Introduction
Peripheral nerves enable communication between the
brain and muscles, skin and organs, and peripheral nerve injury
often results in the loss of sensory or motor function (1,2). The
majority of the functions of the larynx depend on healthy vocal
fold motion, which in turn relies on an intact recurrent laryngeal
nerve (RLN) (3). Laryngeal palsy
often occurs as a result of recurrent laryngeal or vagal nerve
injury during oncological surgery of the head and neck (4). Despite a standardized surgical
approach, it is difficult to locate the RLN or protect it when scar
tissue, inflammatory changes, malignancies of the thyroid, or
anatomical variations in the RLN are present (5). During surgery, the RLN may be injured
as a result of stretching, compression, cutting or cauterizing
(6). Although the injured RLN
regenerates, even across a gap (7,8),
absolute normal function is not recovered due to laryngeal muscle
atrophy, motor neuron loss, decreased motor fiber density, or
synkinesis (9–12). Furthermore, the regeneration
potential of the RLN is associated with the severity of the injury
(13).
The symptoms and signs of laryngeal paralysis vary
(14). Although a number of
patients with unilateral vocal fold paralysis are completely
asymptomatic, others may experience disabling symptoms, such as
serious hoarseness, dysphagia and dysphonia (15). In addition to affecting the quality
of life, RLN injury may impose psychosocial and economic burdens
(16). The vocal folds are fragile
structures, and even a slight injury may result in extreme
complications. Early intervention is important for complete
recovery and preservation of laryngeal function (17). Reinnervation techniques, such as
neural anastomosis, nerve grafting and creation of a laryngeal
muscle pedicle have been reported, however, they have had little
impact and are not widely accepted as treatment methods (18).
Spontaneous reinnervation is normal following RLN
injury (19–22). In patients, RLN reinnervation
improves muscle tone and enables vocal fold medicalization
(23–25). However, as it cannot restore
motion, RLN reinnervation is not beneficial for patients with
unilateral paralysis (19).
Although the rat larynx is small, it has been used
as a model for investigating RLN injury and recovery (6). In previous studies, peripheral nerve
injury models involved crushing, transecting, cutting, compression,
stretching and cauterizing the nerve (26–39).
The present study selected the transection model, as it utilizes a
simple and reproducible technique, and is ideal for analyzing the
characteristics of spontaneous reinnervation.
Neurotrophic factors are a group of proteins that
have been demonstrated to prevent motor neuron loss, and promote
differentiation and reinnervation (40–45).
Neurotrophic factors are observed in neural tissue and in skeletal
muscles, where they provide a retrograde trophic influence on
innervating motor neurons (45).
Previous studies determined the effects of various neurotrophic
factors, such as brain-derived neurotrophic factor (BDNF), ciliary
neurotrophic factor, neurotrophin-4, and nerve growth factor, in
RLN injury (4,17,19,22,46–51);
treatment with neurotrophins may stimulate nerve regeneration
(48).
The aim of the present study was to characterize the
features of spontaneous reinnervation in rats following RLN injury.
Therefore, based on previous studies, two neurotrophic factors,
BDNF and glial cell line-derived neurotrophic factor (GDNF) were
selected to investigate their expression levels following nerve
injury.
Materials and methods
Experimental animals
The present study was conducted on 25 male
Sprague-Dawley rats (weight, 320–350 g) purchased from the Shanghai
SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The rats were
maintained in the animal laboratory of the Shanghai Jiao Tong
University affiliated Shanghai First People's Hospital (Shanghai,
China) and all surgical procedures were performed in the animal
operating room. The experiment was approved in accordance with the
Institutional Animal Care and Use guidelines of Shanghai Jiao Tong
University by the Animal Experimental Committee of First People's
Hospital affiliated with the Shanghai Jiao Tong University of
Medicine. The animals were treated humanely. All rats were
maintained under standardized laboratory conditions in groups of 5
rats, with food and water available ad libitum. The humidity
was 60% and the air temperature was 25°C, with a 12 h light/dark
cycle.
Surgical procedure
Following intraperitoneal anesthesia with sodium
pentobarbital (40 mg/kg body weight; Shanghai Yuanye Biotechnology
Co., Ltd., Shanghai, China), telescopic video laryngoscopy
(BF-1T160; Olympus Corporation, Tokyo, Japan) was performed to
confirm normal motion of the vocal fold with breathing. Following
removal of the hair on the neck, a vertical midline cervical
incision was made with a sharp blade (Tongkang Medical Instrument
Co., Ltd., Shanghai, China). To expose the tracheoesophageal
groove, the salivary glands and infrahyoid muscles were reflected
laterally using iris hooks (Tongkang Medical Instrument Co., Ltd.).
Subsequently, the right RLN was carefully isolated from the
tracheoesophageal groove. The animals were divided into two groups,
with 5 rats/group. For the denervation group, the right RLN was
resected at the level of the seventh tracheal ring, with the
removal of a 5-mm segment. The muscle layers and skin were sutured
separately. For the control group, the incision was sutured
following identification of the right RLN. Telescopic video
laryngoscopy was repeated to confirm paralysis of the right vocal
fold. On occasions where the right vocal fold exhibited movement,
the rat was excluded from further investigation. An intraperitoneal
injection of benzylpenicillin sodium (105 U/kg body
weight; North China Pharmaceutical Co., Ltd., Shijiazhuang, China)
was administered to all rats to prevent infection. All the rats
were monitored following surgery, fed a standard diet, and allowed
to heal for 3, 6, 10 or 16 weeks (n=5 for each group and time
period).
Vocal fold movement
At 3, 6, 10 and 16 weeks, five rats with transection
injuries were anesthetized again, as described above. Previous
studies have demonstrated that the effects of anesthesia on vocal
fold mobility are minimal (52,53).
Video laryngoscopy was performed to assess the vocal fold motion
and glottis morphology during spontaneous breathing. The movements
observed via transoral endoscopy were divided into five different
categories ranging from grade 0 to 4 using the following criteria:
grade 0, no vocal fold movement; grade 1, slight vocal fold
movement; grade 2, <50% abduction of vocal fold; grade 3,
>50% abduction of vocal fold; and grade 4, normal vocal fold
movement. The final scores were obtained by two investigators who
were blinded to the animal grouping and time since denervation. In
case of disagreement, a third investigator's score was obtained.
Normal movement of the vocal fold was measured using five rats and
a double-blind assay was performed to prevent subjective bias.
