Introduction
Receptor tyrosine-protein kinase ErbB-2 is a protein
that is encoded by the ERBB2 gene in humans. The
ERBB2 gene is also termed human epidermal growth factor
receptor 2 (HER2). The ErbB-2/HER2 protein is a member of
the epidermal growth factor receptor (EGFR/ErbB) family.
Amplification or overexpression of HER2 has been demonstrated to be
important in the development and progression of certain types of
cancer, including gastric cancer (1–3).
Similar to other members of the ErbB family, HER2
contains an extracellular ligand binding domain, a transmembrane
domain and an intracellular domain that can interact with a
multitude of signaling molecules and exhibit ligand-dependent and
ligand-independent activity (4).
However, to date, no ligand of HER2 has been identified in
mammalian cells. Instead of being activated by binding with the
ligand, HER2 is activated predominantly through heterodimerizing
with any of the other three members of the ErbB family, with
preference to EGFR (5).
Dimerization results in the autophosphorylation of tyrosine
residues within the cytoplasmic domain of HER2 and initiates a
variety of signaling pathways, including mitogen-activated protein
kinase, phosphoinositide 3-kinase/Akt, phospholipase C/protein
kinase C (PKC) and signal transducer and activator of
transcription-mediated pathways (6).
Our previous study demonstrated that type II cyclic
guanosine monophosphate (cGMP)-dependent protein kinase (PKG II)
inhibited the activation of EGFR through binding with and causing
phosphorylation of the receptor (7,8).
Since HER2 has a similar structure to EGFR and forms a dimer with
EGFR, whether PKG II has an inhibitory effect on HER2 warrants
further investigation. Therefore, the present study was designed to
investigate the possible inhibition of HER2 by PKG II.
Materials and methods
Cell line and reagents
The human gastric cancer cell line HGC-27 was
provided by the Institute of Cell Biology (Shanghai, China).
Adenoviral vectors encoding the cDNA β-galactosidase (Ad-LacZ) and
PKG II (Ad-PKG II) were provided by Dr Gerry Boss and Dr Renate
Pilz (University of California, San Diego, CA, USA). Dulbecco's
modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were
obtained from Gibco (Thermo Fisher Scientific Inc., Waltham, MA,
USA). The polyclonal rabbit anti-human PKG II antibody was obtained
from Abgent Biotechnology (San Diego, CA, USA; cat. no. AP8001a;
dilution, 1:200). The horseradish peroxidase (HRP)-conjugated
monoclonal mouse anti-human β-actin antibody was obtained from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA; cat. no.
sc-47778; dilution, 1:1,000). Polyclonal rabbit anti-p-ErbB2 (T686;
cat. no. ab11717; dilution, 1:1,000) and polyclonal rabbit
anti-human phosphoserine/threonine (cat. no. ab17464; dilution,
1:1,000) were purchased from Abcam (Cambridge, MA, USA). Polyclonal
rabbit anti-human p-HER2 (Tyr1248) antibody (cat. no. BS4090;
dilution, 1:500) and polyclonal rabbit anti-human ErbB2/HER2 (cat.
no. BS1169; dilution, 1:500) were obtained from Bioworld
Technology, Inc. (St. Louis Park, MN, USA). Monoclonal mouse
anti-flag antibody (cat. no. F1804; dilution, 1:1,000) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). The
HRP-conjugated polyclonal anti-mouse and anti-goat IgG secondary
antibodies (cat. nos. 115-035-003 and 111-035-003, respectively;
dilution, 1:10,000) were purchased from Jackson ImmunoResearch
Laboratories (West Grove, PA, USA). The Bioepitope®
protein A+G Agarose IP was obtained from Bioworld Technology, Inc.
The cellular permeable cGMP analog
8-(4-chlorophenylthio)guanosine-3′,5′-cyclic monophosphate
(8-pCPT-cGMP) was acquired from Calbiochem (San Diego, CA, USA).
EGF was purchased from Sigma-Aldrich. The cell transfection reagent
Lipofectamine™ 2000 and E. coli BL-21DE3 were obtained from
Invitrogen (Thermo Fisher Scientific, Inc.). The QuikChange
Lightning Site-Directed Mutagenesis kit was purchased from Agilent
Technologies (Santa Clara, CA, USA) and the SanPrep Column Plasmid
Mini-Preps kit was obtained from Sangon Biotech Shanghai Co. Ltd.
