Introduction
Glioma is a common type of brain tumor, accounting
for 40–50% of all intracranial tumors, which vary in size and are
highly invasive (1,2). Generally, gliomas are treated via
surgery, radiotherapy and chemotherapy; however, it is difficult to
remove them completely due to the resistance of tumor cells to
radiotherapy. This may lead to a relapse of the residual nidus,
resulting in high recurrence, high mortality and low cure rates
(3,4). Therefore, it is important to develop
novel agents for more effective treatment.
Eupatilin is a pharmacologically active flavonoid
extracted from Artemisia asiatica Nakai (Asteraceae) and a
primary active component of DA-9601 for mucosal protection
(5,6). It has anti-inflammatory properties
and is widely used for treatment of gastritis and peptic ulcers
(7). Additionally, it has
anti-oxidative effects against gastric mucosal damage and may
enhance regeneration of damaged mucosa (8). Recently, eupatilin was identified to
exhibit an antitumor effect. Cheong et al (9) reported that eupatilin inhibits
angiogenesis in gastric cancer cells by blocking the expression of
signal transducer and activator of transcription 3, and the
expression of vascular endothelial growth factor (VEGF). Park et
al (10) determined that
eupatilin may be used as a chemo-preventive and antimetastatic
agent in human gastric cancer. Eupatilin also suppressed the growth
of human endometrial cancer cells via arrest of the cell cycle at
the G2/M phase through upregulation of p21 (11).
However, to the best of our knowledge, there have
been no reports regarding the effects of eupatilin on glioma.
Therefore, in the present study aimed to investigate the effects of
eupatilin on glioma mechanisms underlying these effects. The
results demonstrated that eupatilin has inhibitory effects on
proliferation, invasion and migration, and promotes the apoptosis
of glioma cells via suppression of the Notch-1 signaling pathway.
Additionally, knockdown of Notch-1 enhanced the inhibitory effects
of eupatilin on glioma cell growth and invasion.
Materials and methods
Cell culture
The LN229 and U87MG human glioma cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and then cultured at 37°C in Dulbecco's modified Eagle's
medium (Bio-Rad Laboratories, Inc., Hercules, CA, USA) supplemented
with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO,
USA), 100 U/ml penicillin (Sigma-Aldrich) and 100 mg/ml
streptomycin (Sigma-Aldrich) in a 5% CO2 saturated
humidity incubator.
Cell viability assay
The LN229 and U87MG cells were seeded in 96-well
culture plates at a density of 5×104 cells/well.
Following 24 h, they were treated with 12.5, 25 or 50 µM
eupatilin (Sigma-Aldrich) for 24, 48, 72 or 96 h. Control group
cells were treated with 0.1% dimethylsulfoxide (DMSO;
Sigma-Aldrich) in culture medium. Subsequent to treatment
application, all cells were incubated with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT;
Sigma-Aldrich) solution for 24 h at 37°C. Then DMSO was added in
each well and shaken for 10 min at room temperature. The optical
density was measured with an enzyme-linked immunosorbent assay
reader (BioTek Instruments, Inc., Winooski, VT, USA) at a
wavelength of 570 nm. Each experiment was performed at least three
times.
Cell invasion and migration assays
Cell invasion and migration assays were performed
with a Transwell chamber (EMD Millipore, Boston, MA, USA) that was
placed in a 24-well plate. The cells were pretreated with 0, 12.5,
25 and 50 µM eupatilin for 24 h and then suspended in 50
µl serum-free medium (Sigma-Aldrich). The cells were also
used for invasion assays, cells at a density of 6×103
cells/well were added to the upper chamber and complete medium
(Sigma-Aldrich) was added to the lower chamber. The chambers were
separated with a polycarbonate membrane was coated with 20
µl Matrigel (BD Biosciences, San Jose, CA, USA). The cells
were then incubated for 36 h at 37°C, those remaining in the upper
chamber were removed with cotton swabs and the ones on the bottom
surface of the membrane were fixed and stained with methanol and
Giemsa (Sigma-Aldrich), respectively, and then counted under an
optical microscope (×200; CX31; Olympus Corporation, Tokyo, Japan).
The migration assay was performed as described above, except that
Matrigel was not applied to the membrane.
