Introduction
Tanshinone IIA (Tan-IIA;
C19H18O3) is extracted from
Danshen (Salviae miltiorrhizae radix) (1,2).
Tan-IIA exerts antitumor activity in a variety of human cancer
cells, such as lung (3), breast
(4,5), hepatic (6), pancreatic (7) and colon (8). These studies (3–8)
suggest that Tan-IIA may be administered as a complementary
therapeutic agent to treat gastric cancer. Furthermore, our
previous studies demonstrated that Tan-IIA inhibits the
proliferation of AGS cells in a time- and dose-dependent manner, by
decreasing the level of protein expression of binding
immunoglobulin (Ig) protein, myeloid cell leukemia 1 protein,
B-cell lymphoma-extra-large and translationally-controlled tumor
protein, and increasing caspase-12, C/EBP-homologous protein,
Bcl-2-associated X protein, and caspase-12, -9 and -3 to induce
apoptosis (9). The half-maximal
inhibitory concentration was 5.5, 3.7 and 3.5 µg/ml at 24,
48 and 72 h, respectively (9). In
addition, Tan-IIA was demonstrated to inhibit AGS human gastric
cancer cells by increasing the protein expression level of
phosphorylated (p)-p38 and p-Jun-amino-terminal kinase, and
decreasing that of p-extracellular-signal-regulated kinases to
induce G2/M phase arrest. In addition, Tan-IIA increased
the protein expression levels of tumor necrosis factor-α, FAS
ligand, and caspase-8 and -3 to induce apoptosis (10). It is well documented that nuclear
factor (NF)-κB functions as a tumor promoter in
inflammation-associated cancer and is, therefore, a potential
target for cancer prevention in chronic inflammatory diseases
(11). During malignant tumor
metastasis, the degradation of the extracellular matrix (ECM) is
mediated by matrix metalloproteinases (MMPs) (12–14).
As the underlying mechanisms of Tan-IIA inhibiting the migration
ability of gastric cancer cells remain to be elucidated, this was
the aim of the present study, as well as evaluating the levels of
metastatic-associated protein expression in AGS human gastric
cancer cells.
Materials and methods
Materials
The AGS human gastric adenocarcinoma cell line
[Bioresource Collection and Research Center (BCRC); BCRC no. 60102]
was obtained from the Food Industry Research and Development
Institute (Hsinchu, Taiwan). Tan-IIA (CAS no. 568-72-9) was
obtained from Sigma-Aldrich (St. Louis, MO, USA). Ham's F-12K
(Kaign's) Medium, fetal bovine serum (FBS), 1%
penicillin/streptomycin and glutamine were obtained from Thermo
Fisher Scientific, Inc. (Gibco; Waltham, MA, USA). The polyclonal
rabbit anti-human NF-κB-p65 (cat. no. 6956; molecular weight,
65kDa), monoclonal rabbit anti-human cyclooxygenase (COX)-2 (cat.
no. 12282; molecular weight, 74kDa) and monoclonal rabbit
anti-human MMP-2 (cat. no. 13132; molecular weight, 72kDa)
antibodies were all obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Polyclonal goat anti-human MMP-7 (cat. no.
NB600-1069; molecular weight, 28kDa) and polyclonal rabbit
anti-human MMP-9 (cat. no. NBP1-57940; molecular weight, 78kDa)
antibodies were obtained from Novus Biologicals, LLC (Littleton,
CO, USA). Sodium deoxycholate, leupeptin, Triton X-100, Tris-HCl,
ribonuclease-A, sodium pyruvate, HEPES, dimethyl sulfoxide,
Tween-20 and mouse anti-β-actin antibodies were obtained from
Sigma-Aldrich. BioMax film was obtained from Kodak (Rochester, NY,
USA), and potassium phosphate and 0.2-mm polyvinylidene difluoride
(PVDF) membranes were purchased from Merck Millipore (Darmstadt,
Germany).
Cell culture was conducted as described in a
previous study (9). The AGS cells
were placed into 75-cm2 tissue culture flasks and
maintained in Ham's F-12K Medium containing 10% heat-inactivated
FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells
were grown for 48–72 h at 37°C in a humidified atmosphere of 5%
CO2.