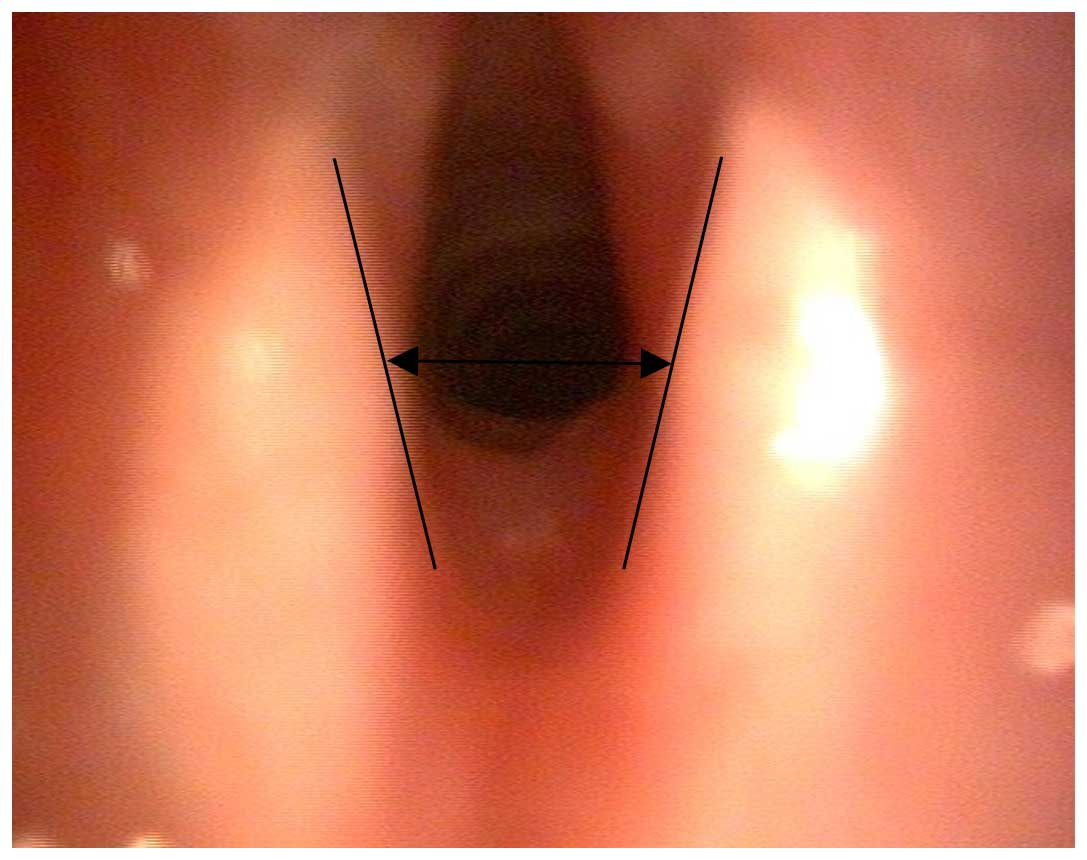
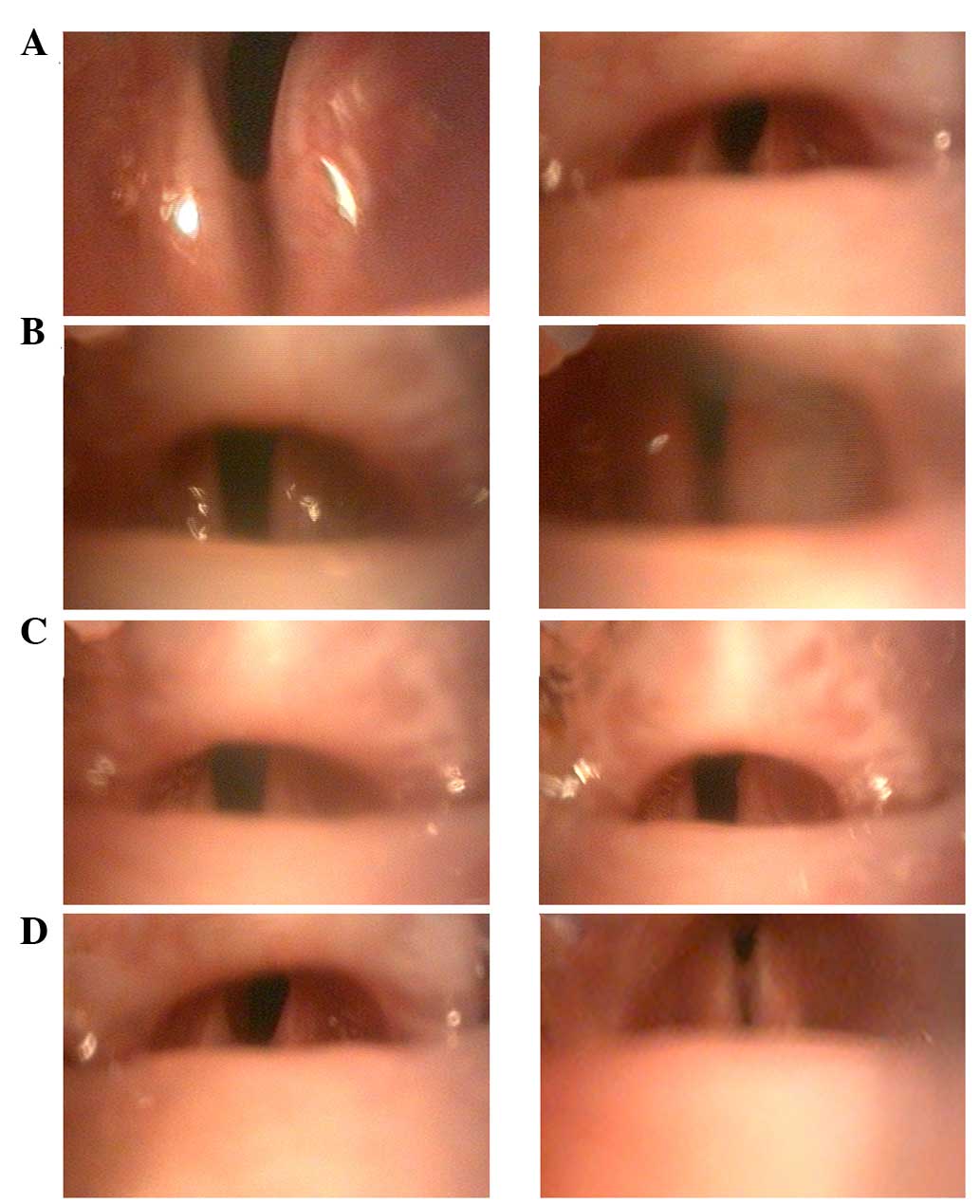
Images were captured for measurement of the
arytenoid cartilages (Fig. 1). The
angles between the arytenoid cartilage structures were calculated
(54) using images of maximal
adduction and abduction (Fig.
2).
Measurement of vocalization
Animals were maintained in a quiet environment for
one day prior to euthanasia. Vocalization lasting 20 sec was
recorded by stimulating the right hind limb using forceps every 4 h
following surgery. The procedures were performed in an identical
environment by the same investigator using the same equipment,
crush pressure (5 N), and distance between the recorder and mouth
(10 cm). A capture tool (Cool Edit Pro v2.1; Adobe Systems, Inc.,
San Jose, CA, USA) was used to record the sound fragments, followed
by acoustic space analysis using Photoshop CC software, version
14.0 (Adobe Systems, Inc.). Calculations were performed as follows:
Spectral area (%) = area of postoperative rats/area of control
rats. Amplitude (%) = amplitude of postoperative rats/amplitude of
control rats.