Cell apoptosis assay
Cell apoptosis was detected with Annexin
V-fluorescein isothiocyanate (FITC; Abcam, Cambridge, MA, USA) and
propidium iodide (PI; Abcam) staining followed by flow cytometric
analysis. Briefly, U87MG cells were seeded in 24-well culture
plates at a density of 4×104 cells/well. Eupatilin at
concentrations of 0, 12.5, 25 and 50 µM was added to the
plates after 24 h. The cells were cultured for 48 h at 37°C and
then harvested via centrifugation at 1,000 × g for 10 min. The
cells were incubated with Annexin V-FITC and PI for 15 min at room
temperature. Apoptosis was analyzed using flow cytometry (FC500; BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the
eupatilin-treated U87MG cells with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in accordance
with the manufacturer's protocol. cDNA synthesis was conducted
using 5 µg of the total RNA with M-MuLV reverse
transcriptase (Clontech Laboratories, Inc., Palo Alto, CA, USA).
The genes of interest were amplified with the following primers:
Forward: 5′-TCAGCGGGATCCACTGTGAG-3′ and reverse:
5′-ACACAGGCAGGTGAACGAGTTG-3′ for Notch-1; and forward:
5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse: 5′-GCTGTCACCTTCACCGTTCC-3′
for β-actin. β-actin was used as a control. The PCR was run for 30
cycles at 94°C (denaturation) for 30 sec, at 55°C (annealing) for
30 sec and at 72°C (extension) for 20 sec. The experiment was
performed for three times. The data obtained was calculated using
the comparative Cq method (2−ΔΔCq) as previously
described (12).
Western blot analysis
The U87MG cells were treated with 0, 12.5, 25 and 50
µM eupatilin for 24 h and then immersed in a lysis buffer
containing 40 mmol/l Tris-HCl, 1 mmol/l EDTA, 150 mmol/l KCl, 100
mmol/l NaVO3, 1% Triton X-100, and 1 mmol/l
phenylmethylsulfonyl fluoride (pH 7.5). The protein was separated
by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(Sigma-Aldrich) and then transferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc.). The membranes were treated with 5%
non-fat milk in Tris-buffered saline (TBS) at room temperature for
1 h and then incubated overnight at 4°C with primary mouse
monoclonal anti-human Notch-1 (1:1,500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA; cat. no. sc-373944) or β-actin (1:1,000;
Santa Cruz Biotechnology, Inc.; cat. no. sc-8432). The membranes
were then washed three times with TBS and Tween-20 (TBST) for 10
min at room temperature. Subsequently, the membranes were incubated
with a bovine anti-mouse horseradish peroxidase-conjugated
secondary antibody (1:3,000; Santa Cruz Biotechnology, Inc.; cat.
no. sc-2370) for 1 h at room temperature and then washed three
times for 10 min with TBST and once with TBS. Immunoreactive bands
were detected by enhanced chemiluminescence (GE Healthcare Life
Sciences, Freiburg, Germany). The optical densities of the bands
were quantified using a Gel-Pro Analyzer, version 4.0 (Media
Cybernetics, Inc,, Rockville, MD, USA).
Small interfering RNA (siRNA)-Notch-1 and
cell transfection
The siRNA sequences were as follows: Sense
5′-ACGAAGAACAGAAGCACAAAGGCGG-3′ and antisense
5′-CCGCCUUUGUGCUUCUGUUCUUCGU-3′ for Notch-1; and sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense
5′-ACGUGACACGUUCGGAGAATT-3′ for scramble control. Prior to
transduction (24 h), U87MG cells, at a density of 5×104
cells/well, were seeded into 6-well plates and then cultured in 2
ml basic culture medium containing 5% FBS until the cells were 70%
confluent. The cells were then transfected with Notch-1 siRNA or
the scramble control siRNA, using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the protocol described
in Côté et al (13).
Subsequently, the transfected cells were treated with 0, 12.5, 25
and 50 µM eupatilin for 48 h.
