Introduction
Chronic myeloid leukemia (CML) is a cancerous
disease in which blood cells lose the ability to perform their
normal roles (1), increasing the
risk of bleeding more easily, infection and anemia in patients with
CML (2). CML is associated with
the oncogenic BCR-ABL gene mutation in the Philadelphia
chromosome, where chromosomal translocation, t (9;22) (q34;q11.2)
causes a fusion of Abelson (ABL) at chromosome 9q34 with the
breakpoint cluster region (BCR) at chromosome 22q11.2
(3). It was revealed that the
BCR-ABL gene product increases the expression of MDM2, a
negative regulator of p53 (4).
MDM2, a regulator of p53, is an E3 ubiquitin-ligase, regulating the
stability of p53 (5). Loss of p53
is associated with the progression of CML (6) and p53 stabilization in CML cells
causes apoptosis (7–9).
Butein (3,4,2′,4′-tetrahydroxychalcone), extracted
from Rhus verniciflua stokes, stem-bark of cashews
(Semecarpus anacardium) or the heartwood of Dalbergia
odorifera (10–13), exerts an anticancer effect in
various types of cancer, including breast cancer (14,15),
prostate cancer (16), lymphoma
(11) and leukemia (17). In leukemia cells, butein has been
demonstrated to induce tumor necrosis factor-related
apoptosis-inducing ligand-mediated apoptosis (17). However, while chalcones, including
butein, caused the apoptosis of mouse melanoma cells independently
of p53 (18), p53 dependency in
butein-mediated apoptotic cell death remains to be elucidated.
The present study assessed the apoptotic effect of
butein on two different CML cell lines, KBM5 and K562. The KBM5
cells express wild-type p53 and the K562 cells express no p53
(19,20). Therefore, these cell lines provided
a clear model to determine whether the butein effect on apoptotic
cell death of CML cells was associated with the expression of p53.
Understanding the mechanisms underlying butein treatment is useful
for developing drugs to inhibit the progression of CML.
Materials and methods
Reagents and cell lines
Butein (3,4,2′,4′-tetrahydroxychalcone) was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). MG132 and cycloheximide were purchased from Calbiochem (La
Jolla, CA, USA). The caspase inhibitor, Z-VAD-FMK, was purchased
from Promega (Madison, WI, USA). The KBM5 and K562 cell lines were
kindly given by Dr Bharat B Aggarwal (University of Texas M.D.
Anderson Cancer Center, Houston, TX, USA) and from Dr Dong-Hoon Jin
(Asan Medical Center, Seoul, Korea), respectively. The cells were
cultured in Iscove's modified Dulbecco's medium, supplemented with
10% fetal bovine serum and 1% antibiotics (Welgene, Inc., Daegu,
Korea).
Cell viability and trypan blue assay
A total of 2×104 cells (for either the
KBM5 or the K562 cell line) were seeded into each well of 96-well
plates and were subsequently treated with butein at different
concentrations for 24 h. The cell viability was measured using an
EZ-Cytox Enhanced Cell Viability assay kit (DoGen, Seoul, Korea),
according to the manufacturer's instructions. Trypan blue assays
were performed to measure cell growth. The cells were treated with
various concentrations of butein for 72 h and the viable cell
numbers were quantified daily.
Western blotting
Whole cell extracts were lysed in cell lysis buffer
(Biosesang, Inc., Seongnam, Korea). Equal quantities of protein (30
µg) were separated on 8–12% SDS-PAGE gels and were
subsequently transferred onto a polyvinylidene difluoride membrane
(GE Healthcare Life Sciences, Freiburg im Breisgau, Germany). After
blocking the membranes with 1% bovine serum albumin and 2% skimmed
milk for 1 h, the membranes were incubated at 4°C overnight with
the appropriate primary antibody, and were washed three times in
phosphate buffered saline with 0.01% Tween-20. The membranes were
incubated at room temperature for 1 h with horseradish
peroxidase-conjugated secondary antibodies. In order to visualize
the protein bands, the membranes were treated with enhanced
chemiluminescence kit solution (DoGen) and exposed to X-ray film
(AGFA Healthcare, Mortsel, Belgium). Anti-PARP, caspase-3,
caspase-9, cyclin-dependent kinase (CDK)4, phosphorylated (p-)p53
and p-murine double minute 2 (MDM2) antibodies were obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-CDK1,
CDK2, cyclin E, cyclin A, cyclin B, p21, p53 and Bcl-2 antibodies
were purchased from Santa Cruz Biotechnology, Inc. Anti-cyclin D
and Bcl-xL antibodies were obtained from BD Biosciences (San Jose,
CA, USA). The anti-tubulin antibody was obtained from
Sigma-Aldrich, Inc. (St. Louis, MO, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
The total RNA was extracted using a total RNA
Extraction kit (Intron Biotechnology, Inc., Seongnam, Korea). The
cDNA was synthesized using a cDNA synthesis kit (Takara Bio, Inc.,
Shiga, Japan), according to manufacturer's instructions.