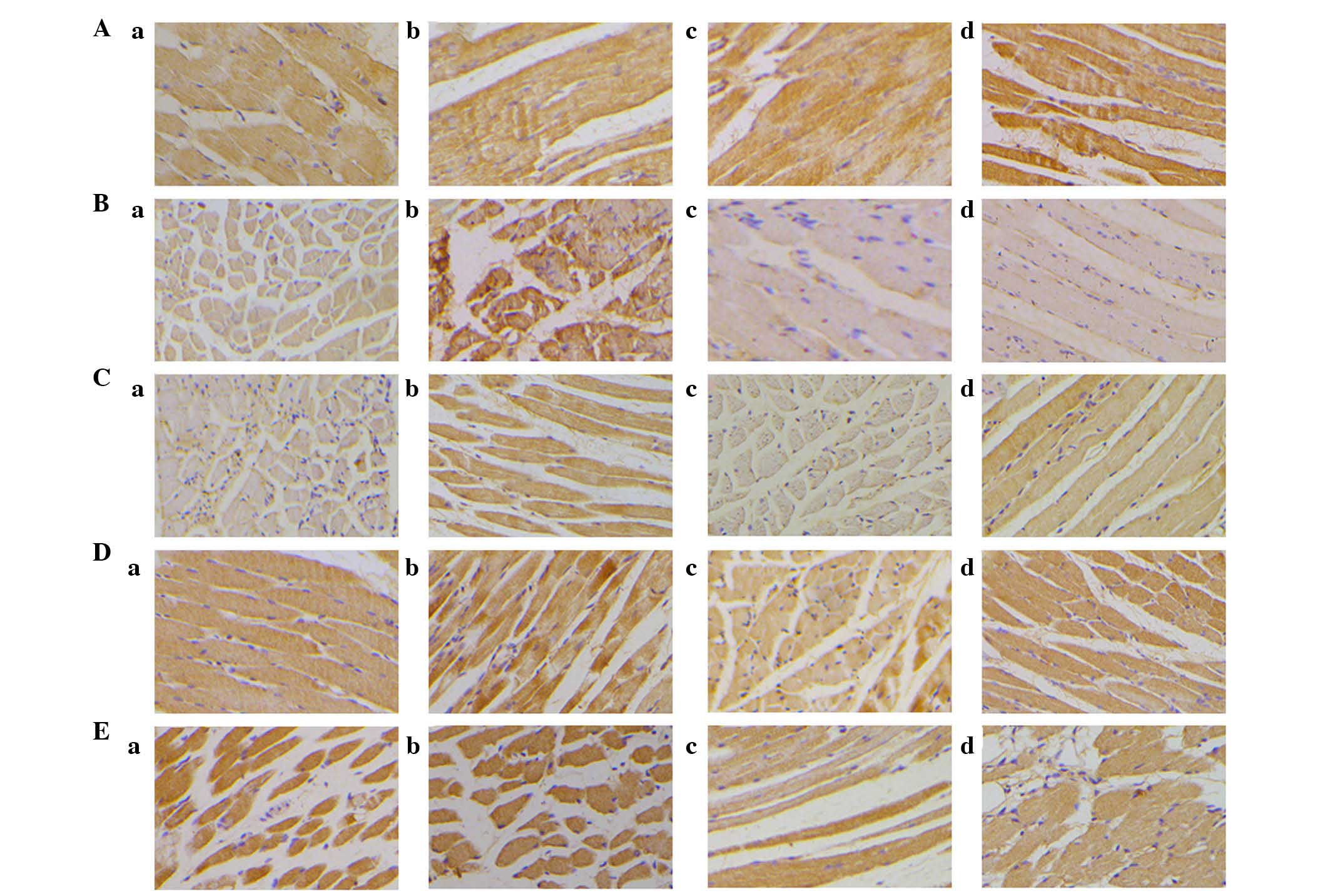
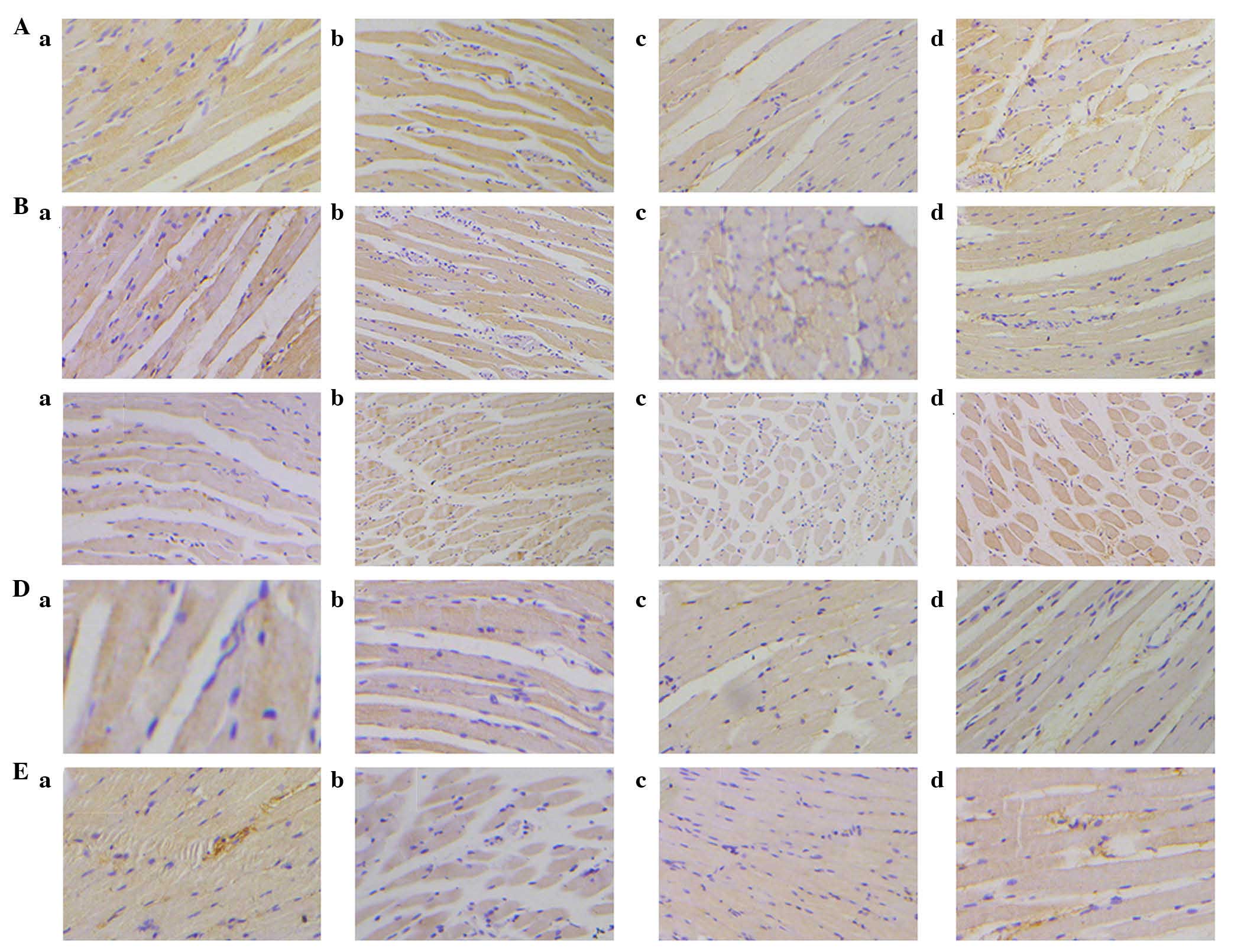
Histologic examination
Following euthanasia using sodium pentobarbital (120
mg/kg body weight), the larynx was excised. Under a dissecting
microscope (SZ51-ILST-SET; Olympus Corporation), the thyroarytenoid
(TA) and posterior cricoarytenoid (PCA) muscles were removed for
immunostaining of BDNF and GDNF. The TA and PCA muscles were
selected for the current study as the TA muscles are hypothesized
to be the most affected laryngeal muscles following resection of
the RLN, and the PCA muscle is the sole abductor muscle (55). At each time-point, six sections (7
µm thick) from each rat were prepared for staining. Sections
were pretreated with 0.3% H2O2 and
preincubated in 5% bovine serum albumin (Wuhan Boster Biological
Technology, Co., Ltd., Hubei, China). The sections were then
incubated with a rabbit monoclonal antibody against BDNF (dilution,
1:75; ab108319; Abcam, Cambridge, MA, USA) or a rabbit polyclonal
antibody against GDNF (dilution, 1:100; sc-328; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), overnight at 4°C, followed
by incubation for 15 min at 37°C with the horseradish
peroxidase-conjugated secondary antibody (GK500711; Genetic
Technology Co., Ltd., Shanghai, China) according to the
manufacturer's instructions. Following incubation with
3,3′-diaminobenzidine, the sections were dehydrated, dried and
coverslipped.
Measurement of immunostaining
Two observers blinded to the grouping examined each
section separately using a morphometric workstation running Image
Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA). Following calibration of the optical density, the images were
recorded at a final magnification of ×100. The area, mean density
and relative optical density of immunolabeling were obtained for
each specimen. The microscope illumination was calibrated to ensure
that the relative optical density values were within the linear
response range.
The right RLN was harvested. The left nerve served
as a control and was harvested at the same level. The RLN was
isolated and fixed in 4% (vol/vol) formaldehyde for 48 h, embedded
in paraffin and 7-µm sections were cut from each segment,
and examined by modified Bielschowsky staining (56). The slices were placed in silver
nitrate solution for impregnation for 30 min and restored using
formaldehyde. Silver ammonia was added, which was followed by
another restoration. Finally, the sections were toned with gold
chloride and fixed by sodium thiosulfate (Shanghai Yuanye
Biotechnology Co., Ltd.). As a result, the axons appeared black.
For each time-point, six sections from each rat were prepared for
imaging. The diameter and number of axons were calculated using
Photoshop CC software, version 14.0 (Adobe Systems, Inc.), and the
longest and shortest axons in addition to the mean axon diameter
were analyzed.