Statistical analysis
All experiments were performed at least three times
and the data were expressed as the mean ± standard deviation. The
differences between the sample means were compared using one-way
analysis of variance using SPSS, version 19 (IBM SPSS, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Eupatilin inhibits the viability of
glioma cells
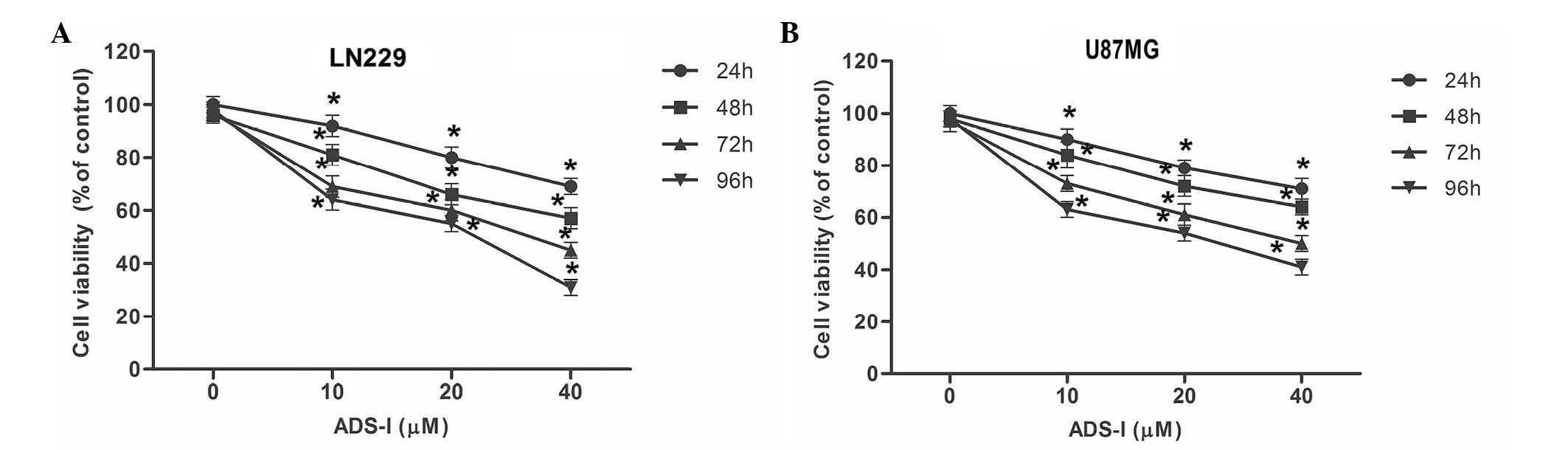
The effect of eupatilin on the viability of glioma
cells was investigated using an MTT assay. As demonstrated by
Fig. 1, higher doses of eupatilin
significantly reduced LN229 and U87MG cell viability (P<0.05;
Fig. 1A and B) compared with the
control group. Additionally, exposing the cells to eupatilin for a
longer time period enhanced its effect on cell viability. These
observations indicate that eupatilin may inhibit the viability of