(Shanghai, China). Electrochemiluminescence (ECL) reagents were
acquired from EMD Millipore (Billerica, MA, USA).
Cell culture and preparation of cell
extracts
HGC-27 cells were cultured in DMEM supplemented with
10% FBS and maintained at 37°C in a humidified incubator with 95%
air and 5% CO2. On the day prior to infection, cells
were planted into 6-well plates. To observe the phosphorylation of
HER2, the cells were infected with Ad-LacZ or Ad-PKG II for 24 h
and serum starved overnight. Subsequently, in the Ad-LacZ + EGF and
Ad-PKG II + EGF groups, the cells were incubated with EGF (100
ng/ml) for 5 min; in the Ad-PKG II + cGMP + EGF groups, the cells
were incubated with 8-pCPT-cGMP for 1 h and then with EGF (100
ng/ml) for 5 min. To observe PKGII binding with HER2 directly and
causing the serine/threonine phosphorylation of HER2, the cells
were infected with Ad-PKG II for 24 h, serum starved overnight and
incubated with 8-pCPT-cGMP for 1 h. At the end of the treatments,
the cells were harvested by aspiration of the media and direct
addition of heated 2X SDS sample buffer. The cell lysate was
scraped and transferred to tubes, heated for 5 min at 100°C and
stored at −20°C.
Co-immunoprecipitation (Co-IP)
The cells growing on the 100-mm culture plate were
washed two times with cold PBS and lysed by adding 1 ml
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China; 50 mM Tris-HCl pH 7.4, 1% Triton
X-100, 1 mM EDTA, 1 mM leupeptin, 1 mM phenylmethylsulfonyl
fluoride, 10 mM NaF, 1 mM Na3VO4) per plate.
An antibody against tag protein flag was used for
immunoprecipitation. The precipitates were probed with antibodies
against target proteins.
Western blotting
Proteins were separated by SDS-PAGE (10%) gel (EMD
Millipore) according to the molecular size and transferred onto a
polyvinylidene difluoride membrane (EMD Millipore). Blots were
blocked with 5% (w/v) non-fat milk in Tris-buffered saline with
Tween 20 for 1 h at room temperature and then incubated at 4°C
overnight with the primary antibodies (including anti-p-HER2,
anti-β-actin, anti-p-ErbB2/HER2, anti-phosphoserine/threonine and
anti-flag), followed by incubation with the secondary antibodies
(including goat anti-mouse and goat anti-rabbit HRP-conjugated
antibodies) at room temperature for 1 h. The signal was visualized
using ECL detection reagents. To perform densitometry analysis,
digital images of the positive bands were obtained with Chemidoc
XRS and analyzed using the image analysis program Quantity One,
version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
results are presented as the ratio of target protein/loading
control.
Construction of mutant plasmid
The cDNA encoding human HER2 was cut off by
HindIII from the plasmid CMV-HER2-WT (cat. no. 16257;
Addgene, Cambridge, MA, USA) and was cloned into the expression
vector p3XFlag-myc-CMV-24. Mutants of HER2 were generated using the
QuikChange Site-Directed Mutagenesis kit (Stratagene, San Diego,
CA, USA). Threonine 686 was mutated to glutamic acid (T686E) and
alanine (T686A). The following primers were used: Mutant HER2
(T686E), forward 5′-GAAGATCCGGAAGTACGAGATGCGGAGACTGCTG-3′ and
reverse 5′-CAGCAGTCTCCGCATCTCGTACTTCCGGATCTTC-3′; mutant HER2
(T686A), forward 5′-GAAGATCCGGAAGTACGCGATGCGGAGACTGCTG-3′ and
reverse CAGCAGTCTCCGCATCTCGTACTTCCGGATCTTC. The mutant plasmids
were sequenced and the mutations were confirmed.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical significance was performed using a
two-tailed analysis of variance with SPSS statistical software,
version 19 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
PKG II inhibits EGF-induced activation of
HER2
The activation of HER2 is dependent on the
ligand-receptor binding of other ErbB receptors, particularly EGFR.
