Introduction
Graft-versus-host disease (GVHD), mediated by mature
T cells in the donor graft, remains a major complication following
allogeneic bone marrow transplantation. Alloreactive T cells
involved in the generation of acute GVHD can be derived from a
naïve T cell pool (1). The core of
the GVHD reaction is donor T cell activation, in which donor T
cells proliferate, differentiate and produce cytokines. Several
studies have focussed more attention on the role of the
CD4+ T cell subsets involved in the development of acute
GVHD. Th1 cells secreting pro-inflammatory cytokines, including
interferon (IFN)-γ and tumor necrosis factor (TNF)-α, which are
considered to be responsible for driving cellular immune responses,
have been demonstrated to be etiological factors in the induction
of GVHD (2,3). Th17 cells, which are characterized by
the production of interleukin (IL)-17A, IL-17F, IL-21 and IL-22,
have been demonstrated to be sufficient, but not necessary, to
induce GVHD (4,5). In addition experimental data in mice
and humans have demonstrated the potential of Th cell subsets to
exhibit plasticity, shifting between phenotypes (6). The above observations suggest that
the Th1 and Th17 cells involved in GVHD have roles in the network.
Although efforts have been focussed on investigating the solitary
function of these cells, the correlation of Th1 and Th17 cells in
GVHD process remain to be fully elucidated.
It is important to considered that, despite the
prominent role of CD4+ T cells in the pathogenesis of
GVHD, the function of CD8+ T cells in GVHD cannot be
ignored.
Several studies have reported the function of
CD8+ T cells as cytotoxic T lymphocytes in the final
cellular and inflammatory effector phase III of GVHD. It has been
reported that donor cytotoxic double deficient
(perforin−/− and FasL−/−) CD8+ T
cells expand continuously and caus life-threatening GVHD (7,8).
However, during phase II of GVHD, characterized by T cell
activation and polarization, evidence of the effects of
CD8+ T cells remains limited. Furthermore, certain
findings support the hypothesis that donor anti-host cytotoxicity
via the two major pathways (perforin and FasL) is not a
prerequisite for the induction of GVHD (9). Therefore, the role of donor
CD8+ T cells in the pathogenesis of GVHD is not only
limited to the perforin or FasL pathways.
Classically, CD8+ T cells have been
assigned to Tc1 or Tc2 lineages, based on the cytokines produced by
these cells. Notably, it has been reported that naive
CD8+ T cells can also differentiate into IL-17-producing
T cells, termed Tc 17cells, in the same culture conditions as
CD4+ T cells (10).
However, there are no associated reports regarding the generation
and role of Tc17 cells during GVHD. In addition, although a common
idea regarding the activation of CD4+ T subsets and
CD8+ T subsets, the pathophysiological link between them
during the process of GVHD remains poorly understood (11). Therefore, the involvement of
alloreactive donor T-cell responses, and whether T cell
polarization leads to distinct targeted tissue damage, remain to be
fully elucidated and require investigation.
In the present study, the dynamic changes of
alloantigen specific effector CD4+ T and CD8+
T cell subsets, which produce inflammatory cytokines involved in
the multistep GVHD pathogenesis progress were investigated. These
cells and cytokines act as critical links between the occurrence
and progression of GVHD. The present study may provide novel
information to clarify the mechanism of GVHD.
Materials and methods
Mice
Male C57BL/6 (H-2Kb) mice, as donors, and BALB/c
(H-2Kd) mice, as recipients, aged between 6–8 weeks were purchased
from SLRC Laboratory Animal Centre, Co., Ltd. (Shanghai, China).
The mice were housed in individual cages (5 mice/cage) under
controlled temperature (21–23°C) and relative humidity (60–65%).
The mice underwent a 12/12 h light/dark cycle, and had ad
libitum access to a standard mouse diet and water. The mice
were bred in a specific pathogen-free facility at Xuzhou Medical
College (Xuzhou, China). All experiments were performed according
to the guidelines of the Institutional Animal Committee of Xuzhou
Medical College. The study was approved by the Laboratory Animal
Ethics Committee of Xuzhou Medical College (Jiangsu, China).
Induction and assessment of GVHD
The procedures used for the induction of GVHD were
as described as our previous study (12). Briefly, the BALB/c recipients
underwent 7.5 Gy total body irradiation (TBI) from a
60Co source (Beijing Kang Keda Technology Co., Ltd.,
Beijing, China), and bone marrow transplantation (BMT) was
performed via injecting the mice intravenously with bone marrow
cells (5×106/mouse) from the donor mice 4 h
post-irradiation. Bone marrow was flushed from the medullary cavity
of the femur and tibia using phosphate-buffered saline (PBS). For
the induction of GVHD, donor splenic T cells, which were isolated
using flow cytometry (FACSCalibur; BD Pharmingen, San Diego, CA,
USA), were co-transferred (5×105/mouse) with the bone
marrow cells to the recipient mice (BS; bone marrow cells+splenic T
cells). The transplantation day was set as day 0. The experimental
and control groups were established at the same time and
experiments were replicated 2–3 times. The recipients were
monitored daily to assess survival, and every 2–3 days to assess
changes in body weight. Mice were sacrificed by cervical
dislocation and specimens (1 cm × 1 cm × 5 mm) of the liver, gut
and lung of the recipients were fixed in formalin (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) prior to being
embedded in paraffin (Sinopharm Chemical Reagent Co., Ltd.) blocks.
The tissue sections were stained with hematoxylin and eosin
(H&E; Sinopharm Chemical Reagent Co., Ltd.) for
histopathological detection. Liver GVHD was evaluated on the
severity of liver cell necrosis and inflammatory infiltration. Gut
GVHD was evaluated on the level of inflammation in the epithelium
and lamina propria. Lung GVHD was evaluated on the severity of
peri-bronchic infiltration and pneumonitis.