MDM2 mRNA amplification was then performed with cDNA (l
µg/µl) and the following primers: forward,
5′-CTGGGGAGTCTTGAGGGACC-3′ and reverse, 5′-CA GGTTGTCTAAATTCCTAG-3′
(21). The cycling conditions used
were as follows: denaturation at 94°C for 30 sec, annealing at 53°C
for 1 min, and elongation at 68°C for 2 min. After 35 cycles, the
MDM2 mRNA band was visualized using a Davinch-Chemi™
Chemiluminescence Imaging system (Davinch-K Co., Ltd., Seoul,
Korea).
Flow cytometry
To assess the cell cycle profile, the cells were
treated with butein and were subsequently fixed in 95% ethanol with
0.5 % Tween-20 at −20°C overnight. The fixed cells were stained
with 50 µg/ml propidium iodide (Santa Cruz Biotechnology,
Inc.). For apoptotic cell death, the cells were treated with butein
and subsequently stained with annexin V and 7-aminoactinomycin D
(7-AAD; Santa Cruz Biotechnology, Inc.). The cells were analyzed
using a FACSCalibur (BD Biosciences, San Jose, CA, USA) with
CellQuest Pro, version 5.2 software.
Statistical analysis
Student's t-test (Microsoft Excel version, 2007) was
used to determine statistically significant differences between the
cell lines. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of butein on the cell viability of
CML cells
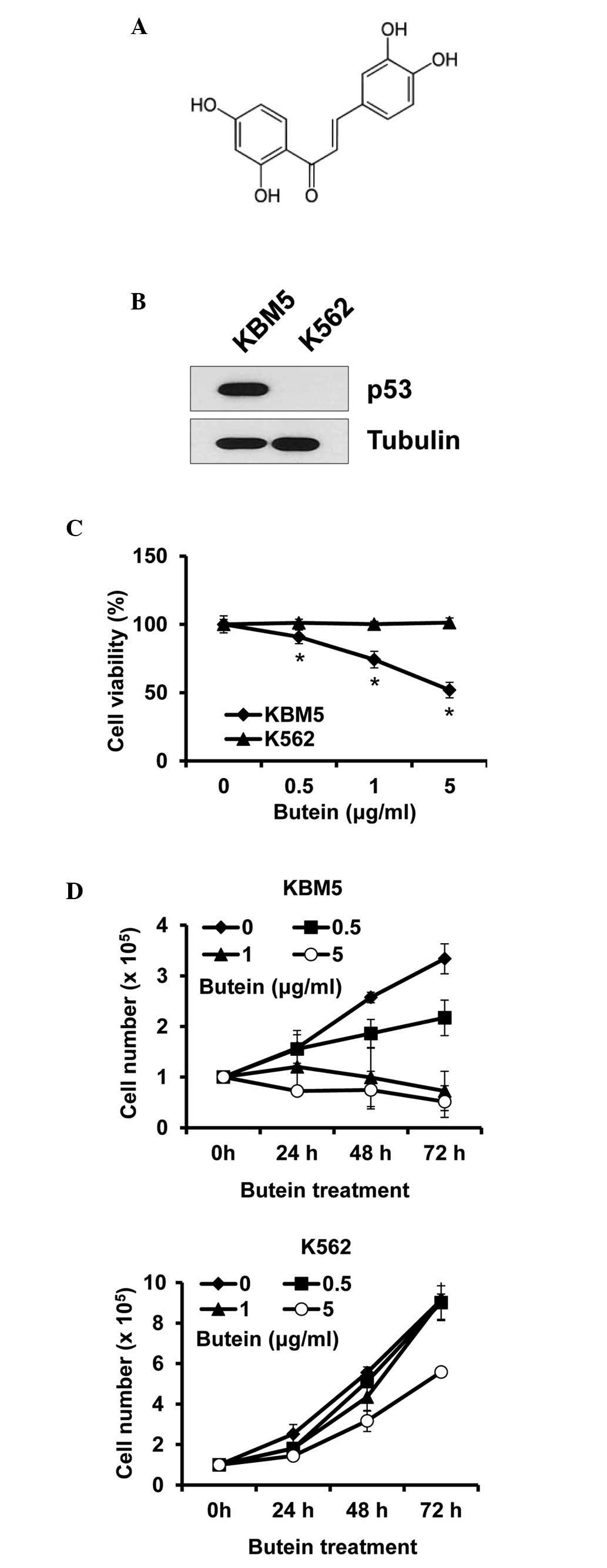