LN229 and U87MG cells.
Eupatilin inhibits the invasion and
migration of glioma cells
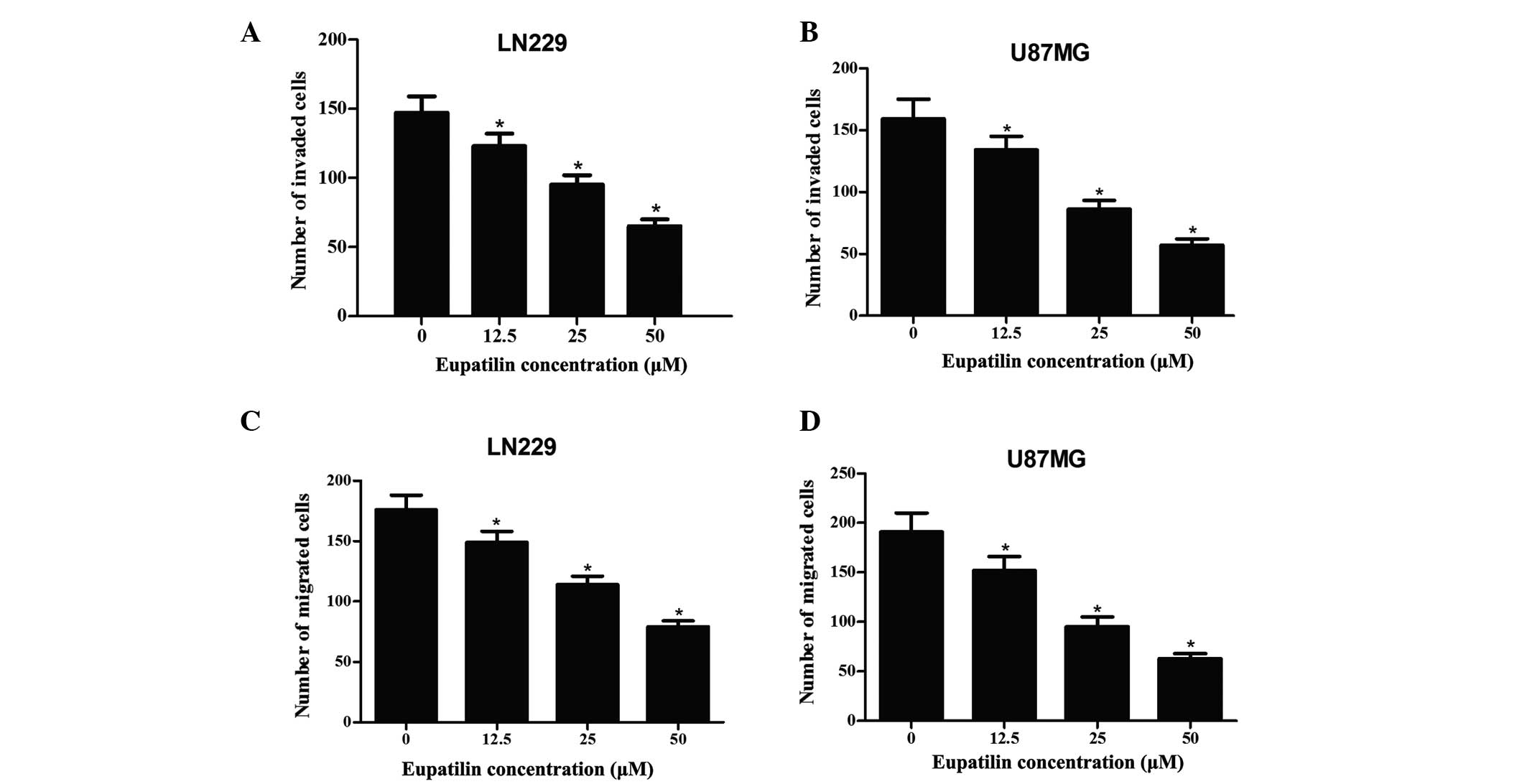
The effect of eupatilin on invasion and migration of
glioma cells was also determined. First, we examined the effect of
eupatilin on invasion of glioma cells using a transwell chamber
with Matrigel. As demonstrated in Fig.
2A and B, the treatment of LN229 and U87MG cells with 0, 12.5,
25 and 50 µM eupatilin for 24 h resulted in inhibition of
cell invasion in a dose-dependent manner. Subsequently, the effect
of eupatilin on migration of glioma cells was also investigated
using a Transwell chamber without Martrigel being applied. Fig. 2C and D demonstrates that the number
of eupatilin-treated cells that migrated into the lower chamber was
significantly reduced, compared with the control group
(P<0.05).