Media and antibodies
RPMI-1640 media was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA) and supplemented with 10%
heat-inactivated fetal calf serum (FCS), 0.1% 2-methoxyestradiol,
100 U/ml penicillin and 100 µg/ml streptomycin. Monoclonal
rat anti-mCD8-fluorescein isothiocyanate (FITC; cat. no. 553031),
rat anti-mCD4-peridinin chlorophyll (PerCP; cat. no. 553052), rat
anti-mIFN-γ-allophycocyanin (APC; cat. no. 554413), and isotype
control antibodies were purchased from BD Pharmingen. The rat
anti-mIL-17a-APC (cat. no. 506916), rat anti-mCD8-phycoerythrin
(PE; cat. no. 100707), rat anti-mCD4-FITC (cat. no. 100510), rat
anti-mCD3-PerCP-Cy5.5 (cat. no. 100328), rat anti-mCD45-PerCP (cat.
no. 103130), rat anti-H-2Kb-PE (cat. no. 116507) and rat
anti-mCD44-PerCP-Cy5.5 (cat. no. 103031) were obtained from
BioLegend, Inc. (San Diego, CA, USA). Rat anti-mCD62L-PE (cat. no.
12-0621) was provided by eBioscience, Inc. (San Diego, CA, USA).
The concentration of all antibodies used was 1 µg/ml
(1:200).
Cytometric bead array analysis for
cytokines
Plasma from each of the each groups was obtained 7,
14, 40 and 50 days following transplantation, respectively. The
production of IL-6, TNF-α, IL-2, IFN-γ, IL-4 and IL-10 cytokines
were measured with a cytometric bead array (CBA) using flow
cytometry (FacsCalibur, BD Pharmingen), according the
manufacturer's instructions. Briefly, six bead populations specific
for IL-6, TNF-α, IL-2, IFN-γ, IL-4 and IL-10 with distinct
fluorescence intensities were coated with capture antibodies
specific for different cytokines. Following incubation of the beads
with 50 µl diluted plasma (2-fold dilution), different
cytokines in the sample were captured by their corresponding beads.
The cytokine-captured beads were then mixed with PE-conjugated
detection antibodies to form sandwich complexes. Following
incubation for 20 min at room temperature in the dark, the
fluorescent samples were washed, harvested and analyzed using
CellQuest version 6 software(BD Pharmingen).
Intracellular cytokine and cell surface
staining
The mice were sacrificed by cervical spine fracture
7, 14, 28, 40, 50 and 60 days following transplantation. The
spleens and bone marrow cells from each mouse were harvested, and
lymphocytes from the spleen were isolated using mouse lymphocyte
separation medium (Dakewe, China), with a purity of 90%. The single
lymphocyte suspension was incubated with or without 50 ng/ml
phorbol myristate acetate (Sigma-Aldrich, St. Louis, MO, USA) with
750 ng/ml ionomycin (Sigma-Aldrich) in the presence of 10
µg/ml brefeldin A (Invitrogen Life Technologies) at 37°C for
5 h. The cells were washed, fixed with 4% paraformaldehyde
(Sinopharm Chemical Reagent Co., Ltd.) and permeabilized in PBS
containing 0.1% saponin (Sigma-Aldrich), 0.1% bovine serum albumin
and 0.05% NaN3 overnight at 4°C. The cells were then