Butein is a polyphenolic compound obtained from
plants (Fig. 1A). To determine
whether the cytotoxic effect of butein on CML cells was
p53-dependent, CML cells expressing p53 (KBM5) or not (K562) were
treated with butein at different concentrations and were subjected
to viability assays. The expression patterns of p53 were confirmed
in the KBM5 and K562 cells, to ensure that the p53 status was as
described (Fig. 1B). Treatment
with 5 µg/ml butein for 24 h reduced the viability of the
KBM5 cells by ~48.1%, whereas it caused no effect on the viability
of the K562 cells (Fig. 1C). Cell
growth was subsequently measured for 72 h treatment. In accordance
with the data from the cell viability assays, butein reduced KBM5
cell numbers, however, not K562 cell numbers. Additionally, the
treatment prevented cell growth, even at 0.5 µg/ml, while
butein at 5 µg/ml marginally reduced K562 cell growth
(Fig. 1D). Therefore, these data
indicated that the expression of p53 in CML cells was crucial for
butein sensitivity.
Effect of butein on the CML cell
cycle
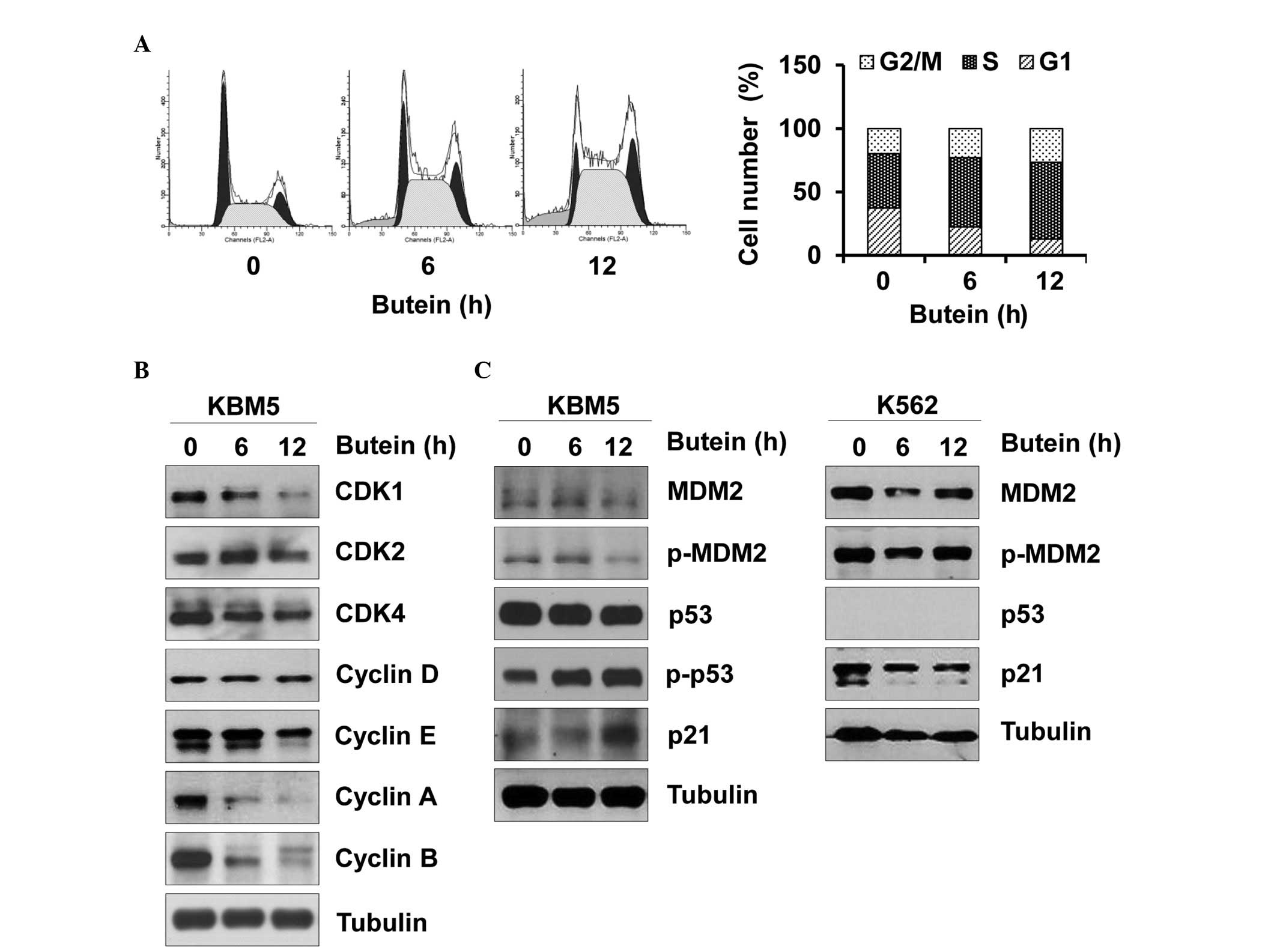
Next, whether butein affected the cell cycle of CML
cells was assessed, since an abnormal cell cycle is tightly
associated with cell death. Butein arrested the KBM5 cells in