Statistical analysis
Each outcome value was summarized as the mean ±
standard deviation. A one-way analysis of variance with Tukey's
multiple comparison tests was used for statistical analysis of the
measures of vocal fold movement and vocalization using SPSS version
20.0 (IBM SPSS, Armonk, NY, USA). The immunostaining and
histological results were analyzed using the independent-samples
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Animal survival and recovery
All animals survived until the predetermined
endpoint. All incisions healed well without infection. Nerve ends
were surrounded by scar adhesions, with no significant bulges.
Effect of denervation on vocal fold
motion
All denervated animals demonstrated immediate right
vocal fold paralysis. No obvious vocal cord movement was recorded
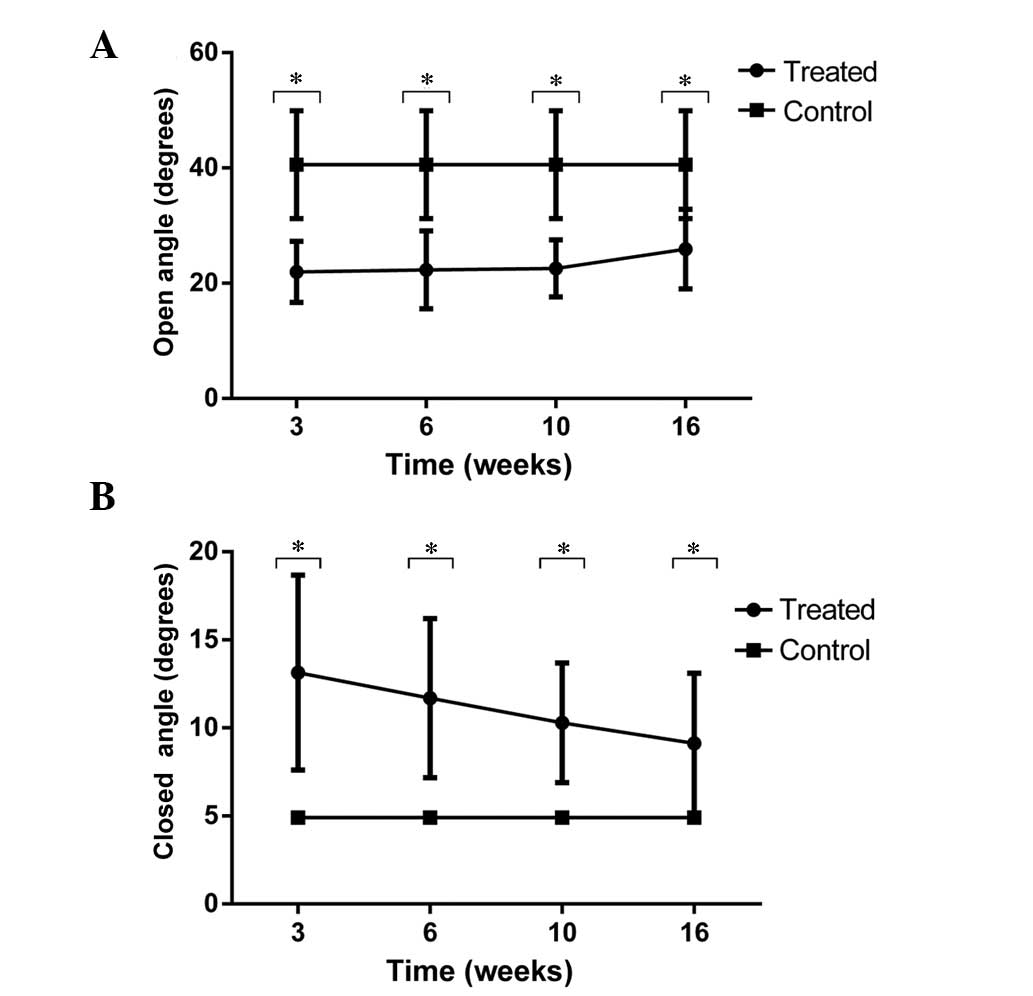
at any of the time intervals following transection of the RLN. A
significant difference was indicated for angles between the
arytenoid cartilage structures for the denervation and control
groups (P<0.01). The angles of maximal abduction were
21.97±5.30, 22.34±6.79, 22.56±4.94, 25.94±6.92 and 40.60±9.36, at
3, 6, 10 and 16 weeks and in the control group, respectively
(Fig. 3A), and the maximal
adduction angles were 13.14±5.54°, 11.70±4.52°, 10.29±3.41°,
9.12±3.99° and 4.90±0.22°, at 3, 6, 10 and 16 weeks and in the
control group, respectively (Fig.
3B). The vocal fold movements on the side of nerve injury did
not return to normal during the study.
Effect of denervation on
vocalization
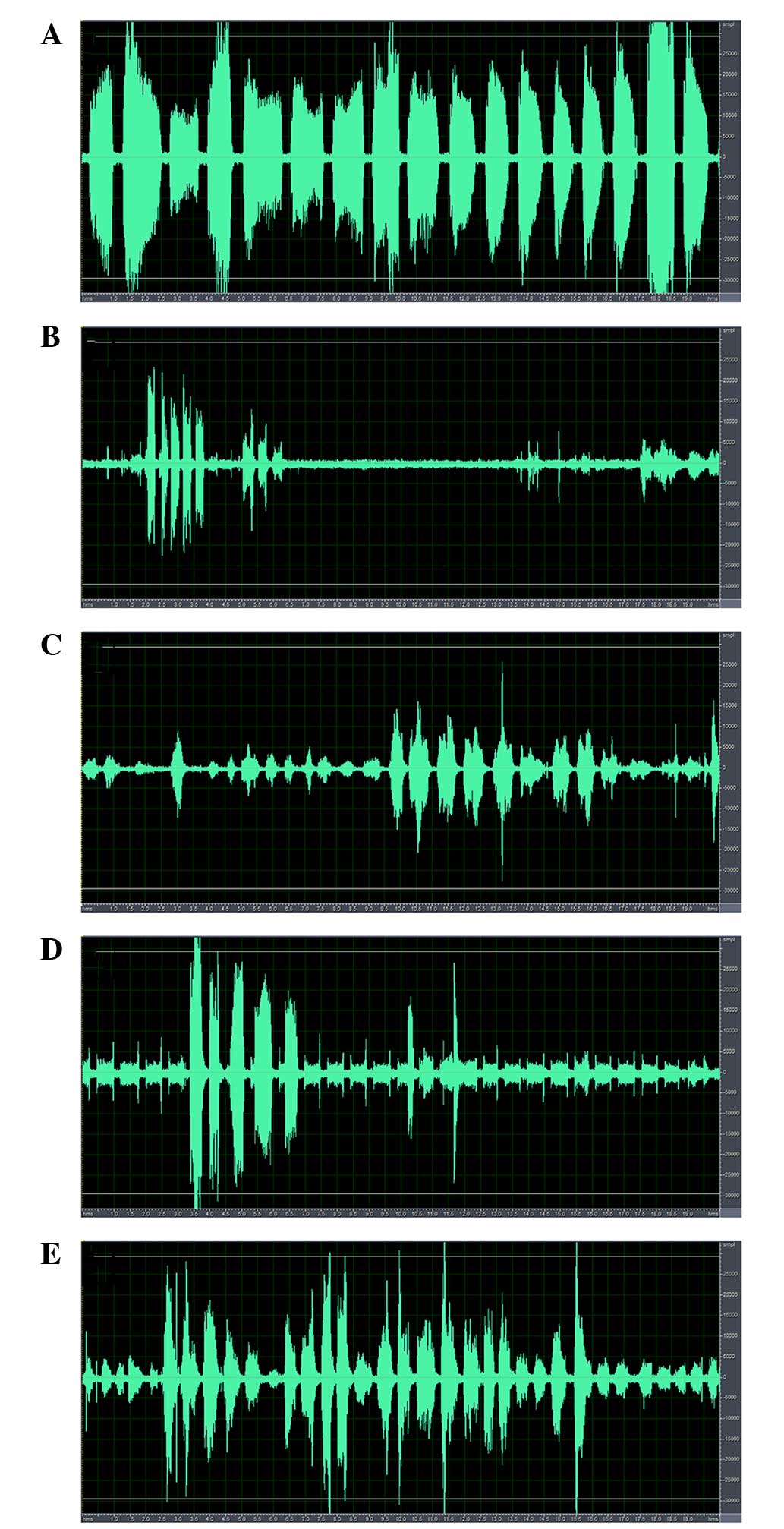
The voice spectra of the denervation and control
groups are presented in Fig. 4.