stained with conjugated monoclonal antibodies for the CD3, CD4,
CD8, CD62L and CD44 surface markers and IFN-γ and IL-17a
intracellular cytokines for 20 min in the dark. The cells were
acquired using a flow cytometer (FacsCalibur, BD Pharmingen) and
data were analyzed using CellQuest Pro software (version 6.0; BD
Pharmingen). Isotype controls were included for each staining.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons were performed using a non-parametric t-test for
two-group comparisons, or one-way analysis of variance for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
GVHD evaluation
GVHD was routinely induced in BALB/c mice
(H-2Kd) by transplantation of bone marrow cells combined
with splenic T cells from C57BL/6 mice (H-2Kb). The
reconstituted immune system was assessed 7 and 14 days following
transplantation using flow cytometry. Bone marrow cells were
collected and stained with anti-mouse CD45 and anti-mouse
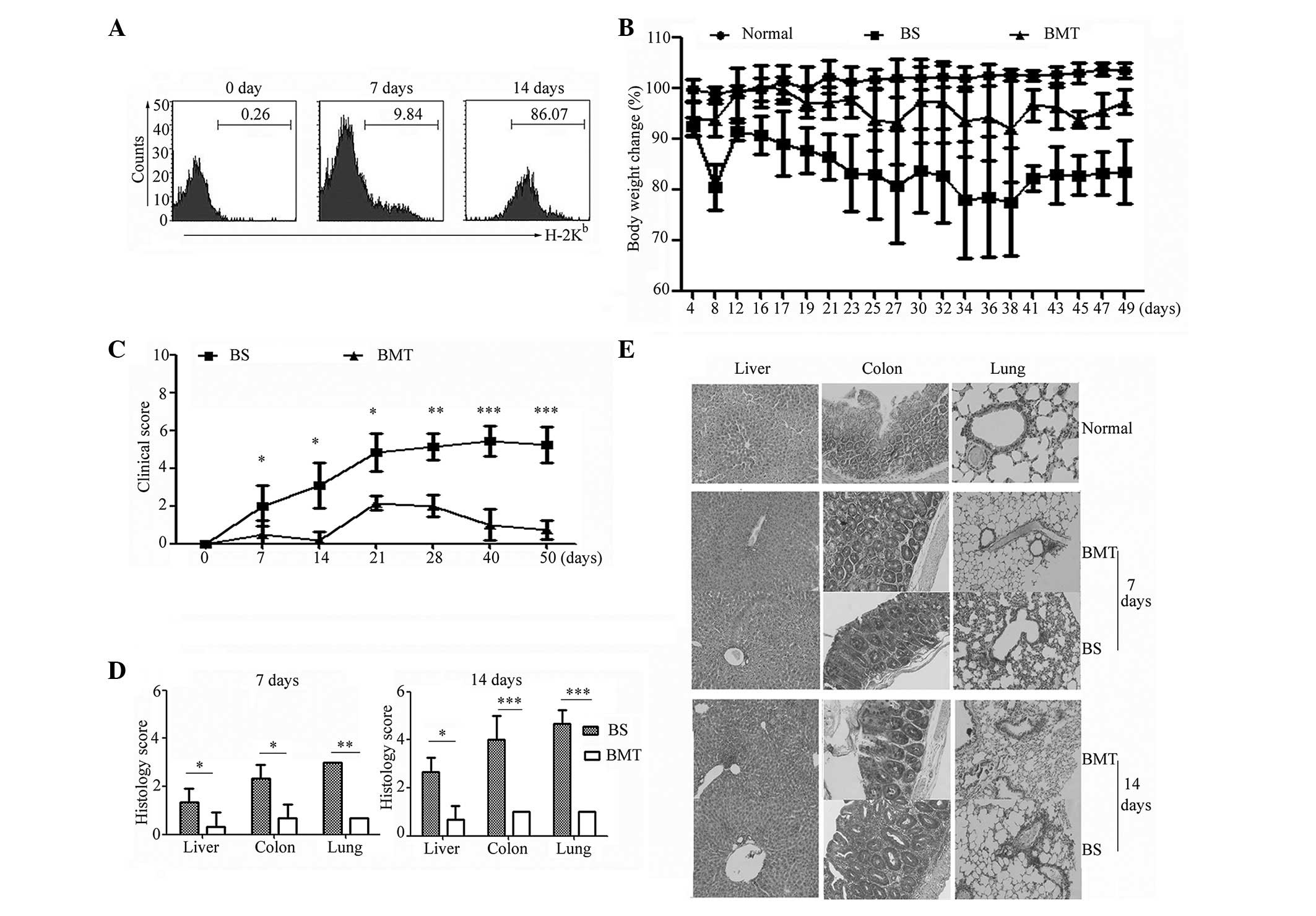
H-2Kb antibodies. As shown in Fig. 1A, the results demonstrated that a
low level of donor-derived cells was detected on day 7, whereas the
CD45+ cells were almost all donor origin
(H-2Kb) on day 14 in the BS groups, indicating a
successful reconstituted immune system inthe recipients.
Subsequently, the onset and progression of GVHD were observed. As
shown in Fig. 1B, the results
revealed that the recipients in the BS group exhibited more severe
weight loss, compared with the recipients in the BMT and normal
groups. As shown in Fig. 1C and D,
the clinical score and histopathology score of the GVHD targets,
including the liver, colon and lung, were analyzed on day 7 and 14.
A higher score was detected from day 7 in the BS mice, compared
with the BMT mice, and statistical analysis demonstrated that this
was a significant difference (P<0.05). As shown in Fig. 1E, marginal perivascular
infiltration was detected in the lung of the BS mice 7 days
post-transplantation. As time progressed, higher levels of severe
tissue damage and higher histopathology scores were detected in the
BS mice than the BMT mice on day 14. In the BS groups, the liver
exhibited marked portal and lobular inflammation with mononuclear
and granulocytic infiltrates. The colon specimens from the BS mice
exhibited marked infiltration of mononuclear and granulocytic cells
into the lamina propria, with extensive goblet cell depletion. More
severe perivascular cuffing, vasculitis and peribronchiolar cuffing
were observed in the lung specimens of the BS recipients, compared
with the BMT mice (Fig. 1E). The
tissue sections from the normal control mice exhibited no
abnormality. Taken together, these results demonstrated that the
GVHD model was successfully established in the BS mice in the
present study, and that more severe tissue lesions were detected on
day 14 following allogenic transplantation.