S-phase of the cell cycle (Fig.
2A). Furthermore, butein reduced the expression levels of CDK1,
CDK4, cyclin A, cyclin B and cyclin E, and caused no affect on the
expression levels of CDK2 and cyclin D (Fig. 2B). Therefore, butein-mediated
changes in the expression levels of CDK/cyclin appeared to cause
S-phase arrest. Furthermore, butein increased the phosphorylation
of p53 and in turn, increased the expression of p21, while reducing
phosphorylated and total expression levels of MDM2 in the KBM5
cells (Fig. 2C). In the K562
cells, butein marginally reduced the expression levels of p21 and
MDM2 (Fig. 2C). Therefore, these
data suggested that butein may impair the CML cell cycle by
targeting p53 and its downstream effectors.
Effect of butein on the expression of
MDM2 in CML cells
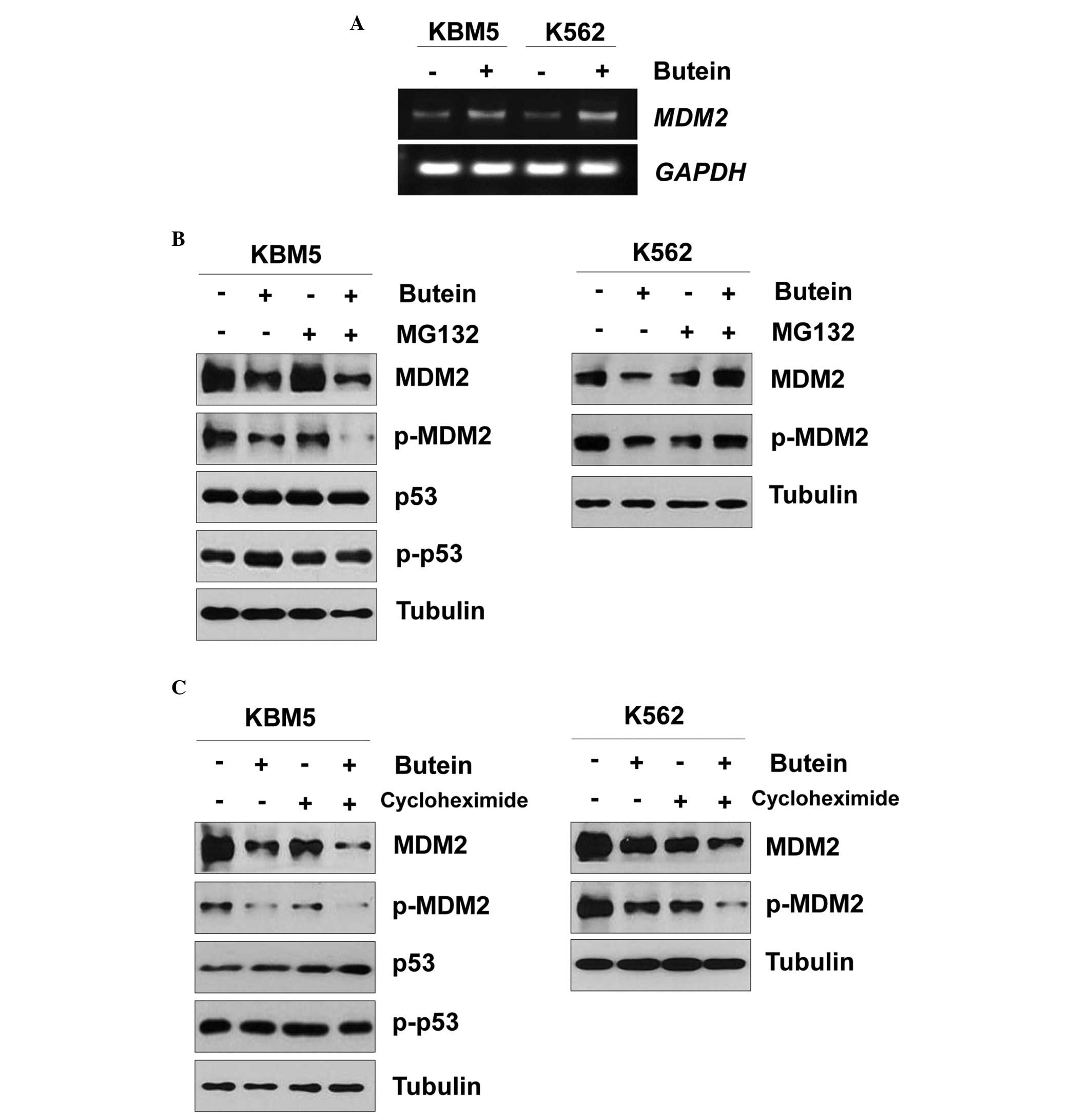
Butein reduced the expression of MDM2 independent of
the p53 status (Fig. 2B).
Therefore, the present study further examined the effect of butein
on MDM2. When the KBM5 and K563 cells were treated with butein, the
mRNA expression of MDM2 was increased (Fig. 3A). Since butein reduced the protein
expression of MDM2, whether butein affected MDM2 protein stability
was assessed. When the KBM5 cells were pretreated with MG132 and
subsequently treated with butein, the protein expression of MDM2