Wound-healing assays were performed as described in
a previous study (15). Cells were
plated at a density of 1×106 cells per 60-mm Petri dish
in complete medium for 16–20 h. Different concentrations (0, 2.0,
3.7 and 5.5 µg/ml) of Tan-IIA were administered for 0, 24,
and 48 h and, once the cells reached confluency, a plastic pipette
tip was drawn across the center of the plate to produce a clean,
wide, wound area. Cell movement into the wound area was examined
under an Olympus 1X81 microscope (Olympus Corporation, Tokyo,
Japan).
Western blot analysis
The western blotting procedures were conducted as
described in previous studies (9,10).
The cells treated with Tan-IIA were lysed in ice-cold
radioimmunoprecipitation assay buffer (Merck Millipore) containing
a protease inhibitor cocktail (Gibco; Thermo Fisher Scientific,
Inc.). The lysate was agitated for 30 min at 4°C and centrifuged at
12,281 × g for 10 min. The protein concentration was measured using
the Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
Equal quantities of proteins (10 µg) were subjected to
electrophoresis using 12% sodium dodecyl sulfate-polyacrylamide
gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA; stacking gel:
70 V, 400 mA, 30 min; separating gel: 100 V, 400 mA, 90 min). To
verify equal protein loading and transfer, the proteins were
transferred to PVDF membranes, which were blocked for 1 h at 4°C
using blocking buffer [5% dried, skimmed milk in solution
containing 50 mM Tris-HCl (pH 8.0), 2 mM CaCl2, 80 mM
sodium chloride, 0.05% Tween-20 and 0.02% sodium azide (Merck
Millipore)]. The membranes were subsequently incubated for 2 h at
room temperature with the following primary antibodies: Rabbit
polyclonal anti-NF-κB-p65, monoclonal rabbit anti-COX-2, monoclonal
rabbit anti-MMP-2, polyclonal goat anti-MMP-7, polyclonal rabbit
anti-MMP-9 (all diluted to 1:1,000), followed by incubation at room
temperature for 1 h with anti-rabbit (cat. no. sc:2004) or
anti-mouse (cat. no. sc:2005) IgG-horseradish peroxidase-conjugated
secondary antibodies (1:5,000; Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA). The membranes were washed three times for 10 min
with 1X phosphate-buffered saline with 0.05% (v/v) Tween 20. The
protein bands were visualized on X-ray film using an enhanced
chemiluminescence detection system (PerkinElmer, Inc., Waltham, MA,
USA).
Statistical analysis
All data presented are from a minimum of three