Alloreactive effector IFN-γ+
and IL-17+ T cell subsets are involved at different
time-points
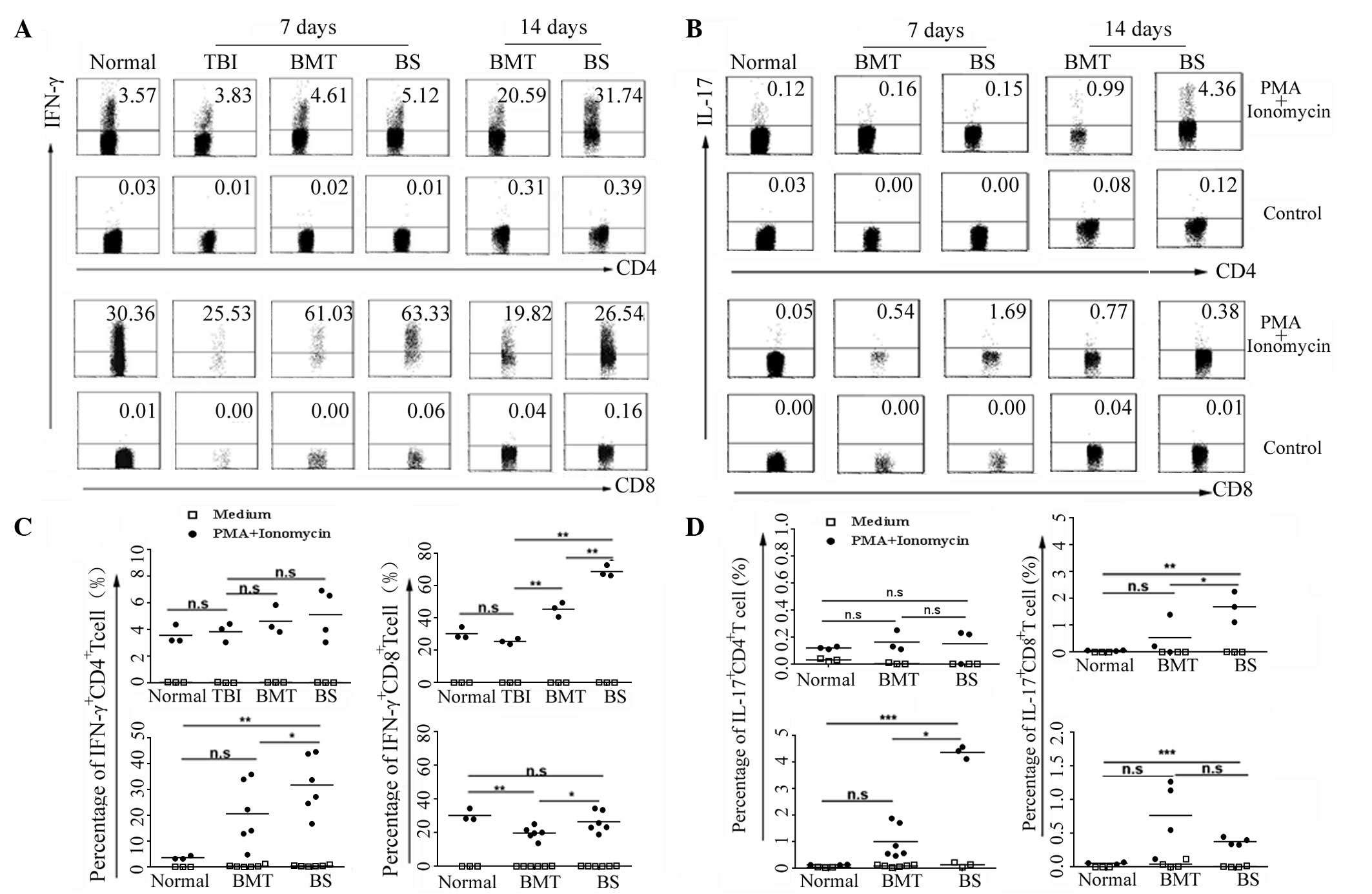
To examine the correlation between IFN-γ-producing
and IL-17-producing T cells following allogeneic transplantation,
the present study isolated splenic lymphocytes from normal, TBI,
BMT and BS mice on days 7 and 14 respectively. Lymphocytes were
cultured for 5 h with or without phorbol 12-myristate 13-acetate
and ionomycin, and were stained with anti-CD3, anti-CD4 and
anti-CD8 antibodies to set gates. Subsequent intracellular cytokine
staining was performed using anti-mouse-IFN-γ antibody. The
CD4+ IFN-γ+ T cells represented Th1 cells and
the CD8+ IFN-γ+ T cells represented Tc1
cells. The percentages of Th1 cells and Tc1 cells were compared 7
days post-transplantation. As shown in Fig. 2A and B, the percentages of the Th1
cells in the normal, TBI, BMT and BS mice were at a similar level,
and statistical analysis revealed no significant differences
(P>0.05). However, the percentage of Tc1 cells in the BS mice
was significantly higher than those in the normal, TBI and BMT mice
(P<0.01). An increased percentage of Th1 cells and a reduced
percentage of Tc1 cells were observed in the BS mice on day 14
post-transplantation, which were higher than those in the BMT mice
(P<0.05). These data suggested that Tc1 cells were induced in
the initial phase of GVHD, and the involvement of Th1 cells in the
process of GVHD was later than that of the Tc1 cells.
With the exception of Th1 cells, the Th17 cell as a
novel Th cell subset has been demonstrated to be crucial in the
induction and development of GVHD. A novel population of
IL-17-expressing CD8+ effector T cell (Tc17) has been
identified in several diseases (13), however, its involvement in acute
GVHD have not been reported. The present study aimed to detect Th17
cells and Tc17 cells at different time following transplantation.
At 7 days post-transplantation for, the proportion of Th17 cells in
the BS mice was at a low level and was not significantly different
to the normal or BMT mice (P>0.05; Fig. 2C and D). However, the percentage of
Th17 cells in the BS mice increased markedly on day 14, whereas no
marked IL-17+CD4+ T cell involvement was
observed in the normal mice or BMT mice without GVHD. As shown in
Fig. 2D, increased production of
IL-17 in CD8+ T cells was observed in the BS mice, but
not in the BMT mice, on day 7. Compared with the normal and BMT
mice, the percentage of Tc17 cells induced in the BS mice was
higher (P<0.05). Further investigation demonstrated that the
proportion of Tc17 cells decreased marginally with the development
of GVHD, although it remained higher than that in the normal mice
(Fig. 2D). The above results
indicated that Th17 and Tc17 cells were induced in GVHD at
different phases and the predominant cellular source of IL-17 may
change during GVHD by Tc17 and Th17 cells.