was reduced (Fig. 3B), as earlier.
Additionally, butein reduced the protein expression of MDM2 even in
KBM5 cells treated with cycloheximide (Fig. 3C). Therefore, the MDM2 protein may
be degraded in a proteasome-independent manner. Notably, treatment
with MG132 rescued the butein-mediated MDM2 reduction in K562 cells
(Fig. 3B). In addition, the
protein expression of MDM2 was reduced even in the K562 cells
treated with butein and cycloheximide (Fig. 3C). Therefore, these data suggested
that butein-mediated MDM2 degradation may differ between the KBM5
and K562 cells.
Effect of butein on the apoptosis of CML
cells
To confirm that butein induced apoptotic cell death,
the cells were stained with annexin V and 7-AAD. Treatment with
butein for 24 h induced the apoptosis of KBM5 cells. This apoptotic
effect was not as severe in the K562 cells (Fig. 4A). These data are consistent with
the cell growth and viability assays, and support that p53
sensitizes butein-mediated apoptotic cell death.
Next, the effect of butein on the expression levels
of proteins involved in apoptosis was assessed. Assessing Bcl-2
reduction, cleavage of PARP and the levels of caspase-3, it was
demonstrated that KBM5 cells were more sensitive to butein compared
with the K562 cells (Fig. 4B).