independent experiments. Values are presented as the mean ±
standard deviation. Student's t-test was used to analyze
statistical significance and P<0.05 was considered to indicate a
statistically significant difference.
Results
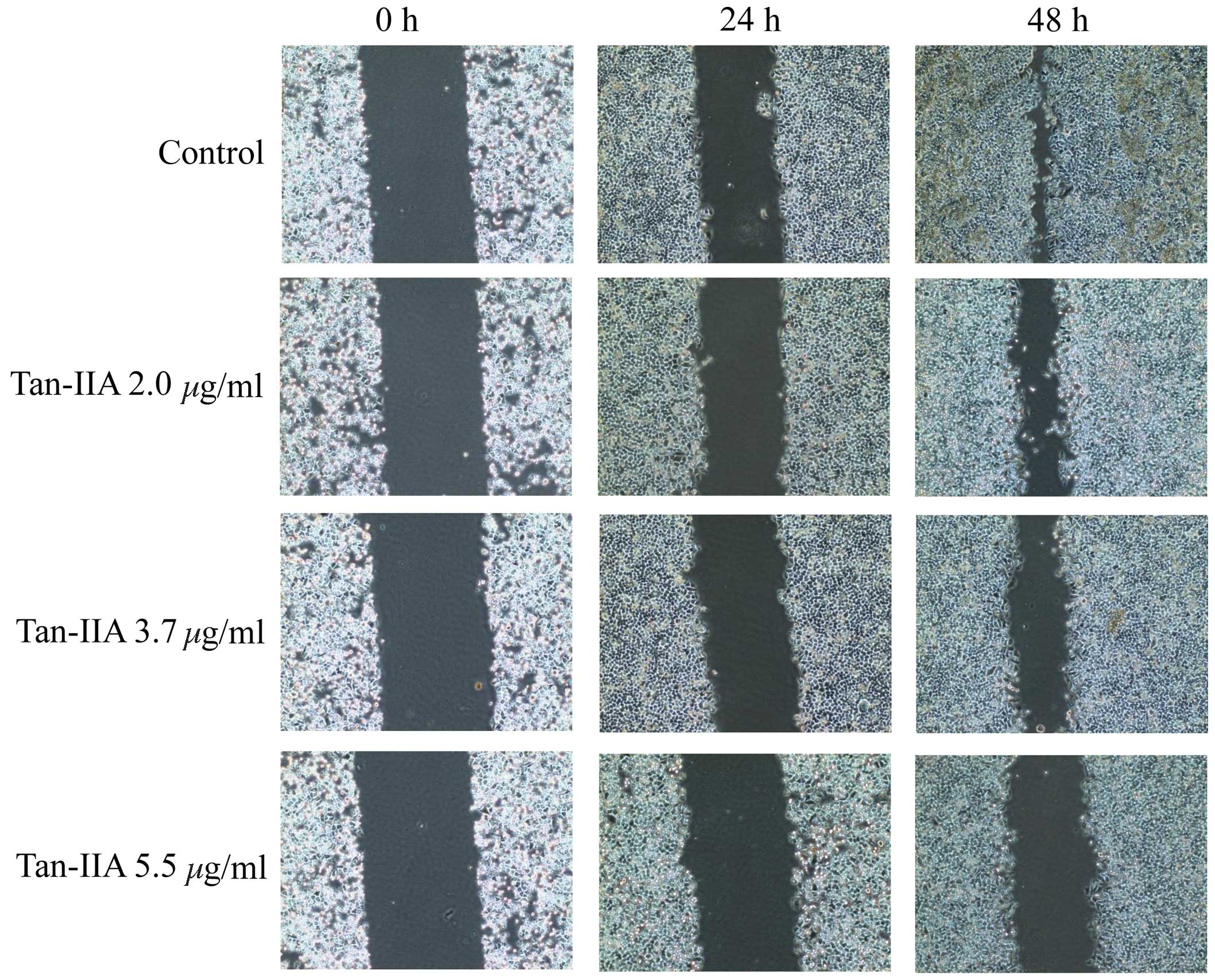
Wound-healing assays
The wound-healing assay is one of the methods used
to investigate cell migration in vitro. A wound was created
in the cell monolayer using a plastic pipette tip and AGS cells
were treated with various concentration of Tan-IIA (0, 2.0, 3.7 and
5.5 µg/ml) for 24 or 48 h. Images were captured to compare
the cell migration for wound closure. The results indicate that
Tan-IIA inhibits the migration of AGS cells in a time- and
dose-dependent manner (Fig.
1).
Effects of Tan-IIA concentration on the
protein expression levels of NF-κB-p65, COX-2, MMP-2, -7, and -9
and β-actin in AGS cells
The AGS cells were treated with various
concentrations of Tan-IIA (0, 2.0, 3.7 and 5.5 µg/ml) for 24
or 48 h and the protein expression levels of NF-κB-p65, COX-2,
MMP-2, -7, and -9 and β-actin were evaluated by western blot
analysis. The results demonstrate that Tan-IIA significantly
decreases the protein expression levels of NF-κB-p65 (Fig. 2A), COX-2 (Fig. 2B), and MMP-2 (Fig. 2C), -7 (Fig. 2D) and -9 (Fig. 2E) in a dose-dependent manner.
 | Figure 2Protein expression levels of
NF-κB-p65, COX-2, MMP-2, -7, and -9 and β-actin in AGS cells. The
AGS cells were treated with various concentrations of Tan-IIA (0,
2.0, 3.7 and 5.5 µg/ml) for 24 or 48 h and the protein expression
levels were evaluated by western blot analysis. The results
indicate that Tan-IIA significantly decreases the protein
expression levels of (A) NF-κB-p65, (B) COX-2, (C) MMP-2, (D) MMP-7
and (E) MMP-9 in a dose-dependent manner. Tan-IIA, tanshinone IIA;
NF-κB-p65, nuclear factor κB-p65; COX-2, cyclooxygenase-2; MMP,
matrix metalloproteinase. *P<0.05,
**P<0.01 and ***P<0.001, compared with
the control. |
Effects of Tan-IIA treatment duration on
the protein expression of NF-κB-p65, COX-2, MMP-2, -7, and -9 and
β-actin in AGS cells
The AGS cells were treated with Tan-IIA (3.7
µg/ml) for different durations (0, 24 and 48 h) and the
protein expression levels were evaluated by western blot analysis.
The results demonstrate that Tan-IIA significantly decreases the
protein expression levels of NF-κB-p65, COX-2, and MMP-2, -7 and -9
in a time-dependent manner (Fig.