When EGF binds with EGFR, EGFR forms a dimer with HER2 and this
dimerization causes autophosphorylation/activation of HER2
(5). Tyrosine 1248 (Tyr1248) is
one of the autophosphorylation sites of HER2 and phosphorylation of
this site is associated with downstream signaling (9). The present study investigated the
inhibitory effect of PKG II on Tyr1248 phosphorylation of HER2 in
differently treated HGC-27 cells using western blotting. The
results demonstrated that in Ad-LacZ-infected cells, there was a
pronounced increase in Tyr1248 phosphorylation of HER2 when the
cells were incubated with EGF (100 ng/ml) for 5 min. In cells
infected with Ad-PKG II for 24 h, treated with 8-pCPT-cGMP for 1 h
and then incubated with EGF (100 ng/ml) for 5 min, the Tyr1248
phosphorylation of HER2 was significantly decreased (Fig. 1). This indicated that PKG II could
inhibit EGF-induced Tyr1248 phosphorylation of HER2.
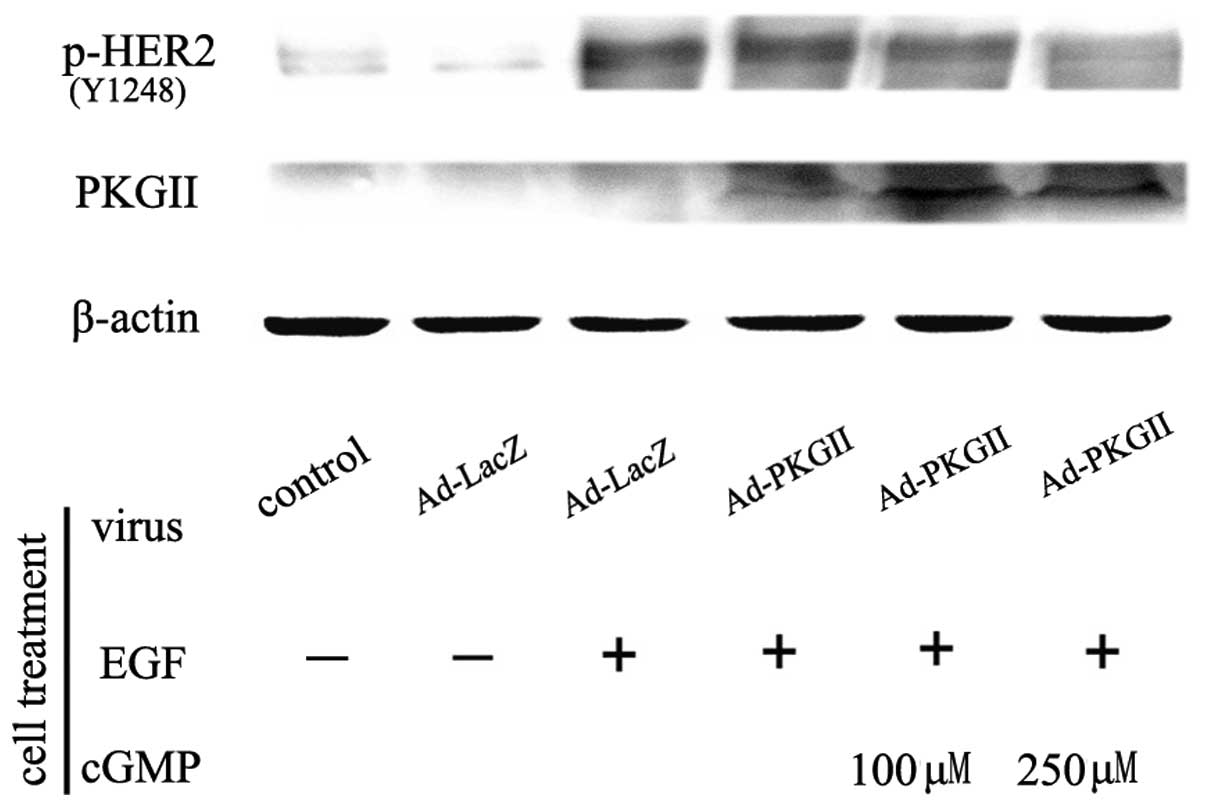 | Figure 1PKG II inhibits EGF-induced tyrosine
1248 phosphorylation of HER2. HGC-27 cells were infected with
Ad-LacZ or Ad-PKG II for 24 h and serum starved overnight.