Eupatilin promotes the apoptosis of
glioma cells
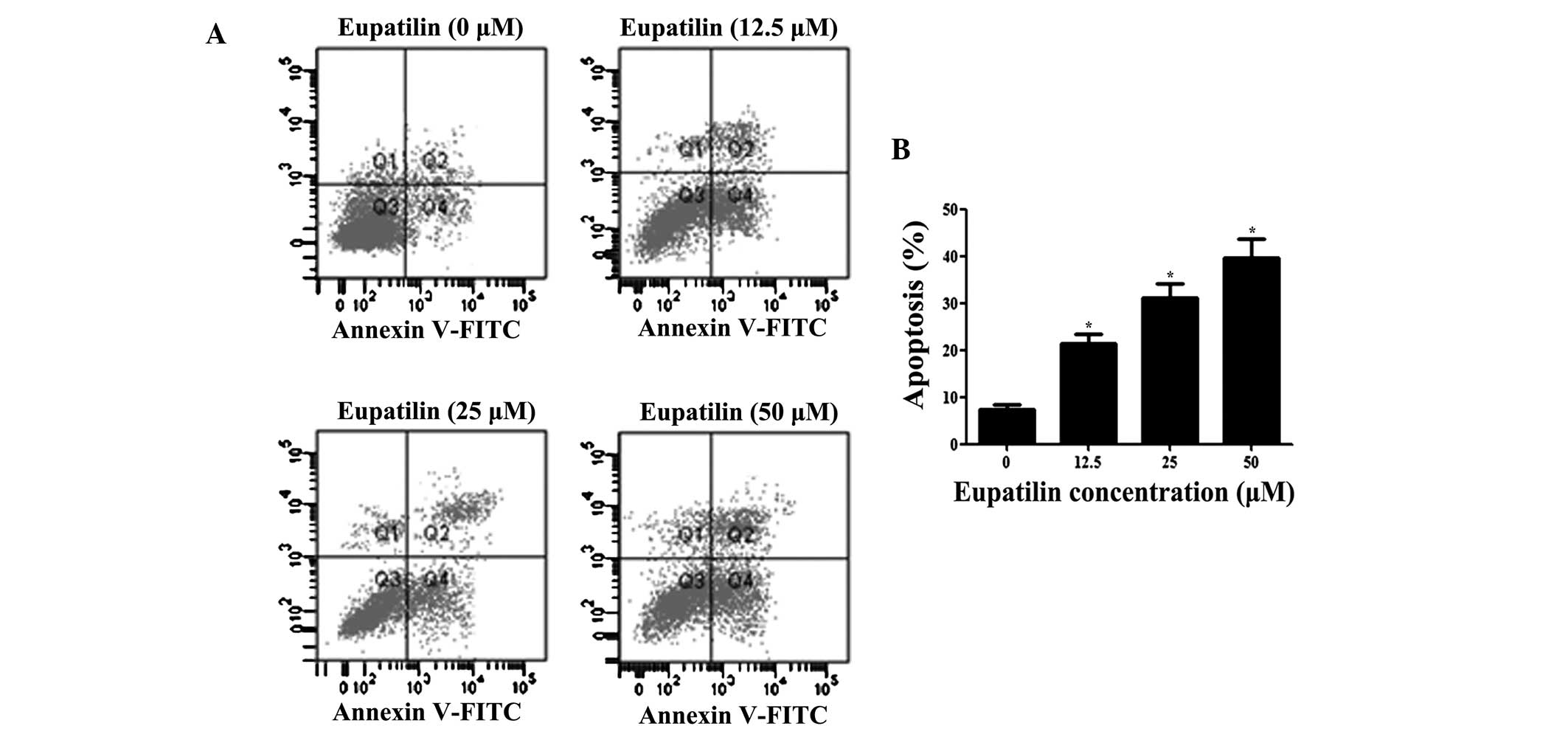
Following treatment with eupatilin for 48 h,
apoptosis of U87MG cells stained with Annexin V-FITC and PI was
determined by flow cytometric analysis. As presented in Fig. 3, the apoptotic rate of the cells in
the eupatilin treatment group was significantly higher than in the
control group, indicating that eupatilin induced the apoptosis of
glioma cells (P<0.05).