Coordinated and dynamic changes in
IFN-γ+ and IL-17+ T cells
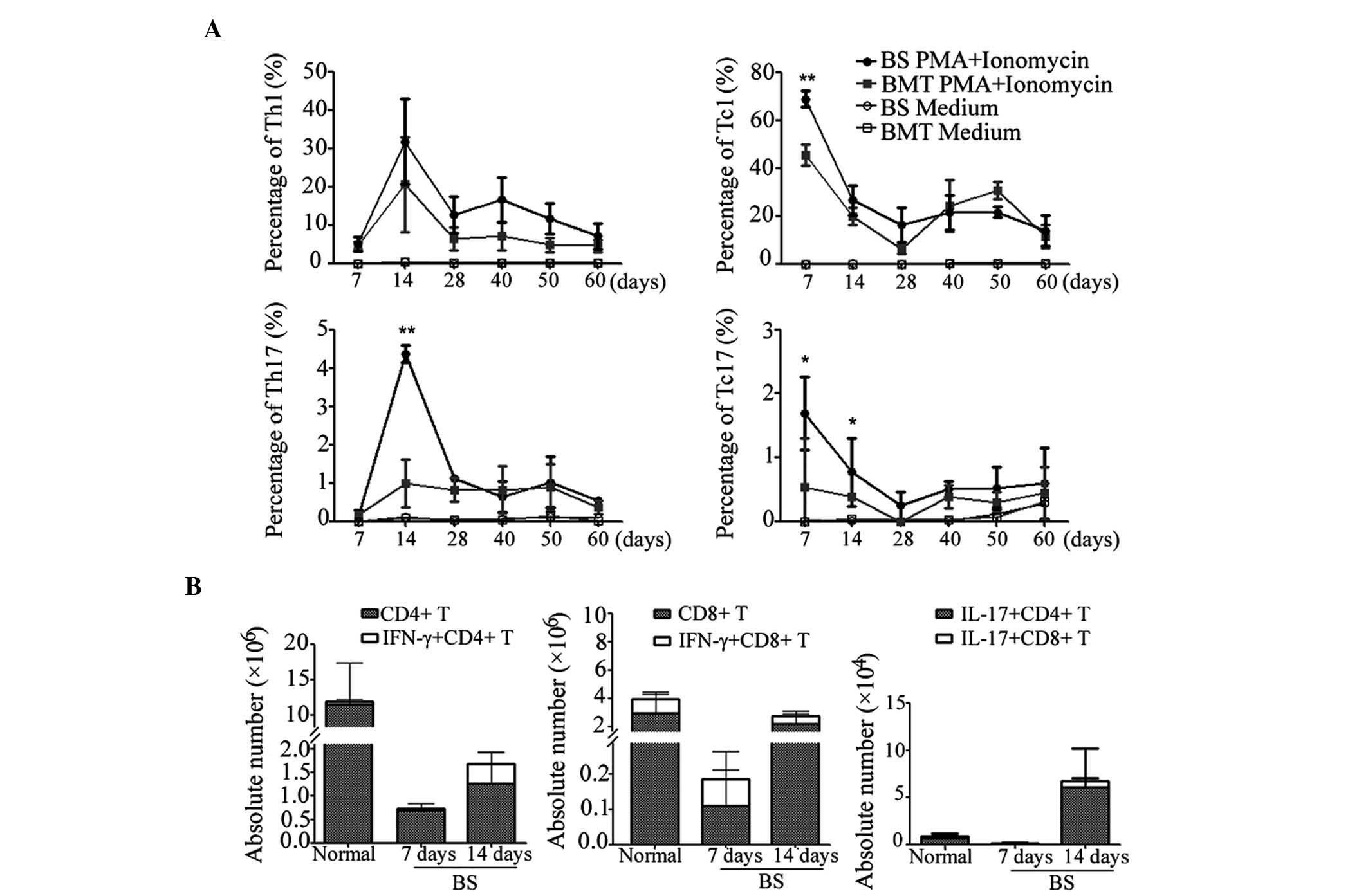
The present study subsequently detected the dynamic
changes in cytokine positive effector T cells through the
progression of GVHD. The percentage of effector CD4+ T
cell and CD8+ T cell subpupulations in the recipient
mice were investigated at different time-points. As shown in
Fig. 3A, the percentage of Th1
cells in the BS mice increased continuously between days 7 and 14,
whereas the Tc1 cells exhibited a decreasing trend. In the BS mice,
the Tc17 cells were sustained at a marginally higher level than in
the BMT mice, whereas the Th17 cells were markedly upregulated on
day 14. Subsequently, both of the effector CD4+ T cells
and CD8+ T cells began to decline following expansion as
time progressed.
The above data confirmed that self-reactive effector
T cells were induced and proliferated in the early phase of GVHD.
Therefore, the absolute number of T cell subsets were assessed in
the BS mice. As shown in Fig. 3B,
the number of total CD4+ and CD8+ T cells
increased between days 7 and 14, indicating T cell expansion. The
number of T cells in the BS mice was markedly lower than that in
the normal mice due to the pre-conditioning irradiation. However,
the numbers of effector T cells, including IFN-γ+ Th1
and Tc1 cells, and IL-17+ Th17 and Tc17 cells, were
higher than those in the normal mice, suggesting that more
activated T cells were generated in GVHD development. In addition,
compared with the CD4+ T cells, which were predominantly
activated on day 14, the CD8+ T cells exhibited activity
earlier.
Phenotype of CD4+ T and
CD8+ T cells in GVHD mice
The above data indicated that the donor-derived T
cells were activated and produced cytokines in the BS mice. The
present study subsequently evaluated the expression of T cell
activation markers in donor T cells 14 days post-transplantation.