Subsequently, in the Ad-LacZ+EGF and Ad-PKG II+EGF groups, cells
were incubated with EGF (100 ng/ml) for 5 min. In the Ad-PKG
II+cGMP+EGF groups, cells were incubated with 8-pCPT-cGMP for 1 h
and then with EGF (100 ng/ml) for 5 min. Cells were harvested and
lysed as described in Materials and methods and the cell lysate was
subjected to western blotting to detect the Tyr1248 phosphorylation
of HER2. The results demonstrated that infection with Ad-PKG II
increased the expression of PKG II. EGF treatment induced a marked
increase in Tyr1248 phosphorylation of HER2. In addition, infection
with Ad-PKG II+cGMP treatment inhibited the EGF-induced
phosphorylation of HER2. The results are representative of three
independent experiments. EGF, epidermal growth factor; PKG II, type
II cGMP-dependent protein kinase; HER2, human epidermal growth
factor receptor 2; Ad, adenovirus; LacZ, β-galactosidase;
8-pCPT-cGMP, 8-(4-chlorophenylthio)guanosine-3′,5′-cyclic
monophosphate. |
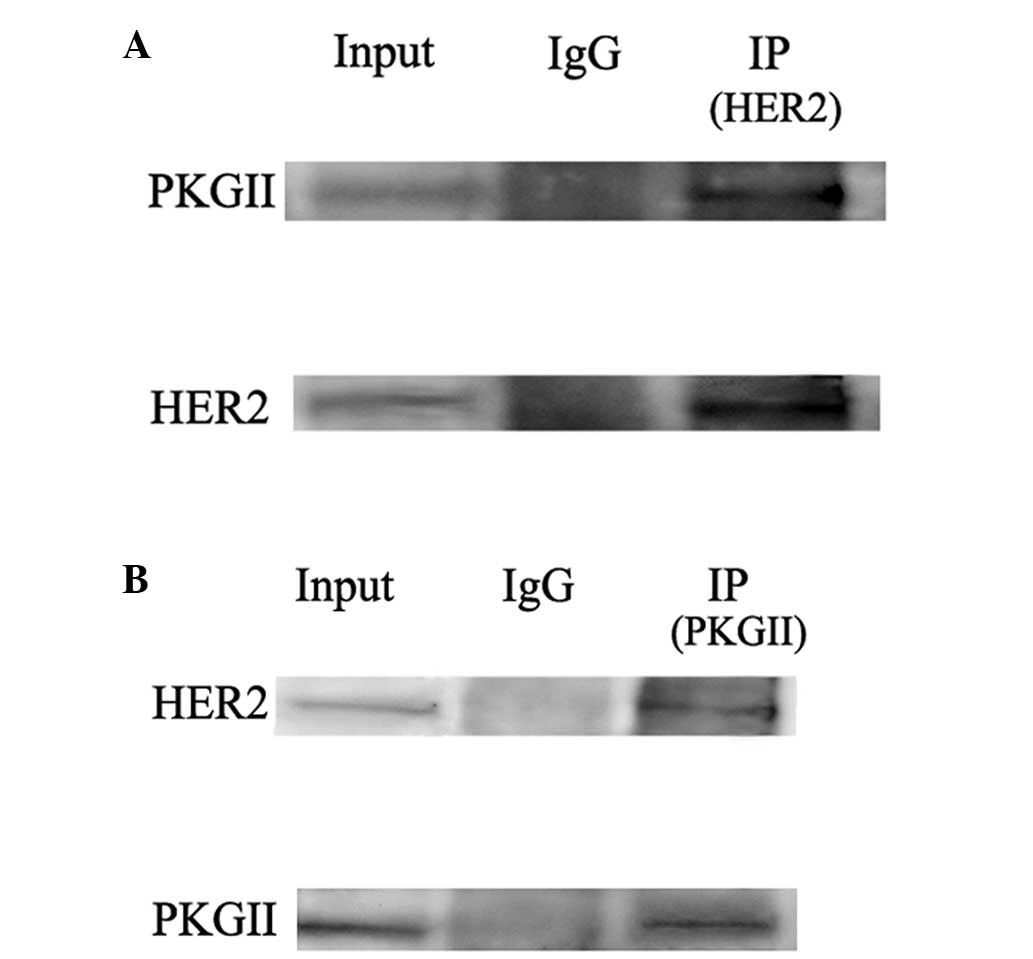
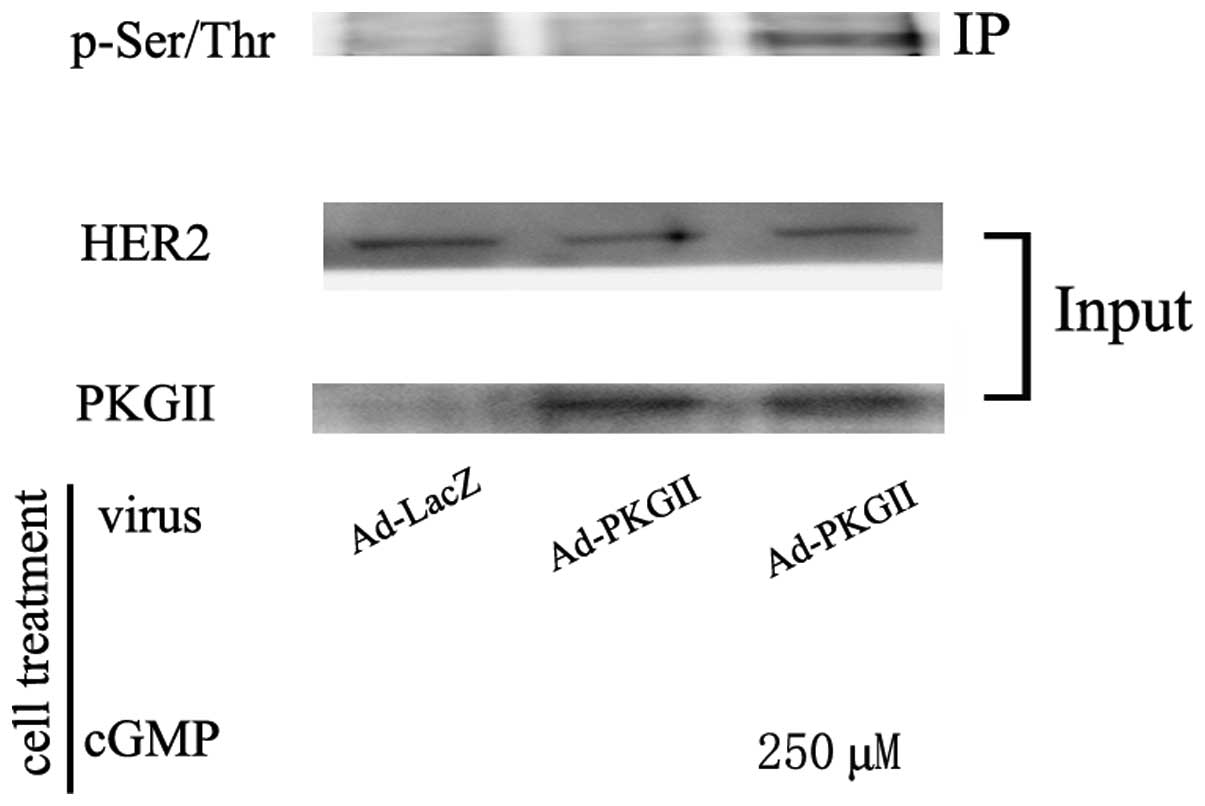
PKG II directly binds with and causes
phosphorylation of HER2
Since the phosphorylation/activation of HER2 was
caused by EGF/EGFR binding and PKG II has been reported to bind
with EGFR (10), whether PKG II
directly inhibits HER2 or the inhibition is subsequent to its
inhibition of EGFR requires further investigation. Co-IP was
performed to detect the possible interaction between PKG II and
HER2. The results demonstrated that in HGC-27 cells infected with
Ad-PKG II and stimulated with 8-pCPT-cGMP, binding between PKG II
and HER2 occurred (Fig. 2).
Western blotting with antibodies against pan serine/threonine
phosphorylation was used to detect the PKG II-induced
serine/threonine phosphorylation of HER2. The results demonstrated
that in cells infected with Ad-PKG II and treated with 8-pCPT-cGMP,
there was a pronounced increase in serine/threonine phosphorylation
of HER2 (Fig. 3). These results
indicated that PKG II inhibited the tyrosine
phosphorylation/activation of HER2 through directly binding with
HER2 and causing phosphorylation.