Next, the effect of butein on apoptotic cell death at different
treatment durations was determined. Butein caused apoptotic
responses in KBM5 cells 6 h after treatment, as indicated by
reduced levels of Bcl-2 and Bcl-xL, and increased cleaved forms of
caspase-9, caspase-3 and PARP (Fig.
4C). To determine whether butein-mediated apoptosis involved
the activation of caspases, Z-VAD-FMK, a caspase inhibitor was
used. This inhibitor failed to reduce PARP cleavage, while it
inhibited the cleavage of caspase-9 and caspase-3 (Fig. 4D). Therefore, these data suggested
that butein may cause CML apoptosis via a p53-dependent and
caspase-independent signaling pathway.
Discussion
The present study demonstrated for the first time,
to the best of our knowledge, that butein-induced apoptosis of CML
cells was dependent on the p53 expression pattern. Furthermore, in
CML cells expressing p53 (KBM5) or those not expressing p53 (K562),
butein mediated the degradation of MDM2 by different mechanisms.
Therefore, while high doses of butein may result in the apoptosis
of CML cells independently of p53 (22), low doses of butein selectively
induced apoptotic cell death of p53-positive CML cells.
As mentioned above, higher doses of butein (>20
µM) caused CML cell death. However, higher doses of butein
appear to cause adverse effects. In the present study, lower doses
of butein only caused apoptosis in p53-expressing CML cells.
Consistently, butein affected the expression of the p53 downstream
effector, p21, and regulated the stability of MDM2. Additionally,
butein mediated cell cycle arrest in S-phase. Therefore, the
butein-activated p53 pathway may arrest cells at S-phase, resulting
in apoptotic cell death.
The present study further demonstrated that butein
reduced the protein expression of MDM2 in both p53-expressing KBM5
and p53-null K562 cells, while butein increased the mRNA expression
of MDM2 independently of p53. The butein-mediated reduction
of MDM2 protein appeared to follow two different mechanisms:
Proteasome-dependent or -independent. Butein reduced the
degradation of MDM2 in a proteasome-independent manner when p53 is
expressed in CML cells. It was revealed that hispolon, a chemical
compound from Phellinus species, reduces the protein
expression of MDM2 via a lysosomal degradation pathway (23), which is similar to the butein
effect on CML cells. Therefore, while the molecular mechanisms by
which butein causes proteasome-independent degradation of MDM2 in
p53-expressing cells remains to be elucidated, the present study
suggested that p53 may decide the fate of MDM2, at least in CML
cells.
In conclusion, the present study revealed the
molecular mechanism by which butein caused the apoptosis of CML
cells. While the butein effect on CML cells was revealed previously
(17), the present study revealed
for the first time, to the best of our knowledge, that
butein-mediated apoptotic cell death was dependent on p53, even in
CML. Therefore, it is worth investigating the effect of butein on
cell death or growth retardation in other cancer cell types
expressing wild-type p53.
Acknowledgments
This work was supported by a National Research
Foundation of Korea (NRF) grant funded by the Korea government
(Ministry of Science; no. 2007-0054931).
References
|
1
|
Gavel A: Chronic myelogenous leukemia; a
case report. Can J Med Technol. 8:52–54. 1946.PubMed/NCBI
|
|
2
|
Kantarjian HM, Smith TL, McCredie KB,
Keating MJ, Walters RS, Talpaz M, Hester JP, Bligham G, Gehan E and
Freireich EJ: Chronic myelogenous leukemia: A multivariate analysis
of the associations of patient characteristics and therapy with
survival. Blood. 66:1326–1335. 1985.PubMed/NCBI
|
|
3
|
Frei E III, Tjio JH, Whang J and Carbone
P: Studies of the philadelphia chromosome in patients with chronic
myelogenous leukemia. Ann N Y Acad Sci. 113:1073–1080. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trotta R, Vignudelli T, Candini O, Intine
RV, Pecorari L, Guerzoni C, Santilli G, Byrom MW, Goldoni S, Ford
LP, et al: BCR/ABL activates mdm2 mRNA translation via the La
antigen. Cancer Cell. 3:145–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oliner JD, Kinzler KW, Meltzer PS, George
DL and Vogelstein B: Amplification of a gene encoding a
p53-associated protein in human sarcomas. Nature. 358:80–83. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feinstein E, Cimino G, Gale RP, Alimena G,
Berthier R, Kishi K, Goldman J, Zaccaria A, Berrebi A and Canaani
E: p53 in chronic myelogenous leukemia in acute phase. Proc Natl
Acad Sci USA. 88:6293–6297. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peterson LF, Mitrikeska E, Giannola D, Lui
Y, Sun H, Bixby D, Malek SN, Donato NJ, Wang S and Talpaz M: p53
stabilization induces apoptosis in chronic myeloid leukemia blast
crisis cells. Leukemia. 25:761–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahdi T, Alcalay D, Cognard C, Tanzer J
and Kitzis A: Rescue of K562 cells from MDM2-modulated
p53-dependent apoptosis by growth factor-induced differentiation.
