitlin PL: Sodium
4-phenylbutyrate down-regulates Hsc70: Implications for
intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell
Physiol. 278:C259–C267. 2000.PubMed/NCBI
|
|
17
|
Perlmutter DH: Chemical chaperones: A
pharmacological strategy for disorders of protein folding and
trafficking. Pediatr Res. 52:832–836. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yam GH, Gaplovska-Kysela K, Zuber C and
Roth J: Sodium 4-phenylbutyrate acts as a chemical chaperone on
misfolded myocilin to rescue cells from endoplasmic reticulum
stress and apoptosis. Invest Ophthalmol Vis Sci. 48:1683–1690.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimamatsu K and Wanless IR: Role of
ischemia in causing apoptosis, atrophy, and nodular hyperplasia in
human liver. Hepatology. 26:343–350. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kabeer FA, Sreedevi GB, Nair MS,
Rajalekshmi DS, Gopalakrishnan LP, Kunjuraman S and Prathapan R:
Antineoplastic effects of deoxyelephantopin, a sesquiterpene
lactone from Elephantopus scaber, on lung adenocarcinoma (A549)
cells. J Integr Med. 11:269–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Payton JE, Grieselhuber NR, Chang LW,
Murakami M, Geiss GK, Link DC, Nagarajan R, Watson MA and Ley TJ:
High throughput digital quantification of mRNA abundance in primary
human acute myeloid leukemia samples. J Clin Invest. 119:1714–1726.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hosoi T, Okuma Y and Nomura Y: Expression
of leptin receptors and induction of IL-1beta transcript in glial
cells. Biochem Biophys Res Commun. 273:312–315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kerrigan CL and Stotland MA: Ischemia
reperfusion injury: A review. Microsurgery. 14:165–175. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hatoko M, Tanaka A, Kuwahara M, Yurugi S,
Iioka H and Niitsuma K: Difference of molecular response to
ischemia-reperfusion of rat skeletal muscle as a function of
ischemic time: Study of the expression of p53, p21(WAF-1), Bax
protein, and apoptosis. Ann Plast Surg. 48:68–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuo YR, Jeng SF, Wang FS, Huang HC, Wei FC
and Yang KD: Platelet glycoprotein IIb/IIIa receptor antagonist
(abciximab) inhibited platelet activation and promoted skin flap
survival after ischemia/reperfusion injury. J Surg Res. 107:50–55.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siemionow M and Arslan E:
Ischemia/reperfusion injury: A review in relation to free tissue
transfers. Microsurgery. 24:468–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi X, Hosoi T, Okuma Y, Kaneko M and
Nomura Y: Sodium 4-phenylbutyrate protects against cerebral
ischemic injury. Mol Pharmacol. 66:899–908. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mizukami T, Orihashi K, Herlambang B,
Takahashi S, Hamaishi M, Okada K and Sueda T: Sodium
4-phenylbutyrate protects against spinal cord ischemia by
inhibition of endoplasmic reticulum stress. J Vasc Surg.
52:1580–1586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ono Y, Shimazawa M, Ishisaka M, Oyagi A,
Tsuruma K and Hara H: Imipramine protects mouse hippocampus against
tunicamycin-induced cell death. Eur J Pharmacol. 696:83–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakayama Y, Endo M, Tsukano H, Mori M,
Oike Y and Gotoh T: Molecular mechanisms of the LPS-induced
non-apoptotic ER stress-CHOP pathway. J Biochem. 147:471–483. 2010.
View Article : Google Scholar
|
|
31
|
Vilatoba M, Eckstein C, Bilbao G, Smyth
CA, Jenkins S, Thompson JA, Eckhoff DE and Contreras JL: Sodium
4-phen-ylbutyrate protects against liver ischemia reperfusion
injury by inhibition of endoplasmic reticulum-stress mediated
apoptosis. Surgery. 138:342–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim HJ, Jeong JS, Kim SR, Park SY, Chae HJ
and Lee YC: Inhibition of endoplasmic reticulum stress alleviates
lipopolysaccharide-induced lung inflammation through modulation of
NF-κB/HIF-1α signaling pathway. Sci Rep. 3:11422013. View Article : Google Scholar
|
|
33
|
Wang XB, Huang XM, Ochs T, Li XY, Jin HF,
Tang CS and Du JB: Effect of sulfur dioxide preconditioning on rat
myocardial ischemia/reperfusion injury by inducing endoplasmic
reticulum stress. Basic Res Cardiol. 106:865–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peralta C and Brenner C: Endoplasmic
reticulum stress inhibition enhances liver tolerance to
ischemia/reperfusion. Curr Med Chem. 18:2016–2024. 2011. View Article : Google Scholar : PubMed/NCBI
|