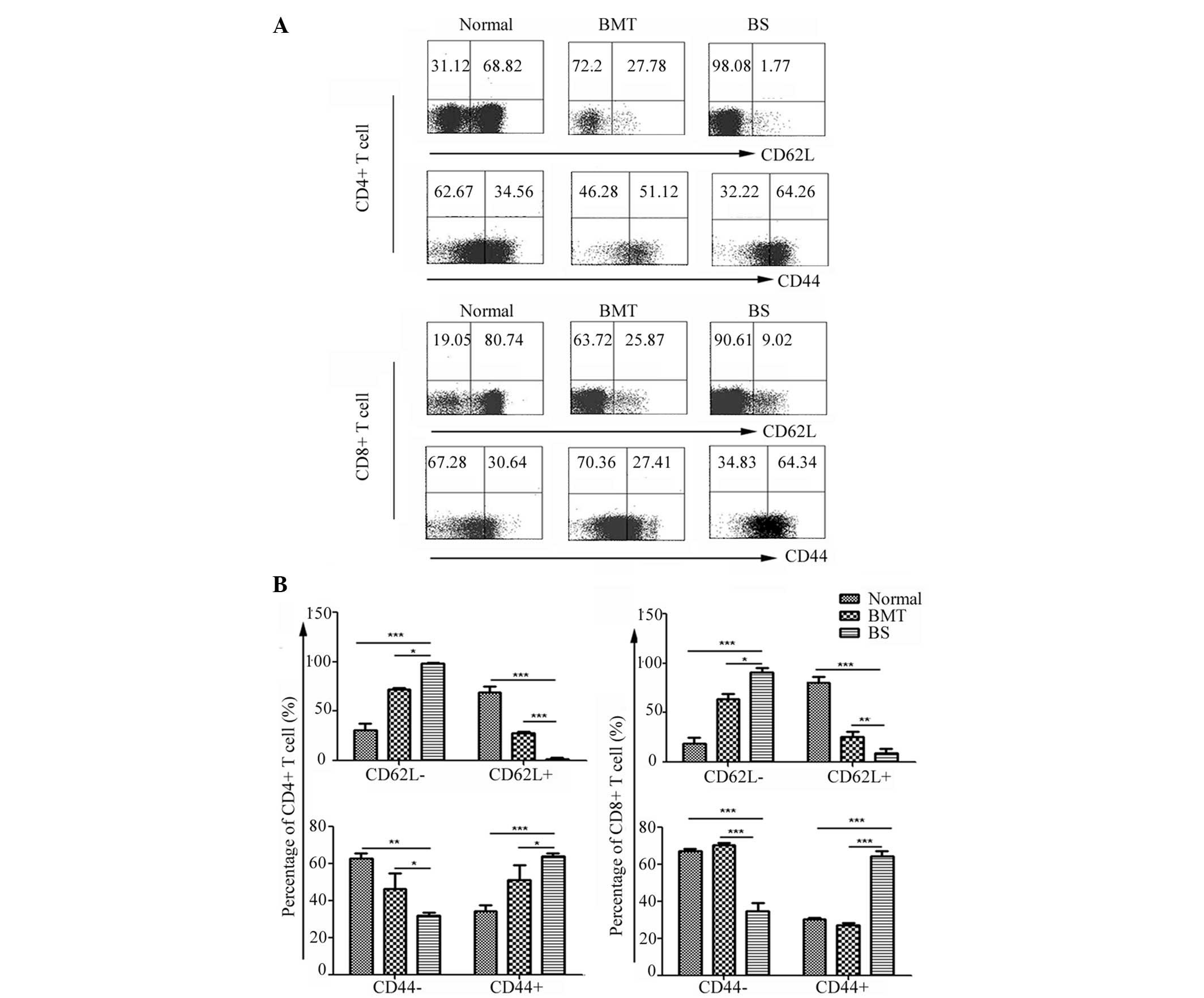
Splenic lymphocytes from the BS mice were isolated and stained, and
the normal and BMT mice were set as controls. CD62L, which is a
lymphocyte homing receptor, is regarded as a marker for naïve or
memory T cells. CD44 has been reported to be a valuable marker for
the detection of activated T cells and adhesion molecules at the
sites of inflammation (14). As
shown in Fig. 4, the expression of
CD62L in CD4+ T cells from the BS mice was markedly
downregulated, with higher levels of CD44 detected in the
CD4+ T cells. It was observed that the majority of the
CD4+ T cells in the BS mice were activated. Regarding
the CD8+ T cells, the expression of CD62L in the BS mice
was lower than that in the normal and the BMT mice, and a
statistically significant difference in the percentage of
CD44+CD8+ T cells between the BS mice and BMT
mice was observed (Fig. 4). These
data indicated that the CD4+ and CD8+ T cells
were activated and involved in different stages of GVHD.
Changes in systemic T cell-associated
cytokines in GVHD mice
To investigate the levels of systemic T
cell-associated cytokines in the process of GVHD, plasma was
collected from each group and cytokines were detected using a CBA.
As shown in Fig. 5A, plasma levels
of IL-6 and TNF-α in the BS mice were higher than in the normal,
TBI and BMT mice, and increased to a peak on day 14. The levels of
IL-6 and TNF-α began to decrease in the later phase of GVHD. IL-2
and IFN-γ, which are considered classical cytokines of Th1 or Tc1
cells were at a high level in the early phase, followed by a
continuous reduction (Fig. 5B).
However, IL-4 and IL-10, as immunosuppressive cytokines of Th2 or
Tc2 cells, increased in a step-wise manner with the development of
GVHD (Fig. 5C). These results
indicated that, during the whole process of GVHD alloantigenic
specific effector T cells, considered key for the pathogenesis of
GVHD, were regulated within the dynamically changing
environment.
Discussion
The present study investigated the characteristics
of transplanted CD4+ and CD8+ T cells and the
subsequent immune responses in the recipients. Based on the
analysis of cytokine production, the results demonstrated that
diverse, but coordinated, T cell immune responses were induced
during the occurrence and development of GVHD. T-cell activation,
proliferation and differentiation in response to host APCs are core
components of the immune reaction of GVHD, and donor
CD4+ and donor CD8+ T cells are crucial in
the pathogenesis of GVHD (15).