3).
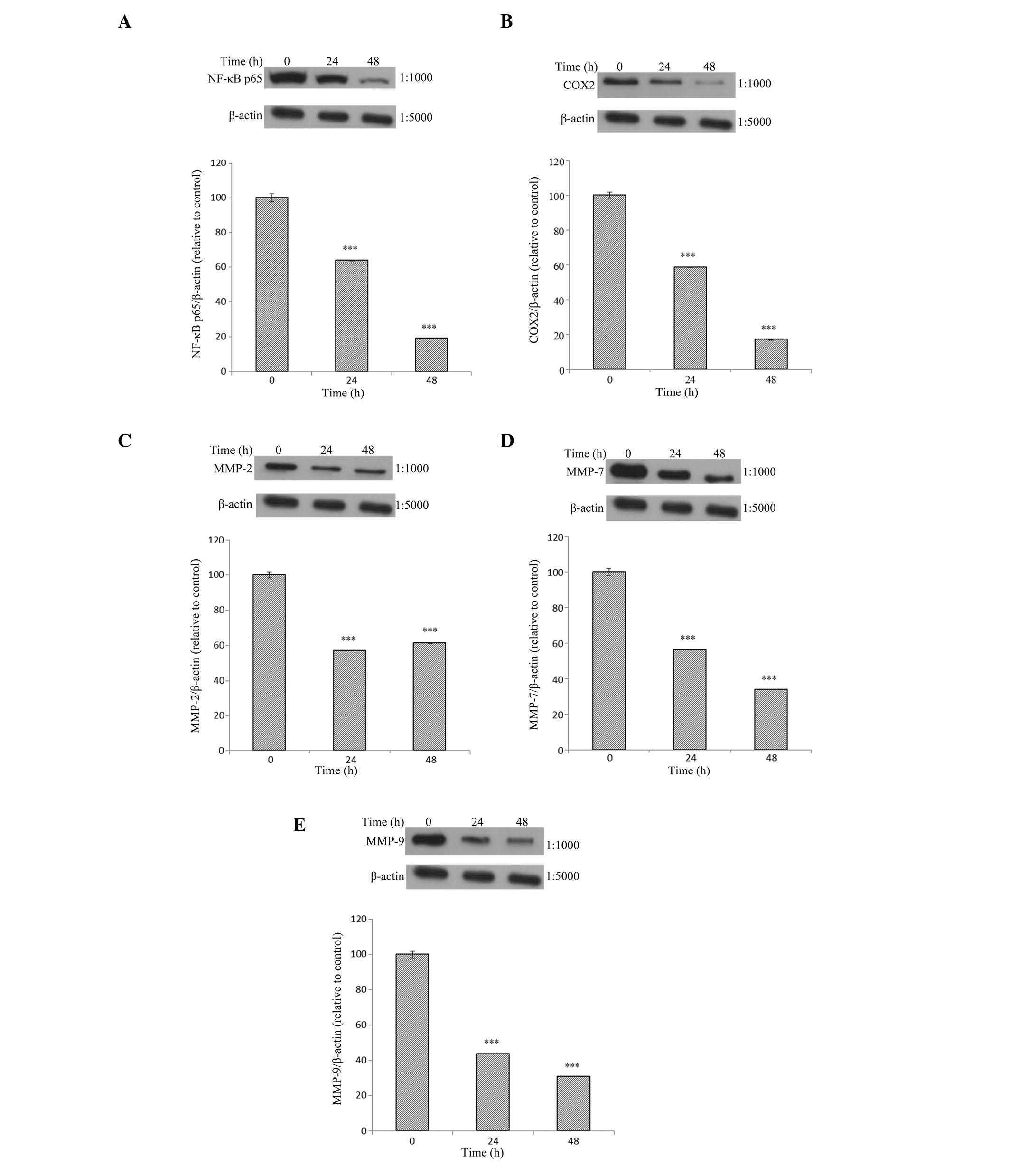 | Figure 3Protein expression levels of
NF-κB-p65, COX-2, MMP-2, -7, and -9 and β-actin in AGS cells. The
AGS cells were treated with Tan-IIA (3.7 µg/ml) for different
durations (0, 24 and 48 h) and the protein expression levels were
evaluated by western blot analysis. The results demonstrate that
Tan-IIA significantly decreases the protein expression levels of
(A) NF-κB-p65, (B) COX-2, (C) MMP-2, (D) MMP-7 and (E) MMP-9 in a
time-dependent manner. Tan-IIA, tanshinone IIA; NF-κB-p65, nuclear
factor κB-p65; COX-2, cyclooxygenase-2; MMP, matrix
metalloproteinase. *P<0.05, **P<0.01,
and ***P<0.001, compared with the control. |
Discussion
Cell migration in vitro is commonly evaluated
using a wound-healing assay. A wound is created in the cell
mono-layer using a plastic pipette tip. Images are captured upon
initiation of the assay and at regular time-points during cell
migration (that is taking place to close the wound), and these
images are compared to measure the cell migration. This method aims
to mimic cell migration during wound healing in vivo
(16). The present study indicates
that Tan-IIA inhibits the migration ability of AGS human gastric
cancer cells in a time- and dose-dependent manner in vitro.