Eupatilin reduces Notch-1 expression in
glioma cells
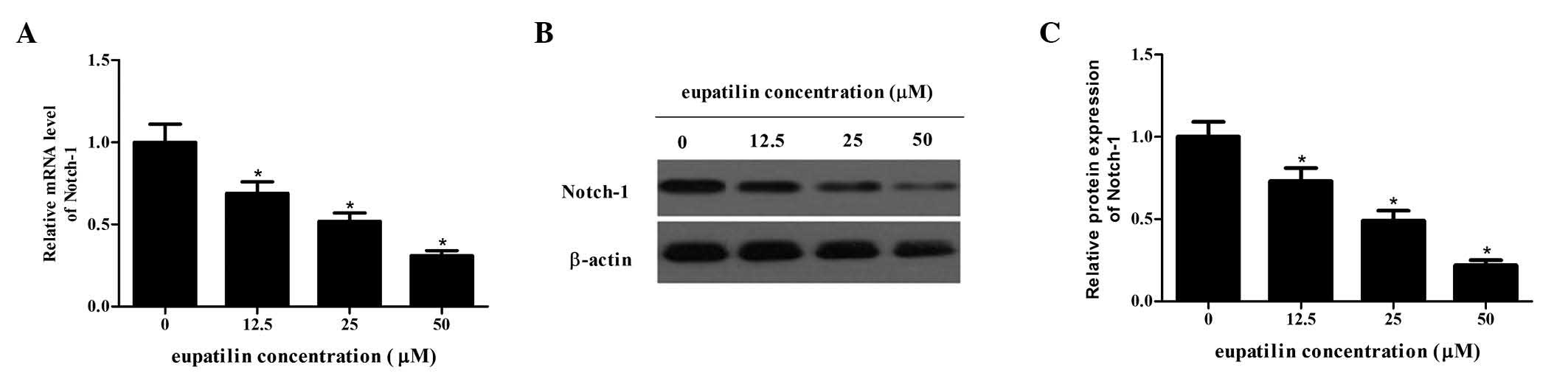
Notch-1 is a transmembrane receptor which is often
important for the proliferation and invasion of tumor cells
(14). The current study
determined how eupatilin affects Notch-1 expression using RT-PCR
and western blot analysis. The protein and mRNA expression levels
of Notch-1 following treatment with different concentrations of
eupatilin were significantly reduced compared with the control
group in a dose-dependent manner (P<0.05; Fig. 4). Therefore, eupatilin may suppress
Notch-1 expression in glioma cells.
Downregulation of Notch-1 by siRNA
potentiates the eupatilin-induced inhibition of proliferation and
invasion of glioma cells
In order to further examine the effect of Notch-1
glioma cells, Notch-1 was downregulated in U87MG cells using siRNA.
The transfected cells were treated with eupatilin of different
concentrations for 48 h. The protein expression levels of Notch-1
were detected by western blot analysis (Fig. 5A). The combination of Notch-1 siRNA
and eupatilin treatment led to inhibition of proliferation and
invasion of glioma cells to a greater extent than that observed
following treatment with eupatilin only (Fig. 5B and C).
Discussion
The current study determined that eupatilin has an
anticancer effect on glioma cells. This was demonstrated by the
inhibition of cell viability, decreased migration and
proliferation, and increased apoptosis of glioma cells.
Additionally, eupatilin suppressed Notch-1 expression and when
combined with a knockdown of Notch-1 by siRNA its anticancer effect
was increased.
Cell proliferation is important for cell survival
and it is also an essential biological feature of tumor formation.
Therefore, one aim of tumor treatment is to inhibit tumor
proliferation. The present study determined that eupatilin may
suppress the proliferation of viable glioma cells in a
dose-dependent manner. Consistent with these results, Son et
al (15) reported that
eupatilin also exhibited an inhibitory effect on the proliferation
of human aortic smooth muscle cells. In addition, eupatilin
inhibited the proliferation of ras-transformed human breast
epithelial cells (16).
Reducing metastasis may also be a promising method
for tumor treatment, as a high rate of metastasis often results in
a poor prognosis. In order to reduce metastasis, invasion and
migration of tumor cells should be inhibited. The present study
aimed to observe the effect of eupatilin on invasion and migration
of glioma cells using Transwell assays. Overall, eupatilin
decreased the migration and invasion abilities of glioma cells in a
dose-dependent manner. These results were consistent with previous
studies that focused on gastric and aortic cells (10,15).
Therefore, eupatilin may be used to suppress the invasion and
migration of glioma cells.
Triggering apoptosis in cancer cells may be an
important method for treating cancer (17,18).
Seo and Surh (19) revealed that
eupatilin may induce apoptosis in human promyelocytic leukemia
cells. In addition, Kim et al (20) demonstrated that eupatilin may
induce apoptosis in human gastric cancer cells. In accordance with
these studies, the present study identified that eupatilin may
promote apoptosis in glioma cells in a concentration-dependent
manner.