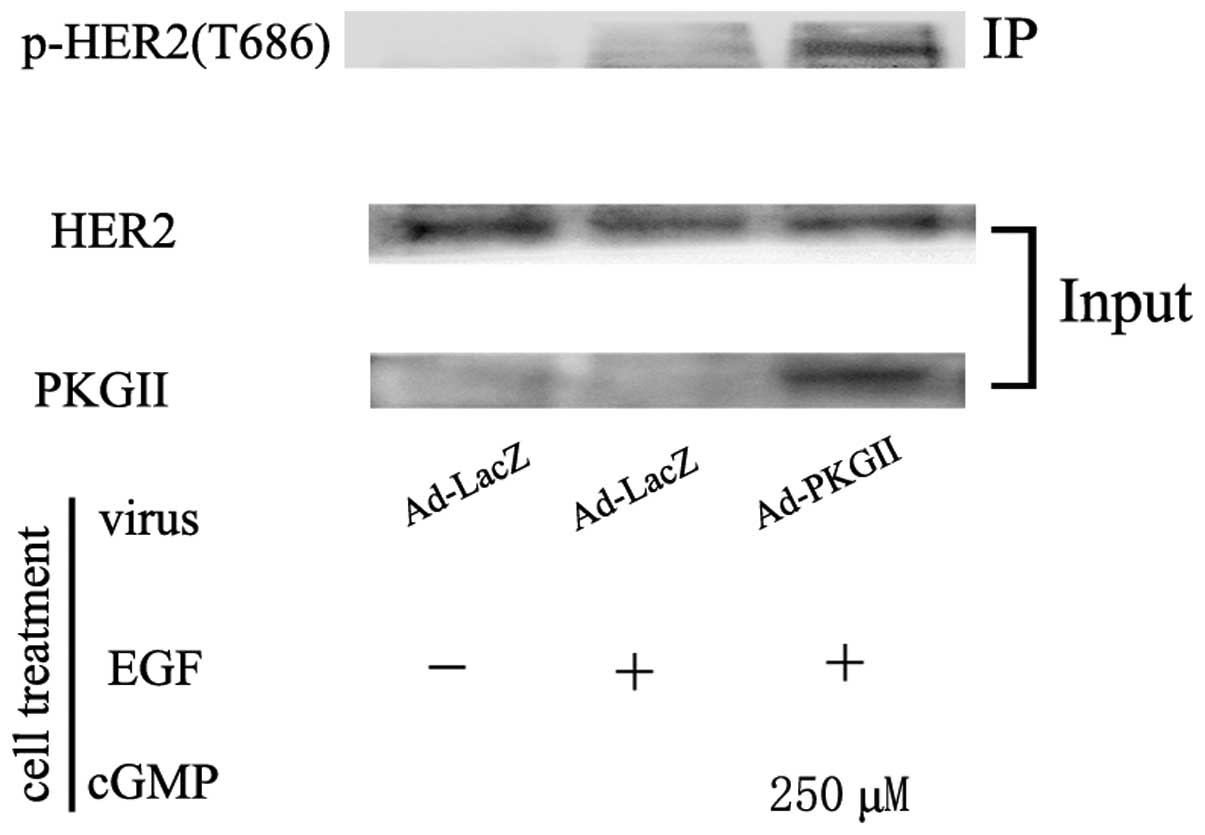
Threonine 686 is a PKG II-specific
phosphorylation site of HER2
In order to reveal the PKG II-specific
phosphorylation site of HER2, western blotting with antibodies
against p-HER2 (T686) was used to detect the phosphorylation of
threonine 686 on HER2 in cells infected with Ad-PKG II and treated
with 8-pCPT-cGMP. The results demonstrated that PKG II caused a
pronounced increase in phosphorylation of threonine 686 (Fig. 4). To further determine if this site
was the main site for PKG II-induced phosphorylation, plasmids
encoding the cDNA of flag-tagged wild-type and mutant HER2 were
constructed. The cells were transfected with the plasmids to
express the mutants of HER2 and the proteins were isolated by
immunoprecipitating with antibody against flag. Western blotting
with antibody against pan serine/threonine phosphorylation was
applied to detect the phosphorylation of the precipitated HER2
proteins. The results demonstrated that PKG II did not cause
serine/threonine phosphorylation of the T686A mutant of HER2 and
had no inhibitory effect on HER2 tyrosine
phosphorylation/activation, confirming that threonine 686 was the
main phosphorylating site of PKG II. Furthermore, EGF had no
stimulating effect on the T686E mutant of HER2, indicating that the
mimic of threonine 686 phosphorylation prevented the activation of
HER2 by EGF (Fig. 5). These
results confirmed that threonine 686 was the PKG II-specific
phosphorylation site of HER2 and the phosphorylation of this site
was crucial for the inhibition of HER2 activation by PKG II.