This finding is consistent with previous studies, which
demonstrated that Tan-IIA markedly decreased migratory and invasive
abilities in SGC7901 gastric cancer cells (15). It is well known that MMP-2
overexpression is significantly associated with poor overall
survival (OS) of gastric cancer patients, and has a negative impact
on OS in Asian and European countries (17). A previous meta-analysis indicated
that MMP-2 overexpression may be a predictive factor for poor
prognosis in gastric cancer (17).
de la Peña et al (18)
demonstrated that MMP-2 expression may present as a potential
molecular marker for advanced human gastric cancer. An additional
meta-analysis indicated that abnormal MMP-2 expression may be
markedly associated with poor prognosis in gastric cancer patients
(19). Yang et al (20) demonstrated that the MMP-7-181
A>G polymorphism may contribute to gastric cancer
susceptibility. Furthermore, Huang et al (21) demonstrated that interleukin
(IL)-1β-induced p38 activation significantly increased the
messenger (m)RNA and protein expression, and activity of MMP-2 and
-9. This indicated the IL-1β/p38/activator protein 1 (c-Fos)/MMP-2
and -9 signaling pathway may be important in metastasis in MKN-45
and AGS cells. In our previous study, AGS cells were treated with
Tan-IIA and it was demonstrated that treatment increased p-p38
expression levels in a time- and dose-dependent manner (10). In the present study, the results
indicate that Tan-IIA decreases MMP-2, -7 and -9 expression levels
in a time- and dose-dependent manner. The results indicate that
Tan-IIA may inhibit the metastasis of AGS cells by decreasing
MMP-2, -7 and -9 expression levels.
Carcinogen exposure and chronic inflammation are
significant underlying conditions resulting in tumor development.
It is well documented that NF-κB is essential in promotion of
inflammation-associated cancer, and is therefore being considered
as a potential target for cancer prevention in chronic inflammatory
diseases (11). MGC803 gastric
cancer cells treated with chloroquine inhibited the mRNA expression
levels of COX-2, MMP-2 and -7 and NF-κB-p65. This prevented the
migration of MGC803 cells in a dose-dependent manner. Furthermore,
the results indicated that the Toll-like receptor 9/NF-κB signaling
pathway was involved in gastric cancer cell migration (22). The results from the current study
indicate that Tan-IIA decreases NF-κB-p65 and COX-2 expression
levels in a time- and dose-dependent manner. In addition, the
results from the wound-healing assay indicate that Tan-IIA inhibits
the migration of AGS cells for wound closure in a time- and
dose-dependent manner. These results suggest that Tan-IIA inhibits
the migration ability of AGS cells by decreasing the protein
expression of NF-κB-p65, COX-2, and MMP-2, -7 and -9.
In conclusion, to the best of our knowledge, this is
the first study to report that Tan-IIA inhibits the migration
ability of AGS cells via decreasing the protein expression of
NF-κB-p65, COX-2 and MMPs. H owever, these findings warrant further
investigation in the future.
Acknowledgments
The present study was supported by a grant from the
Research Section of the Changhua Christian Hospital (Changhua,
Taiwan; grant no. 102-CCH-IRP-066).
References
|
1
|
Che AJ, Zhang JY, Li CH, Chen XF, Hu ZD
and Chen XG: Separation and determination of active components in
Radix Salviae miltiorrhizae and its medicinal preparations by
nonaqueous capillary electrophoresis. J Sep Sci. 27:569–575. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
4
|
Su CC and Lin YH: Tanshinone IIA inhibits
human breast cancer cells through increased Bax to Bcl-xL ratios.