The vocalization sounded sharp and high-pitched in the control
rats, whereas it was hoarse and deep in the denervation groups. The
spectral analysis of the control group demonstrated a high
amplitude with a wide, continuous waveform (Fig. 4A). Following RLN injury, the
spectrum became narrow with a low amplitude. However, the amplitude
and width of the continuous waveform increased gradually with time
(Fig. 4B–E).
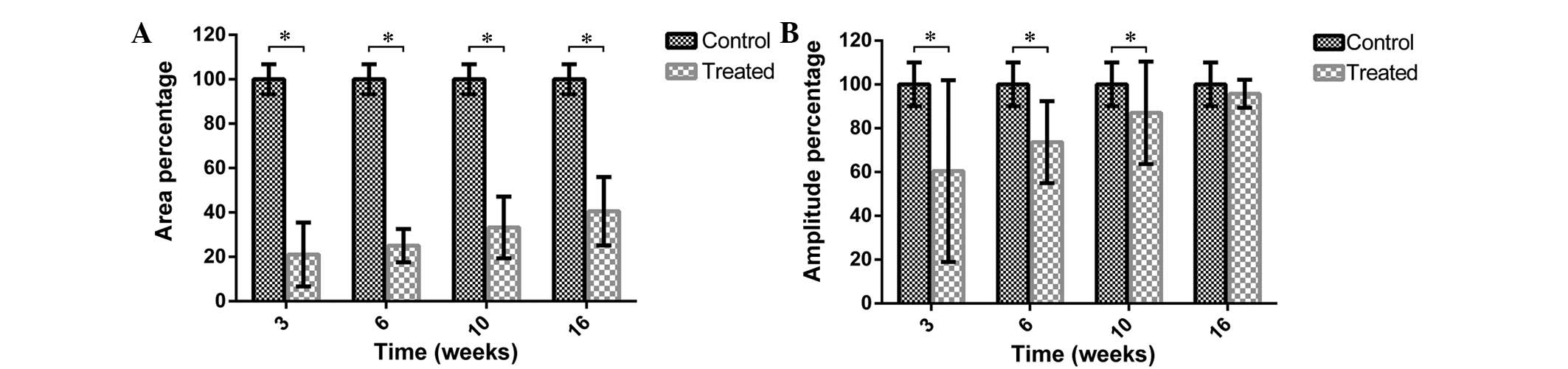
The spectral area (calculated as a percentage) in
the denervation group increased gradually over time, with values at
3, 6, 10 and 16 weeks of 21.07±14.41, 25.04±7.50, 33.22±13.89 and
40.55±15.36%, respectively (Fig.
5A; P<0.01 vs. the control group). The postoperative
amplitudes at 3, 6, 10, and 16 weeks were 60.43±41.49, 73.62±18.72,
87.02±23.40 and 95.74±6.38%, respectively (Fig. 5B). Over time, the amplitude
percentages increased (P<0.05), although there was no
significant difference between the control group and the 16-week
denervation group (P=0.0540).
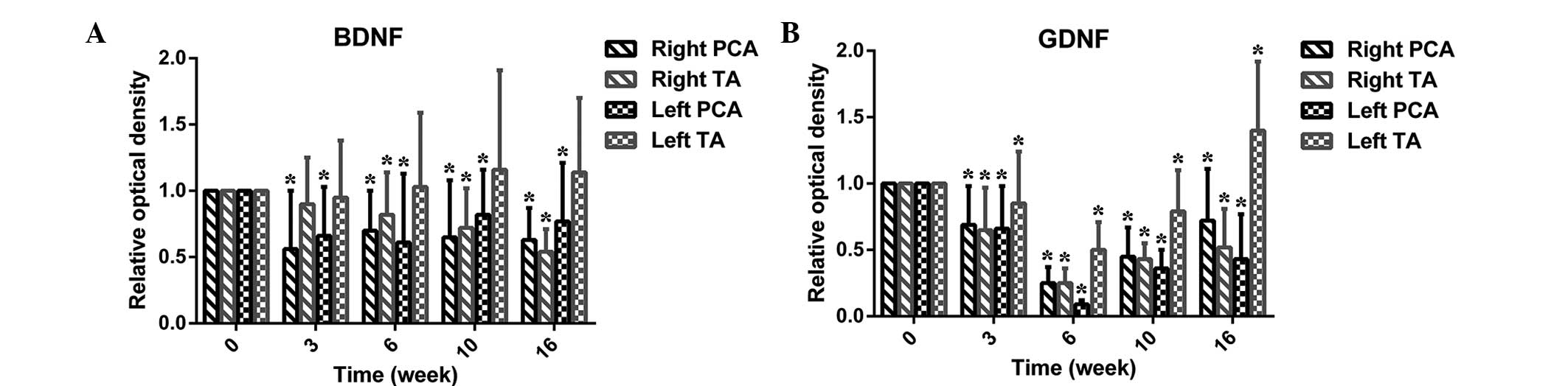
Effect of denervation on neurotrophic
factor expression
Figs. 6 and
7A present data for the expression
of BDNF in the TA and PCA muscles. In the TA muscles, BDNF
expression levels decreased slowly over time. However, the
expression of BDNF significantly decreased at 3 weeks following
injury. Although the expression increased at 6 weeks, it did not
reach the baseline level.
Expression of GDNF (Figs. 7B and 8) in the TA was similar to that in the
PCA muscle from 3 to 10 weeks; however, at rest, there was a
marginal increase in GDNF expression levels in the PCA muscle.