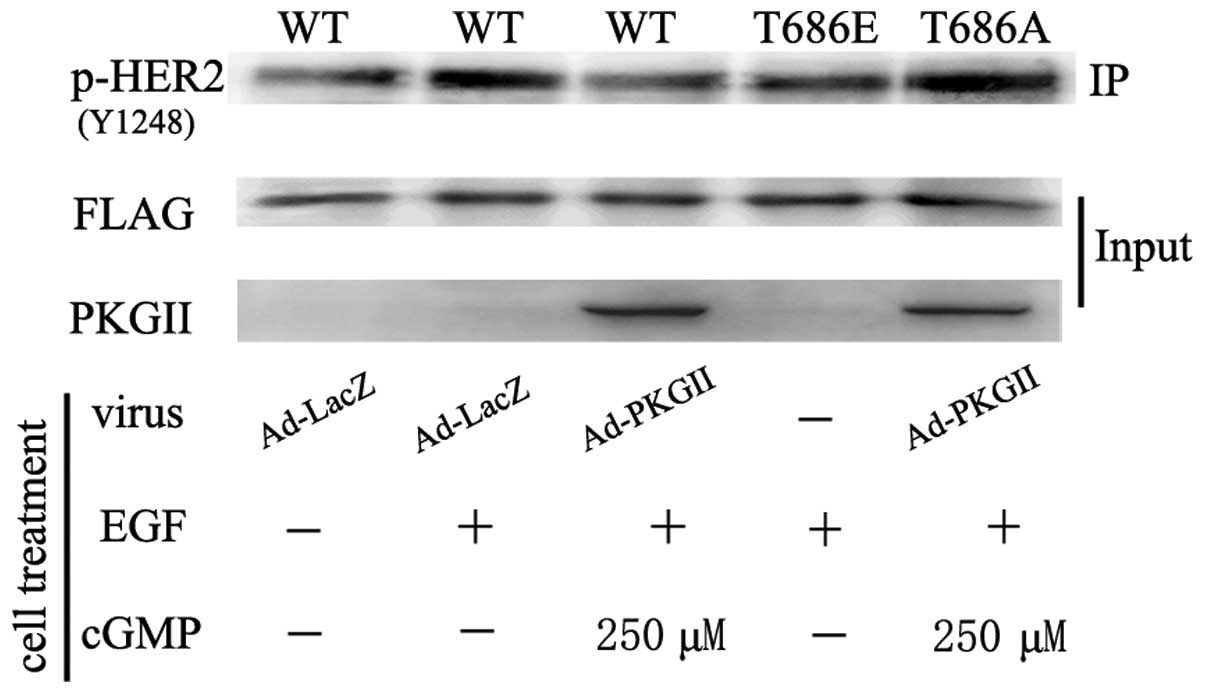 | Figure 5Threonine 686 phosphorylation is
associated with HER2 activation. HGC-27 cells were transfected with
plasmids encoding cDNA of WT HER2, threonine 686-glutamic acid
mutant HER2 (T686E) and threonine 686A-alanine mutant HER2 (T686A).