Biol Cell. 90:615–627. 1998. View Article : Google Scholar
|
|
9
|
Rizzo MG, Zepparoni A, Cristofanelli B,
Scardigli R, Crescenzi M, Blandino G, Giuliacci S, Ferrari S, Soddu
S and Sacchi A: Wt-p53 action in human leukaemia cell lines
corresponding to different stages of differentiation. Br J Cancer.
77:1429–1438. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang EB, Zhang K, Cheng LY and Mack P:
Butein, a specific protein tyrosine kinase inhibitor. Biochem
Biophys Res Commun. 245:435–438. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JC, Lee KY, Kim J, Na CS, Jung NC,
Chung GH and Jang YS: Extract from Rhus verniciflua Stokes is
capable of inhibiting the growth of human lymphoma cells. Food Chem
Toxicol. 42:1383–1388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Selvam C, Jachak SM and Bhutani KK:
Cyclooxygenase inhibitory flavonoids from the stem bark of
Semecarpus anacardium Linn. Phytother Res. 18:582–584. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan SC, Chang YS, Wang JP, Chen SC and
Kuo SC: Three new flavonoids and antiallergic, anti-inflammatory
constituents from the heartwood of Dalbergia odorifera. Planta Med.
64:153–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho SG, Woo SM and Ko SG: Butein
suppresses breast cancer growth by reducing a production of
intracellular reactive oxygen species. J Exp Clin Cancer Res.
33(51)2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JO, Moon JW, Lee SK, Kim SM, Kim N, Ko
SG, Kim HS and Park SH: Rhus verniciflua extract modulates survival
of MCF-7 breast cancer cells through the modulation of
AMPK-pathway. Biol Pharm Bull. 37:794–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan N, Adhami VM, Afaq F and Mukhtar H:
Butein induces apoptosis and inhibits prostate tumor growth in
vitro and in vivo. Antioxid Redox Signal. 16:1195–1204. 2012.
View Article : Google Scholar :
|
|
17
|
Kim N: Butein sensitizes human leukemia
cells to apoptosis induced by tumor necrosis factor-related
apoptosis inducing ligand (TRAIL). Arch Pharm Res. 31:1179–1186.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwashita K, Kobori M, Yamaki K and
Tsushida T: Flavonoids inhibit cell growth and induce apoptosis in
B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 64:1813–1820.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lubbert M, Miller CW, Crawford L and
Koeffler HP: p53 in chronic myelogenous leukemia. Study of
mechanisms of differential expression. J Exp Med. 167:873–886.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sen S, Takahashi R, Rani S, Freireich EJ
and Stass SA: Expression of differentially phosphorylated Rb and
mutant p53 proteins in myeloid leukemia cell lines. Leuk Res.
17:639–647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zell JA, Ramakrishnan R and Rathinavelu A:
Regulation of mdm2 mRNA expression in human breast tumor-derived
GI-101A cells. Life Sci. 71:2331–2339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harikumar KB, Kunnumakkara AB, Ahn KS,
Anand P, Krishnan S, Guha S and Aggarwal BB: Modification of the
cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by
xanthohumol leads to suppression of NF-kappaB-regulated gene
products and potentiation of apoptosis in leukemia cells. Blood.
113:2003–2013. 2009. View Article : Google Scholar
|
|
23
|
Lu TL, Huang GJ, Wang HJ, Chen JL, Hsu HP
and Lu TJ: Hispolon promotes MDM2 downregulation through
chaperone-mediated autophagy. Biochem Biophys Res Commun.
398:26–31. 2010. View Article : Google Scholar : PubMed/NCBI
|