Effect of denervation on RLN
histology
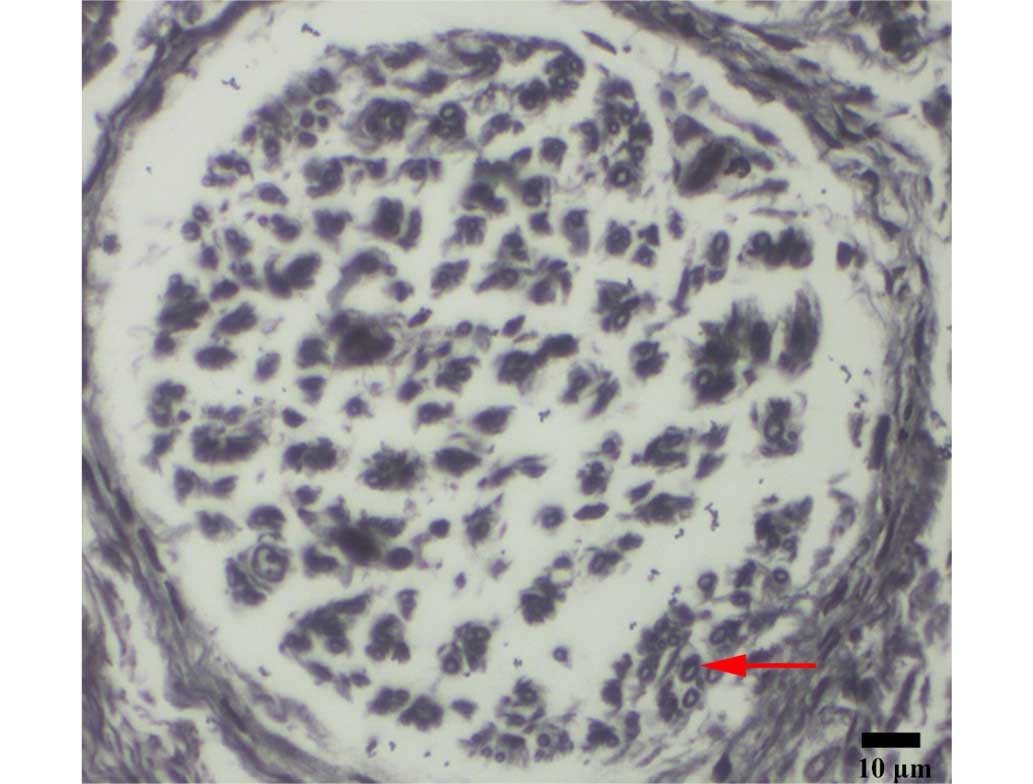
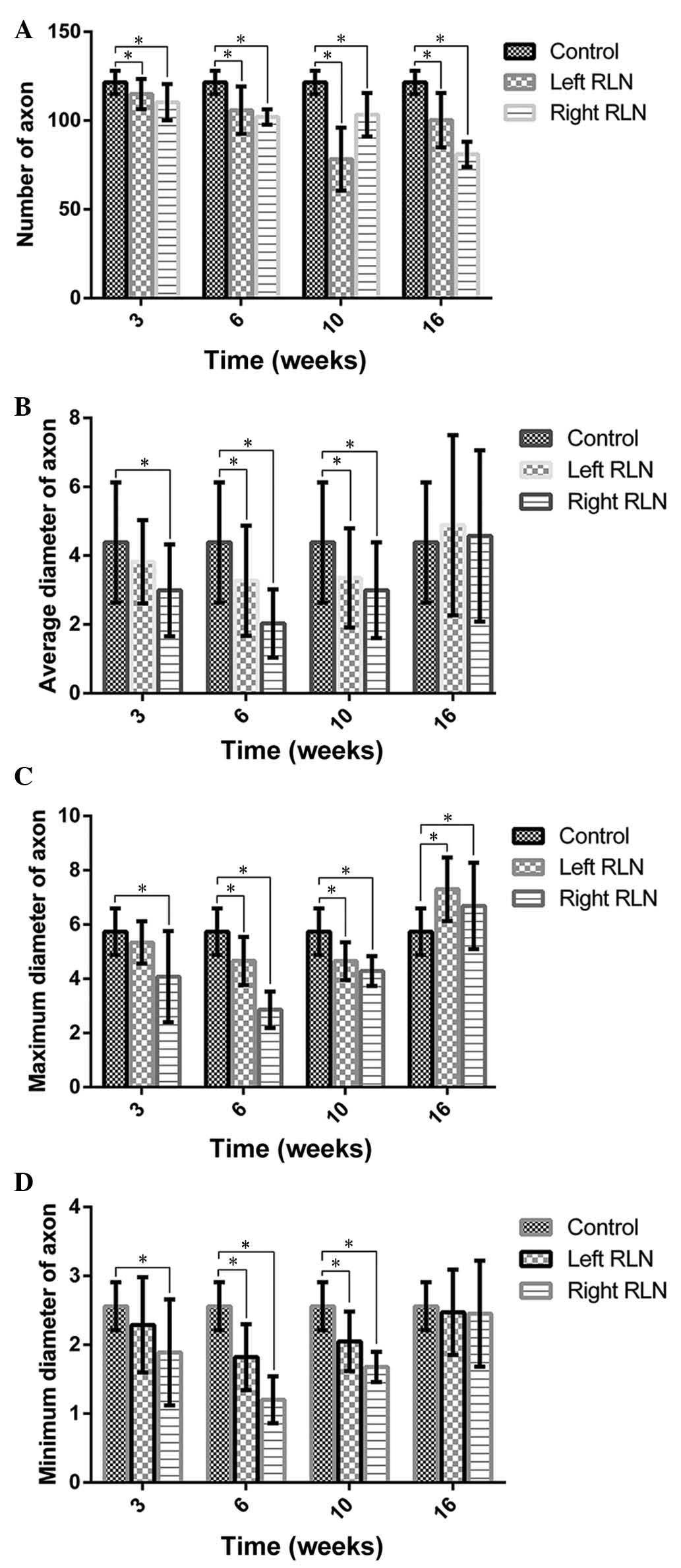
Compressed or degenerating axons were observed in
certain cases. Fig. 9 demonstrates
axons embedded in a fibrous structure. At 16 weeks, the RLN had
fewer axons (~81.00±7.07 axons) than the control nerves (Fig. 10). The axon diameters of the RLN
sections were recorded. The mean diameter of the axons was
4.38±1.1.75 µm in normal rats; the right RLN exhibited a
reduction for 6 weeks, followed by a slight increase. RLN regrowth
was not correlated with vocal fold motion.
Discussion
In the present study, recovery of vocal fold
movement, vocalization, expression levels of BDNF and GDNF, and
morphometry of RLN sections were investigated. When the RLN is
resected, functional recovery of vocal cord mobility is not
achieved. The majority of research studies are limited to examining
the return of airway patency, movement of vocal cords or
reinnervation. The current study offered a vocalization measurement
to reflect the recovery. This method of acoustic wave analysis is
original with, to the best of our knowledge, no previous
investigations conducted in this manner. The data values were
comparable as the same crushing strength was used to stimulate the
rats and an equal distance between the microphones and mouths of
the rats was maintained; furthermore, the same full-size acoustic
window was recorded and equal time was allotted for analysis.
Nerve transection induces Sunderland fifth-degree
injury, which involves injury to the nerve trunk (57). Ischemia causes electrolyte
concentrations to shift and also results in excessive excitatory
amino acid release (58),
triggering cytotoxic signaling cascades, myelin destruction and
restricted regrowth of axons (17). Transection is a common phenomenon
among nerve injuries (59). In the
current study, following transection, a degree of functional
recovery of vocalization was observed. In addition, the spectral
area percentage demonstrated that although voice function had been
restored, it did not return to normal levels. The amplitude
percentage demonstrated that the rats did not recover to normal
levels by 3, 6 and 10 weeks; however they were able to chirp
normally at 16 weeks. Although spontaneous reinnervation did not
restore the motion of the vocal fold, it may provide improved
vocalization compared to complete denervation, perhaps by
maintaining the tonus and bulk of the vocal fold (60).
Following RLN injury, degeneration of axons results
in reduced conduction of action potentials. Furthermore, it is well
known that axons with a larger diameter have stronger regeneration
ability (61). Results from the
present study demonstrated that the diameter and number of axons
was lower in the denervation group than in the control group. The
recovery of axons did not reach the desired level (return to
baseline), which may reflect the limited self-repair capability of
the axons.
The TA and PCA muscles demonstrated a difference in
the expression levels of GDNF; the level of GDNF expression was
lower in the denervated muscles than in the control muscles and
reached a minimum at 6 weeks. BDNF and GDNF were differentially
expressed in the TA and PCA muscles. During the current study,
there was no increase in BDNF expression in either the TA or PCA
muscles, although large differences in BDNF expression levels were
observed between the TA and PCA muscles. In the third week, the
level of BDNF expression was reduced markedly in the right PCA
muscles, possibly as part of a stress response to the surgery,
which triggers catabolism in muscles (62). This requires verification at an
earlier time-point. The contralateral TA may have a compensatory
role. The differences between the TA and PCA muscles may arise from
the various responses to the surgery or may be associated with the
type of muscle fibers (19). The
TA muscles are primarily comprised of fast-twitch fibers, whereas
nearly half of the PCA muscle fibers are slow-twitch (63). Reinnervation of fast-twitch fibers
requires fewer muscle fibers than slow-twitch fibers (60). Based on the results of the present
study, differences in neurotrophin expression may induce
preferential reinnervation of the adductor muscles. This may
explain the adducted position of the vocal fold following RLN
injury, and may be more plausible than the hypothesis of the
present study, that the adducting and abducting forces abrogate
each other, resulting in no net motion. Due to the enhancement of
glottis closure, the preferential reinnervation of the TA muscle
may be favorable in patients with unilateral paralysis, but not in
patients with bilateral paralysis. Further studies are required,
which focus on changes in other neurotrophins, such as nerve growth
factor and neurotrophin-4, and improving PCA muscle function in
patients with bilateral paralysis. The horizontal division of the
PCA and the superior medial division of the TA are predominantly
composed of slow-twitch fibers, whereas the lateral division of the
TA, and the oblique and vertical divisions of the PCA have a higher
percentage of fast-twitch fibers; therefore, future studies are
required to differentiate between the muscle types (64). Due to the specific functions of
slow- and fast-twitch muscle fibers, the expression of BDNF and
GDNF is different (22). It may
therefore be possible to apply neurotrophic factors to promote
nerve regeneration by compensating for the reduction in their
levels following injury.