The Notch signaling pathway is important for
regulating cell proliferation and apoptosis (21,22).
It has been reported that the Notch signaling pathway has a
context-dependent function in tumorigenesis, either acting in an
antiproliferative or oncogenic manner (23). For example, the Notch gene
suppresses proliferation and induces apoptosis in certain tumor
cells, such as lung adenocarcinoma and hepatocellular carcinoma
cells; however, it functions as an oncogene in the majority of
solid tumors, such as glioma and breast cancer (24–27).
For example, Wang et al (28) reported that the Notch signaling
pathway contributes to glioma growth. Additionally, it has been
demonstrated that the Notch signaling pathway is important in the
development of glioma and may regulate proliferation of glioma
cells (29). There is growing
evidence that Notch-1 may affect the growth and invasion of glioma
cells and its downregulation may inhibit proliferation and promote
apoptosis (26,30,31).
The present study determined that eupatilin may reduce Notch-1
expression in glioma cells. When this was combined with knockdown
of Notch-1 by siRNA the inhibitory effect on glioma cell
proliferation and invasion was greater. These results suggested
that eupatilin inhibited proliferation, invasion and migration, and
induced apoptosis through the suppression of the Notch-1 signaling
pathway in glioma cells.
In conclusion, eupatilin exhibited an inhibitory
effect on the proliferation, invasion and migration of glioma
cells, in addition to promoting apoptosis via suppression of the
Notch-1 signaling pathway. Therefore, eupatilin may be a potential
agent for treatment of glioma.
References
|
1
|
Jovčevska I, Kočevar N and Komel R: Glioma
and glioblastoma - how much do we (not) know? Mol Clin Oncol.
1:935–941. 2013.
|
|
2
|
Partap S and Fisher PG: Update on new
treatments and developments in childhood brain tumors. Curr Opin
Pediatr. 19:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yaneva MP, Semerdjieva ML, Radev LR and
Vlaikova MI: Postoperative chemo-radiotherapy with temodal in
patients with glioblastoma multiforme - survival rates and
prognostic factors. Folia Med (Plovdiv). 52:26–33. 2010.
|
|
5
|
Jung J, Ko SH, Yoo Y, Lee JY, Kim YJ, Choi
SM, Kang KK, Yoon HJ, Kim H, Youn J and Kim JM: 5,
7-Dihydroxy-3,4,6-trimethoxyflavone inhibits intercellular adhesion
molecule 1 and vascular cell adhesion molecule 1 via the Akt and
nuclear factor-κB-dependent pathway, leading to suppression of
adhesion of monocytes and eosinophils to bronchial epithelial
cells. Immunology. 137:98–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oh TY, Ahn GJ, Choi SM, Ahn BO and Kim WB:
Increased susceptibility of ethanol-treated gastric mucosa to
naproxen and its inhibition by DA-9601, an Artemisia asiatica
extract. World J Gastroenterol. 11:7450–7456. 2005. View Article : Google Scholar
|
|
7
|
Choi EJ, Lee S, Chae JR, Lee H-S, Jun CD
and Kim SH: Eupatilin inhibits lipopolysaccharide-induced
expression of inflammatory mediators in macrophages. Life Sci.
88:1121–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim
YB, Surh YJ, Cho SW, Lee KM and Hahm KB: Oxidative stress is more
important than acid in the pathogenesis of reflux oesophagitis in
rats. Gut. 49:364–371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheong JH, Hong SY, Zheng Y and Noh SH:
Eupatilin inhibits gastric cancer cell growth by blocking
STAT3-mediated VEGF expression. J Gastric Cancer. 11:16–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park BB, Yoon J, Kim E, Choi J, Won Y,
Choi J and Lee YY: Inhibitory effects of eupatilin on tumor
invasion of human gastric cancer MKN-1 cells. Tumour Biol.
34:875–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho JH, Lee JG, Yang YI, Kim JH, Ahn JH,
Baek NI, Lee KT and Choi J-H: Eupatilin, a dietary flavonoid,
induces G2/M cell cycle arrest in human endometrial cancer cells.