Int J Mol Med. 22:357–361. 2008.PubMed/NCBI
|
|
5
|
Yan MY, Chien SY, Kuo SJ, Chen DR and Su
CC: Tanshinone IIA inhibits BT-20 human breast cancer cell
proliferation through increasing caspase 12, GADD153 and
phospho-p38 protein expression. Int J Mol Med. 29:855–863.
2012.PubMed/NCBI
|
|
6
|
Cheng CY and Su CC: Tanshinone IIA
inhibits Hep-J5 cells by increasing calreticulin, caspase 12 and
GADD153 protein expression. Int J Mol Med. 26:379–385.
2010.PubMed/NCBI
|
|
7
|
Huang CY, Chiu TL, Kuo SJ, Chien SY, Chen
DR and Su CC: Tanshinone IIA inhibits the growth of pancreatic
cancer BxPC 3 cells by decreasing protein expression of TCTP, MCL 1
and Bcl-xL. Mol Med Rep. 7:1045–1049. 2013.PubMed/NCBI
|
|
8
|
Su CC, Chen GW, Kang JC and Chan MH:
Growth inhibition and apoptosis induction by tanshinone IIA in
human colon adenocarcinoma cells. Planta Med. 74:1357–1362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su CC: Tanshinone IIA inhibits human
gastric carcinoma AGS cell growth by decreasing BiP, TCTP, Mcl 1
and Bcl-xL and increasing Bax and CHOP protein expression. Int J
Mol Med. 34:1661–1668. 2014.PubMed/NCBI
|
|
10
|
Su CC: Tanshinone IIA inhibits gastric
carcinoma AGS cells through increasing p-p38, p-JNK and p53 but
reducing p-ERK, CDC2 and cyclin B1 expression. Anticancer Res.
34:7097–7110. 2014.PubMed/NCBI
|
|
11
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu CY, Wu MS, Chen YJ, Chen CJ, Chen HP,
Shun CT, Chen GH, Huang SP and Lin JT: Clinicopathological
significance of MMP-2 and TIMP-2 genotypes in gastric cancer. Eur J
Cancer. 43:799–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elnemr A, Yonemura Y, Bandou E, Kinoshita
K, Kawamura T, Takahashi S, Tochiori S, Endou Y and Sasaki T:
Expression of collagenase-3 (matrix metalloproteinase-13) in human
gastric cancer. Gastric Cancer. 6:30–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu M, Cao FL, Li NY, Liu YQ, Li YP and Lv
CL: Tanshinone IIA reverses the malignant phenotype of SGC7901
gastric cancer cells. Asian Pac J Cancer Prev. 14:173–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodriguez LG, Wu X and Guan JL:
Wound-healing assay. Methods Mol Biol. 294:23–29. 2005.
|
|
17
|
Shen W, Xi H, Wei B and Chen L: The
prognostic role of matrix metalloproteinase 2 in gastric cancer: A
systematic review with meta-analysis. J Cancer Res Clin Oncol.
40:1003–1009. 2014. View Article : Google Scholar
|
|
18
|
de la Peña S, Sampieri CL, Ochoa-Lara M,
León-Córdoba K and Remes-Troche JM: Expression of the matrix
metallo-proteases 2, 14, 24, and 25 and tissue inhibitor 3 as
potential molecular markers in advanced human gastric cancer. Dis
Markers. 2014:2859062014. View Article : Google Scholar
|
|
19
|
Wang HL, Zhou PY, Zhang Y and Liu P:
Relationships between abnormal MMP2 expression and prognosis in
gastric cancer: A meta-analysis of cohort studies. Cancer Biother
Radiopharm. 29:166–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang TF, Guo L and Wang Q: Meta-analysis
of associations between four polymorphisms in the matrix
metalloproteinases gene and gastric cancer risk. Asian Pac J Cancer
Prev. 15:1263–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Q, Lan F, Wang X, Yu Y, Ouyang X,
Zheng F, Han J, Lin Y, Xie Y, Xie F, et al: IL-1β-induced
activation of p38 promotes metastasis in gastric adenocarcinoma via
upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer. 31:13–18.
2014.
|
|
22
|
Zhang Y, Li Y, Li Y, Li R, Ma Y, Wang H
and Wang Y: Chloroquine inhibits MGC803 gastric cancer cell
migration via the Toll-like receptor 9/nuclear factor kappa B
signaling pathway. Mol Med Rep. 11:1366–1371. 2015.
|