During the progress of GVHD, it is hypothesized that
CD4+ T cells are activated by major histocompatibility
complex (MHC) class II molecules, and that naive CD8 T cells
require priming by activated APCs with the assistance of
CD4+ T cells to proliferate and differentiate into
effector T cells (16–18). In the present study, recipient mice
were exposed to 7.5 Gy total body irradiation and were transplanted
with MHC class I mismatched donor cells. The results revealed that
CD8+ T cell alloimmune responses were induced faster and
earlier than CD4+ T cells following allogeneic
transplantation. Consistent with this data, it has been reported
that in heavily-irradiated MHC class I-mismatched mice, purified
CD8+ T cells initiate GVHD without the assistance of
CD4+ T cells (19,20).
This suggests that cooperation between CD4+ T and CD8+ T
cells may not be required for the effective activation of primary
alloimmune responses. Therefore, the present study hypothesized
that pre-conditioning regimens and mismatched cell transplantation
trigger the production of proinflammatory cytokines in
CD8+ T cells.
In the present study, when the phenotype of
alloreactive CD4+ and CD8+ T cells were
assessed 14 days post-transplantation, >90% of the donor
CD4+ T cells were activated, as demonstrated by low
expression levels of CD62L and the upregulated level of CD44 in the
BS mice. These results suggested that CD4+ T cells were
well activated on day 14. However, regarding the CD8+ T
cells, the expression of CD44 in the BS mice was lower than that in
the normal and BMT mice 14 days post-transplantation, which was
consistent with the previous results that the percentages of Tc1
and Tc17 cells reduced between days 7 and 14. It is known that
effector T cells, defined by their secretion of IFN-γ, are short
lived (21). Recent advances have
indicated the presence of a subset of postmitotic, self-renewing
CD44lo/CD62Lhi/CD8+ T cells, which can generate and
sustain all allogeneic T-cell subsets in GVHD reactions, including
central memory, effector memory and effector CD8+ T
cells (22). Thus, the present
study hypothesized that a large quantity of alloreactive
CD8+ T cells secreting high levels of IFN-γ may be
exhausted and a small number persist, becoming central memory or
effector memory cells. Alternatively, it is possible that,
following activation in secondary lymphoid organs, alloreactive
CD8+ T cells migrate into target tissues, thereby
amplifying the GVHD response locally.
IL-17-secreting CD8+ T cells, termed Tc17
cells have been recently identified as a novel subset of
CD8+ T cells, and have been observed to promote
inflammation and mediate immunity to influenza (13). In addition, Tc17 cells can also be
found in mice deficient in T-bet alone, where they appear to be
involved in allograft rejection (23). However, whether IL-17-secreting
CD8+ T cells can be induced in GVHD remains to be fully
elucidated. In an effort to enhance current understanding of GVHD
pathophysiology, the present study performed investigations and
demonstrated for the first time, to the best of our knowledge, that
a large percentage of Tc17 cells were generated in BS mice, which
have received allogenenic transplantation. The results demonstrated
that Tc17 cells had a functional role in the initiation of GVHD. It
has been reported that recipients of Tc17 cells exhibit
infiltration, hemorrhage, widened alveolar septae and
peribronchiolar thickening, and blinded-scoring of these sections
confirmed that inflammation was significantly increased in the mice
receiving the IL-17 secreting Tc17 cells (24). Previous studies have suggested that
IL-17 is critical for GVHD lung pathology (5,25).
Thus, the present study hypothesized that, during the process of
GVHD, lung lesions may be the consequence of not only Th17 cells,
but also Tc17 cells.
The pathophysiological process of GVHD is well known
to cause lesions in host organs to activated donor T cells, which
is caused by an imbalance in cytokine profile following allogeneic
transplantation (26). To compare
the profile of cytokines in GVHD, the present study quantified the
expression of cytokines using CBA, to confirm the expression of
each cytokine in the plasma. The results demonstrated that IL-2 and
IFN-γ were enriched in the early stage following transplantation,
while low levels of IL-4 and IL-10 were detected. It was suggested
that IL-2 and IFN-γ have a prominent role in initiating GVHD,
however, a shift from Th1 to Th2 cytokines indicated the
development of tolerance. Previously, it was reported that the
effect of IFN-γ on acute GVHD may depend on the timing of its
production, as IFN-γ can have immunosuppressive effects when it is
present immediately following HSCT, but can exacerbate disease via
its pro-inflammatory properties at later stages (15). Lai et al reported that
IFN-γ-secreting T cells were enriched in all murine GVHD target
tissue lesions, and Tc1 and Tc2 cells were predominant in human
GVHD colon and liver sections, respectively. However, the
IFN-γ+ Th1, IL-17+ Th17, IFN-γ+
Tc1 and IL-17+ Tc17 cells were more frequent in human
skin lesions, compared with the IL-4+ Th2 and
IL-4+ Tc2 cells (26).
Taken together, the results of the present study indicated that the
regulation of GVHD pathogenesis involves complex cytokine
interactions and variations in cytokine functionality. Cytokine
imbalance may, therefore, be a critical factor in the
pathophysiology of GVHD. To elucidate the various roles of the
complex cytokines and their possible interaction in the GVHD
process, further investigations are required.
Acknowledgments
This study was supported by the National Natural
Sciences Foundation of China (grant. nos. 81200376, 81270637 and
81300377), the China Postdoctoral Science Foundation funded project
(grant. no. 2012M510141) and the Foundation of Jiangsu Province Six
Talents Peak (grant. no. 2012-WSN-082).
References
|
1
|
Ferrara JL, Levine JE, Reddy P and Holler
E: Graft-versus-host disease. Lancet. 373:1550–1561. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu Y and Waller EK: Dichotomous role of
interferon-gamma in allogeneic bone marrow transplant. Biol Blood
Marrow Transplant. 15:1347–1353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Socie G and Blazar BR: Acute
graft-versus-host disease: From the bench to the bedside. Blood.