Food Chem Toxicol. 49:1737–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
13
|
Côté MC, Lavoie JR, Houle F, Poirier A,
Rousseau S and Huot J: Regulation of vascular endothelial growth
factor-induced endothelial cell migration by LIM kinase 1-mediated
phosphorylation of annexin 1. J Biol Chem. 285:8013–8021. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teodorczyk M and Schmidt MH: Notching on
cancer's door: Notch signaling in brain tumors. Front Oncol.
4:341–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Son JE, Lee E, Seo SG, Lee J, Kim JE, Kim
J, Lee KW and Lee HJ: Eupatilin, a major flavonoid of Artemisia,
attenuates aortic smooth muscle cell proliferation and migration by
inhibiting PI3K, MKK3/6, and MKK4 activities. Planta Med.
79:1009–1016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim DH, Na HK, Oh TY, Shin CY and Surh YJ:
Eupatilin inhibits proliferation of ras-transformed human breast
epithelial (MCF-10A-ras) cells. J Environ Pathol Toxicol Oncol.
24:251–259. 2005. View Article : Google Scholar
|
|
17
|
Kang N, Zhang J-H, Qiu F, Tashiro S,
Onodera S and Ikejima T: Inhibition of EGFR signaling augments
oridonin-induced apoptosis in human laryngeal cancer cells via
enhancing oxidative stress coincident with activation of both the
intrinsic and extrinsic apoptotic pathways. Cancer Lett.
294:147–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strasser A, Cory S and Adams JM:
Deciphering the rules of programmed cell death to improve therapy
of cancer and other diseases. EMBO J. 30:3667–3683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seo HJ and Surh YJ: Eupatilin, a
pharmacologically active flavone derived from Artemisia plants,
induces apoptosis in human promyelocytic leukemia cells. Mutat Res.
496:191–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MJ, Kim DH, Na HK, Oh TY, Shin CY and
Surh Ph D Professor YJ: Eupatilin, a pharmacologically active
flavone derived from Artemisia plants, induces apoptosis in human
gastric cancer (AGS) cells. J Environ Pathol Toxicol Oncol.
24:261–269. 2005. View Article : Google Scholar
|
|
21
|
Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ
and He LY: Silencing Notch-1 induces apoptosis and increases the
chemosensitivity of prostate cancer cells to docetaxel through
Bcl-2 and. Bax Oncol Lett. 3:879–884. 2012.
|
|
22
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joutel A and Tournier-Lasserve E: Notch
signalling pathway and human diseases. Semin Cell Dev Biol.
9:619–625. 1998. View Article : Google Scholar
|
|
24
|
Zheng Q, Qin H, Zhang H, Li J, Hou L, Wang
H, Zhang X, Zhang S, Feng L, Liang Y, et al: Notch signaling
inhibits growth of the human lung adenocarcinoma cell line A549.
Oncol Rep. 17:847–852. 2007.PubMed/NCBI
|
|
25
|
Wang M, Xue L, Cao Q, Lin Y, Ding Y, Yang
P and Che L: Expression of Notch1, Jagged1 and beta-catenin and
their clinico-pathological significance in hepatocellular
carcinoma. Neoplasma. 56:533–541. 2009. View Article : Google Scholar
|
|
26
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al:
Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reedijk M, Odorcic S, Chang L, Zhang H,
Miller N, McCready DR, Lockwood G and Egan SE: High-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survival. Cancer Res.
65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Wang C, Meng Q, Li S, Sun X, Bo Y
and Yao W: siRNA targeting Notch-1 decreases glioma stem cell
proliferation and tumor growth. Mol Biol Rep. 39:2497–2503. 2012.
View Article : Google Scholar
|
|
29
|
Xing ZY, Sun LG and Guo WJ: Elevated
expression of Notch-1 and EGFR induced apoptosis in glioblastoma
multiforme patients. Clin Neurol Neurosurg. 131:54–58. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou ZD, Kumari U, Xiao ZC and Tan EK:
Notch as a molecular switch in neural stem cells. IUBMB Life.
62:618–623. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao N, Guo Y, Zhang M, Lin L and Zheng Z:
Akt-mTOR signaling is involved in Notch-1-mediated glioma cell
survival and proliferation. Oncol Rep. 23:1443–1447.
2010.PubMed/NCBI
|