The present study has certain limitations. The study
was conducted over 16 weeks in a small number of rats; therefore,
large-scale, short- and long-term studies are required to confirm
the results. In addition, the lack of vocal fold motion may have
been due to synkinetic reinnervation or failed reinnervation of the
laryngeal muscles. Furthermore, the respiratory cycle of the
animals should be monitored to determine the reason for the lack of
vocal fold motion. Finally, immunohistochemistry was used to
observe the expression of GDNF and BDNF proteins within the
muscles. Although this approach is valuable for localizing specific
proteins in tissues, studies supported by quantitative techniques,
such as western blotting, are required to confirm and expand the
results (19).
The results of the present study demonstrate that
the restoration of the vocal fold is not significant, despite
spontaneous regeneration. In humans, the spontaneous regeneration
of the RLN remains a controversial subject (7,60,65).
Spontaneous laryngeal reinnervation may occur through regeneration
of a healthy residual RLN or from nerves in the surrounding area
(3). Strategies to promote
sufficient reinnervation of the PCA muscle may improve the
effectiveness of spontaneous recovery following RLN injury.
In conclusion, although only vocal function improved
during the present study, spontaneous reinnervation was observed
following RLN injury. Thus, neurotrophic factors may be utilized
following RLN injury to promote repair of neurological
function.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81271067).
References
|
1
|
Zhang N, Yan H and Wen X:
Tissue-engineering approaches for axonal guidance. Brain Res Brain
Res Rev. 49:48–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kingham PJ and Terenghi G: Bioengineered
nerve regeneration and muscle reinnervation. J Anat. 209:511–526.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalgic A, Kandogan T, Koc M, Kulan CA,
Yagci A, Engin O, Aksoy G and Ozuer MZ: Short-term laryngeal
electromyography and histopathological findings after primary
reconstruction of the inferior laryngeal nerve in rabbits:
Prospective study. J Laryngol Otol. 127:48–53. 2013. View Article : Google Scholar
|
|
4
|
Moro K, Shiotani A, Watabe K, Takeda Y,
Saito K, Mori Y and Ogawa K: Adenoviral gene transfer of BDNF and
GDNF synergistically prevent motoneuron loss in the nucleus
ambiguus. Brain Res. 1076:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caldarelli DD and Holinger LD:
Complications and sequelae of thyroid surgery. Otolaryngol Clin
North Am. 13:85–97. 1980.PubMed/NCBI
|
|
6
|
Tessema B, Pitman MJ, Roark RM, Berzofsky
C, Sharma S and Schaefer SD: Evaluation of functional recovery of
recurrent laryngeal nerve using transoral laryngeal bipolar
electromyography: A rat model. Ann Otol Rhinol Laryngol.
117:604–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crumley RL and McCabe BF: Regeneration of
the recurrent laryngeal nerve. Otolaryngol Head Neck Surg.
90:442–447. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shindo ML, Herzon GD, Hanson DG, Cain DJ
and Sahgal V: Effects of denervation on laryngeal muscles: A canine
model. Laryngoscope. 102:663–669. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horsley JS: Suture of the Recurrent
Laryngeal Nerve. Ann Surg. 52:287–288. 1910. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gordon JH and McCabe BF: The effect of
accurate neurorrhaphy on reinnervation and return of laryngeal
function. Laryngoscope. 78:236–250. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crumley RL: Laryngeal synkinesis
revisited. Ann Otol Rhinol Laryngol. 109:365–371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flint PW, Downs DH and Coltrera MD:
Laryngeal synkinesis following reinnervation in the rat.
Neuroanatomic and physiologic study using retrograde fluorescent
tracers and electromyography. Ann Otol Rhinol Laryngol.
100:797–806. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tessema B, Roark RM, Pitman MJ, Weissbrod
P, Sharma S and Schaefer SD: Observations of recurrent laryngeal
nerve injury and recovery using a rat model. Laryngoscope.
119:1644–1651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Woodson GE: Spontaneous laryngeal
reinnervation after recurrent laryngeal or vagus nerve injury. Ann
Otol Rhinol Laryngol. 116:57–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyamaru S, Kumai Y, Ito T and Yumoto E:
Effects of long-term denervation on the rat thyroarytenoid muscle.
Laryngoscope. 118:1318–1323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi JS, Oh SH, An HY, Kim YM, Lee JH and
Lim JY: Functional regeneration of recurrent laryngeal nerve injury
during thyroid surgery using an asymmetrically porous nerve guide
conduit in an animal model. Thyroid. 24:52–59. 2014. View Article : Google Scholar :
|
|
17
|
Araki K, Shiotani A, Watabe K, Saito K,
Moro K and Ogawa K: Adenoviral GDNF gene transfer enhances
neurofunctional recovery after recurrent laryngeal nerve injury.
Gene Ther. 13:296–303. 2006. View Article : Google Scholar
|
|
18
|
Meller SM: Functional anatomy of the
larynx. Otolaryngol Clin North Am. 17:3–12. 1984.PubMed/NCBI
|
|
19
|
Vega-Cordova X, Cosenza NM, Helfert RH and
Woodson GE: Neurotrophin expression of laryngeal muscles in
response to recurrent laryngeal nerve transection. Laryngoscope.
120:1591–1596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kupfer RA, Old MO, Oh SS, Feldman EL and
Hogikyan ND: Spontaneous laryngeal reinnervation following chronic
recurrent laryngeal nerve injury. Laryngoscope. 123:2216–2227.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumai Y, Ito T, Matsukawa A and Yumoto E:
Effects of denervation on neuromuscular junctions in the
thyroarytenoid muscle. Laryngoscope. 115:1869–1872. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halum SL, Bijangi-Vishehsaraei K,
Saadatzadeh MR and McRae BR: Differences in laryngeal neurotrophic
factor gene expression after recurrent laryngeal nerve and vagus
nerve injuries. Ann Otol Rhinol Laryngol. 122:653–663.
2013.PubMed/NCBI
|
|
23
|
Crumley RL: Update: Ansa cervicalis to
recurrent laryngeal nerve anastomosis for unilateral laryngeal
paralysis. Laryngoscope. 101:384–387. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng H, Li Z, Zhou S, Cuan Y and Wen W:
Update: Laryngeal reinnervation for unilateral vocal cord paralysis
with the ansa cervicalis. Laryngoscope. 106:1522–1527. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee WT, Milstein C, Hicks D, Akst LM and
Esclamado RM: Results of ansa to recurrent laryngeal nerve
reinnervation. Otolaryngol Head Neck Surg. 136:450–454. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Unuma K, Chen J, Saito S, Kobayashi N,
Sato K, Saito K, Wakisaka H, Mominoki K, Sano A and Matsuda S:
Changes in expression of prosaposin in the rat facial nerve nucleus
after facial nerve transection. Neurosci Res. 52:220–227. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J and Shi R: A device for the
electrophysiological recording of peripheral nerves in response to
stretch. J Neurosci Methods. 154:102–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tun K, Cemil B, Gurcay AG, Kaptanoglu E,
Sargon MF, Tekdemir I, Comert A and Kanpolat Y: Ultrastructural
evaluation of Pulsed Radiofrequency and Conventional Radiofrequency
lesions in rat sciatic nerve. Surg Neurol. 72:496–501. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zelano J, Plantman S, Hailer NP and
Cullheim S: Altered expression of nectin-like adhesion molecules in
the peripheral nerve after sciatic nerve transection. Neurosci
Lett. 449:28–33. 2009. View Article : Google Scholar
|
|
30
|
Yayama T, Kobayashi S, Nakanishi Y, Uchida
K, Kokubo Y, Miyazaki T, Takeno K, Awara K, Mwaka ES, Iwamoto Y and
Baba H: Effects of graded mechanical compression of rabbit sciatic
nerve on nerve blood flow and electrophysiological properties. J
Clin Neurosci. 17:501–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maarrawi J, Kobaiter-Maarrawi S, Ghanem I,
Ali Y, Aftimos G, Okais N and Samaha E: Pathological effects and
motor response threshold changes following radiofrequency
application at various distances from the L-5 nerve root: An
experimental study. J Neurosurg Spine. 15:285–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rickett T, Connell S, Bastijanic J, Hegde
S and Shi R: Functional and mechanical evaluation of nerve stretch
injury. J Med Syst. 35:787–793. 2011. View Article : Google Scholar
|
|
33
|
Sacharuk VZ, Lovatel GA, Ilha J, Marcuzzo
S, Pinho AS, Xavier LL, Zaro MA and Achaval M: Thermographic
evaluation of hind paw skin temperature and functional recovery of
locomotion after sciatic nerve crush in rats. Clinics (Sao Paulo).