114:4327–4336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yi T, Zhao D, Lin CL, Zhang C, Chen Y,
Todorov I, LeBon T, Kandeel F, Forman S and Zeng D: Absence of
donor Th17 leads to augmented Th1 differentiation and exacerbated
acute graft-versus-host disease. Blood. 112:2101–2110. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carlson MJ, West ML, Coghill JM,
Panoskaltsis-Mortari A, Blazar BR and Serody JS: In
vitro-differentiated TH17 cells mediate lethal acute
graft-versus-host disease with severe cutaneous and pulmonary
pathologic manifestations. Blood. 113:1365–1374. 2009. View Article : Google Scholar :
|
|
6
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage distinct
from the T helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blazar BR, Taylor PA and Vallera DA: CD4+
and CD8+ T cells each can utilize a perforin-dependent pathway to
mediate lethal graft-versus-host disease in major
histocompatibility complex-disparate recipients. Transplantation.
64:571–576. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeda Y, Levy RB, Reddy P, Liu C,
Clouthier SG, Teshima T and Ferrara JL: Both perforin and Fas
ligand are required for the regulation of alloreactive CD8+ T cells
during acute graft-versus-host disease. Blood. 105:2023–2027. 2005.
View Article : Google Scholar
|
|
9
|
Marks L, Altman NH, Podack ER and Levy RB:
Donor T cells lacking Fas ligand and perforin retain the capacity
to induce severe GvHD in minor histocompatibility antigen
mismatched bone-marrow transplantation recipients. Transplantation.
77:804–812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh
CL, Yeh YC, Chou AH, Chang SR, Hsiao KN and Yu FW: Induction of a
distinct CD8 Tnc17 subset by transforming growth factor-beta and
interleukin-6. J Leukoc Biol. 82:354–360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amir AL, Hagedoorn RS, van Luxemburg-Heijs
SA, Marijt EW, Kruisselbrink AB, Frederik Falkenburg JH and
Heemskerk MH: Identification of a coordinated CD8 and CD4 T cell
response directed against mismatched HLA Class I causing severe
acute graft-versus-host disease. Biol Blood Marrow Transplant.
18:210–219. 2012. View Article : Google Scholar
|
|
12
|
Pan B, Zeng L, Cheng H, Song G, Chen C,
Zhang Y, Li Z and Xu K: Altered balance between Th1 and Th17 cells
in circulation is an indicator for the severity of murine acute
GVHD. Immunol Lett. 142:48–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamada H, Garcia-Hernandez Mde L, Reome
JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL and
Dutton RW: Tc17, a unique subset of CD8 T cells that can protect
against lethal influenza challenge. J Immunol. 182:3469–3481. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Surh CD and Sprent J: Homeostasis of naive
and memory T cells. Immunity. 29:848–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blazar BR, Murphy WJ and Abedi M: Advances
in graft-versus-host disease biology and therapy. Nat Rev Immunol.
12:443–458. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teshima T, Ordemann R, Reddy P, Gagin S,
Liu C, Cooke KR and Ferrara JL: Acute graft-versus-host disease
does not require alloantigen expression on host epithelium. Nat
Med. 8:575–581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matte CC, Liu J, Cormier J, Anderson BE,
Athanasiadis I, Jain D, McNiff J and Shlomchik WD: Donor APCs are
required for maximal GVHD but not for GVL. Nat Med. 10:987–992.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chakraverty R, Eom HS, Sachs J, Buchli J,
Cotter P, Hsu R, Zhao G and Sykes M: Host MHC class II+
antigen-presenting cells and CD4 cells are required for
CD8-mediated graft-versus-leukemia responses following delayed
donor leukocyte infusions. Blood. 108:2106–2113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korngold R and Sprent J: Surface markers
of T cells causing lethal graft-vs-host disease to class I vs class
II H-2 differences. J Immunol. 135:3004–3010. 1985.PubMed/NCBI
|
|
20
|
Sprent J, Schaefer M, Lo D and Korngold R:
Properties of purified T cell subsets. II In vivo responses to
class I vs class II H-2 differences. J Exp Med. 163:998–1011. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CY, Kirman JR, Rotte MJ, Davey DF,
Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC and Seder RA:
Distinct lineages of T(H)1 cells have differential capacities for
memory cell generation in vivo. Nat Immunol. 3:852–858. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Joe G, Hexner E, Zhu J and
Emerson SG: Host-reactive CD8+ memory stem cells in
graft-versus-host disease. Nat Med. 11:1299–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burrell BE, Csencsits K, Lu G,
Grabauskiene S and Bishop DK: CD8+ Th17 mediate costimulation
blockade-resistant allograft rejection in T-bet-deficient mice. J
Immunol. 181:3906–3914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yen HR, Harris TJ, Wada S, Grosso JF,
Getnet D, Goldberg MV, Liang KL, Bruno TC, Pyle KJ, Chan SL, et al:
Tc17 CD8 T cells: Functional plasticity and subset diversity. J
Immunol. 183:7161–7168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yi T, Chen Y, Wang L, Du G, Huang D, Zhao
D, Johnston H, Young J, Todorov I, Umetsu DT, et al: Reciprocal
differentiation and tissue-specific pathogenesis of Th1, Th2 and
Th17 cells in graft-versus-host disease. Blood. 114:3101–3112.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai HY, Chou TY, Tzeng CH and Lee OK:
Cytokine profiles in various graft-versus-host disease target
organs following hematopoietic stem cell transplantation. Cell
Transplant. 21:2033–2045. 2012. View Article : Google Scholar : PubMed/NCBI
|