66:1259–1266. 2011. View Article : Google Scholar
|
|
34
|
Wang Y, Tang P, Zhang L, Wan W, He C and
Tang J: Gray-scale contrast-enhanced ultrasonography for
quantitative evaluation of the blood perfusion of the sciatic
nerves with crush injury. Acad Radiol. 18:1285–1291. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu L, Yan Y, Ke K, Wu X, Gao Y, Shen A,
Li J, Kang L, Zhang G, Wu Q and Yang H: Dynamic change of Numbl
expression after sciatic nerve crush and its role in Schwann cell
differentiation. J Neurosci Res. 90:1557–1565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
English AW, Liu K, Nicolini JM, Mulligan
AM and Ye K: Small-molecule trkB agonists promote axon regeneration
in cut peripheral nerves. Proc Natl Acad Sci USA. 110:16217–16222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaya Y, Sarıkcıoğlu L, Aslan M, Kencebay
C, Demir N, Derin N, Angelov DN and Yıldırım FB: Comparison of the
beneficial effect of melatonin on recovery after cut and crush
sciatic nerve injury: A combined study using functional,
electrophysiological, biochemical, and electron microscopic
analyses. Childs Nerv Syst. 29:389–401. 2013. View Article : Google Scholar
|
|
38
|
Li S, Liu Q, Wang Y, Gu Y, Liu D, Wang C,
Ding G, Chen J, Liu J and Gu X: Differential gene expression
profiling and biological process analysis in proximal nerve
segments after sciatic nerve transection. PLoS One. 8:e570002013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schmid AB, Coppieters MW, Ruitenberg MJ
and McLachlan EM: Local and remote immune-mediated inflammation
after mild peripheral nerve compression in rats. J Neuropathol Exp
Neurol. 72:662–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Singleton JR, Dixit VM and Feldman EL:
Type I insulin-like growth factor receptor activation regulates
apoptotic proteins. J Biol Chem. 271:31791–31794. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim B, Leventhal PS, Saltiel AR and
Feldman EL: Insulin-like growth factor-I-mediated neurite outgrowth
in vitro requires mitogen-activated protein kinase activation. J
Biol Chem. 272:21268–21273. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Russell JW, Windebank AJ, Schenone A and
Feldman EL: Insulin-like growth factor-I prevents apoptosis in
neurons after nerve growth factor withdrawal. J Neurobiol.
36:455–467. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Baumgartner BJ and Shine HD: Targeted
transduction of CNS neurons with adenoviral vectors carrying
neurotrophic factor genes confers neuroprotection that exceeds the
transduced population. J Neurosci. 17:6504–6511. 1997.PubMed/NCBI
|
|
44
|
Giménez y Ribotta M, Revah F, Pradier L,
Loquet I, Mallet J and Privat A: Prevention of motoneuron death by
adenovirus-mediated neurotrophic factors. J Neurosci Res.
48:281–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dittrich F, Thoenen H and Sendtner M:
Ciliary neurotrophic factor: Pharmacokinetics and acute-phase
response in rat. Ann Neurol. 35:151–163. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Halum SL, McRae B, Bijangi-Vishehsaraei K
and Hiatt K: Neurotrophic factor-secreting autologous muscle stem
cell therapy for the treatment of laryngeal denervation injury.
Laryngoscope. 122:2482–2496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Heavner SB, Rubin AD, Fung K, Old M,
Hogikyan ND and Feldman EL: Dysfunction of the recurrent laryngeal
nerve and the potential of gene therapy. Ann Otol Rhinol Laryngol.
116:441–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kingham PJ, Hughes A, Mitchard L, Burt R,
Murison P, Jones A, Terenghi G and Birchall MA: Effect of
neurotrophin-3 on reinnervation of the larynx using the phrenic
nerve transfer technique. Eur J Neurosci. 25:331–340. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saito K, Shiotani A, Watabe K, Moro K,
Fukuda H and Ogawa K: Adenoviral GDNF gene transfer prevents
motoneuron loss in the nucleus ambiguus. Brain Res. 962:61–67.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shiotani A, Saito K, Araki K, Moro K and
Watabe K: Gene therapy for laryngeal paralysis. Ann Otol Rhinol
Laryngol. 116:115–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng H, Zhou S and You Z: The expression
and distribution of ciliary neurotrophic factor in laryngeal nerve
regeneration. Zhonghua Er Bi Yan Hou Ke Za Zhi. 34:289–292. 1999.In
Chinese.
|
|
52
|
Aoyama T, Kumai Y, Yumoto E, Ito T and
Miyamaru S: Effects of nerve-muscle pedicle on immobile rat vocal
folds in the presence of partial innervation. Ann Otol Rhinol
Laryngol. 119:823–829. 2010. View Article : Google Scholar
|
|
53
|
Pitman MJ, Weissbrod P, Roark R, Sharma S
and Schaefer SD: Electromyographic and histologic evolution of the
recurrent laryngeal nerve from transection and anastomosis to
mature reinnervation. Laryngoscope. 121:325–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hernández-Morato I, Valderrama-Canales FJ,
Berdugo G, Arias G, McHanwell S, Sañudo J, Vásquez T and
Pascual-Font A: Reorganization of laryngeal motoneurons after crush
injury in the recurrent laryngeal nerve of the rat. J Anat.
222:451–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Iizuka T: Experimental studies on the
nerve interception and atrophy of the intrinsic muscles of the
larynx. J Otolaryng Jap. 69:176–195. 1966.In Japanese.
|
|
56
|
Owens CM, Marga F, Forgacs G and Heesch
CM: Biofabrication and testing of a fully cellular nerve graft.
Biofabrication. 5:0450072013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sunderland S: A classification of
peripheral nerve injuries producing loss of function. Brain.
74:491–516. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Carlson SL, Parrish ME, Springer JE, Doty
K and Dossett L: Acute inflammatory response in spinal cord
following impact injury. Exp Neurol. 151:77–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zábrodský M, Bouček J, Kastner J, Kuchař
M, Chovanec M and Betka J: Immediate revision in patients with
bilateral recurrent laryngeal nerve palsy after thyroid and
parathyroid surgery. How worthy is it? Acta Otorhinolaryngol Ital.
32:222–228. 2012.PubMed/NCBI
|
|
60
|
Blitzer A, Jahn AF and Keidar A: Semon's
law revisited: An electromyographic analysis of laryngeal
synkinesis. Ann Otol Rhinol Laryngol. 105:764–769. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Toya Y, Kumai Y, Minoda R and Yumoto E:
Modulation of nerve fibers in the rat thyroarytenoid muscle
following recurrent laryngeal nerve injury. Acta Otolaryngol.
132:305–313. 2012. View Article : Google Scholar
|
|
62
|
Tetzlaff J, Tanzer L and Jones KJ:
Exogenous androgen treatment delays the stress response following
hamster facial nerve injury. J Neuroendocrinol. 19:383–389. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vega-Cordova X, Cosenza NM, Helfert RH and
Woodson GE: Neurotrophin expression of laryngeal muscles in
response to recurrent laryngeal nerve transection. Laryngoscope.
120:1591–1596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zealear DL and Billante CR:
Neurophysiology of vocal fold paralysis. Otolaryngol Clin North Am.
37:1–23. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Damrose EJ, Huang RY, Blumin JH, Blackwell
KE, Sercarz JA and Berke GS: Lack of evoked laryngeal
electromyography response in patients with a clinical diagnosis of
vocal cord paralysis. Ann Otol Rhinol Laryngol. 110:815–819. 2001.
View Article : Google Scholar : PubMed/NCBI
|