On day 2 after transfection, the cells were infected with Ad-LacZ
or Ad-PKG II overnight and serum starved for 12 h. Subsequently,
the cells were treated with 8-pCPT-cGMP for 1 h and subsequently
incubated with EGF for 10 min. The lysate of the cells was
subjected to western blotting to detect the tyrosine
phosphorylation/activation of HER2. The results demonstrated that
in cells transfected with plasmid of T686E, EGF-treatment did not
cause tyrosine phosphorylation of HER2, indicating that the mimic
of threonine 686 phosphorylation of HER2 could inhibit the
activation of HER2. In cells transfected with the T686A plasmid,
PKG II could not inhibit HER2 activation and did not phosphorylate
HER2 on T686, indicating that PKG II-induced threonine 686
phosphorylation of HER2 was crucial for inhibition of HER2. The
results are representative of three independent experiments. HER2,
human epidermal growth factor receptor 2; EGF, epidermal growth
factor; WT, wild type; PKG II, type II cGMP-dependent protein
kinase; Ad, adenovirus; Ad-LacZ, β-galactosidase; Ad, adenovirus;
IP, immunoprecipitation. |
Discussion
Gastric cancer is the fourth most commonly diagnosed
cancer and the second most common cause of cancer-associated
mortality worldwide (11).
Extensive research is being performed to improve the diagnosis and
treatment of the disease. Amplification of the HER2 gene and
overexpression of the HER2 protein in gastric cancer have been
confirmed by a large number of studies, indicating that HER2 is
important in the occurrence and development of this tumor (2,3).
HER2 forms homo- and heterodimers and serves as a critical
dimerization partner for other members of the HER/ErbB family, and
leads to activation of downstream signaling pathways associated
with cell proliferation, differentiation, survival and angiogenesis
(12). Among the other members of
the HER family, EGFR (HER1) is the most extensively investigated.
When EGF binds with EGFR, the binding causes dimerization of EGFR
with other members of the HER family, with HER2 as the preferential
partner (13). The dimerization
then causes auto-tyrosine phosphorylation of the receptors. The
phosphorylated tyrosine sites can recruit downstream signaling
molecules and initiate several signaling pathways (14). Thus, inhibiting HER2 activity is
important for interfering with the growth and development of
gastric cancer.
Our previous study demonstrated that PKG II
inhibited the activation of EGFR directly, potentially through
binding with EGFR and causing serine/threonine phosphorylation of
EGFR (10). Since HER2 has a
similar structure to EGFR, the present study aimed to investigate
whether PKG II directly inhibits HER2. The Co-IP results
demonstrated that PKG II could bind with HER2. However, since HER2
could dimerize with EGFR and PKG II could bind with EGFR, whether
the binding between PKG II and HER2 was a direct or an indirect one
required further investigation. In order to answer this question,
the phosphorylation of HER2 by PKG II was examined. The results
demonstrated that PKG II caused phosphorylation of HER2 and the
phosphorylation site was threonine 686. This confirmed that PKG II
bound directly with HER2 and phosphorylated it.
Similar to EGFR, HER2 exhibits two kinds of
phosphorylation during its participating signal transduction. One
is tyrosine phosphorylation caused by ligand binding and
dimerizing. The phosphorylated tyrosine residue may act as a
docking site for downstream signaling molecules (14). Another one is serine/threonine
phosphorylation caused by serine/threonine protein kinases,
including protein kinase A (PKA) and PKC. The phosphorylation of
serine/threonine on HER2 is important in regulating its activity.
Threonine 686 is located within the juxtamembrane domain of HER2
and phosphorylation of this site by different protein kinases has
different functions. For example, Gulliford et al reported
that PKC caused T686 phosphorylation of HER2 and stimulated the
internalization and signaling of the ligand-activated receptor
(15). Monje et al reported
that PKA phosphorylated T686 on HER2 and produced synergistic
enhancement of neuregulin-induced HER2-HER3 activation and
proliferation of Schwann cells (16). In the present study, the results
indicated that PKG II-induced T686 phosphorylation of HER2 was
associated with inhibition of this receptor. The causes of these
differences require further investigation in the future.
Our previous results demonstrated that PKG II had an
inhibitory effect on EGFR activation and the results in the present
study demonstrated that PKG II had an inhibitory effect on HER2
activation. These results suggested that PKG II could inhibit EGFR
and HER2 simultaneously. This inhibitory pattern is significant as
dual inhibition is more effective in cancer therapy. For example,
Fink et al reported that a compound with dual inhibitory
effects on EGFR and HER2 demonstrated promising efficacy in EGFR
and HER2-driven human tumor xenograft models (17,18).
Thus, this suggests that PKG II is a potential efficient cancer
inhibitor. This provides new options for cancer therapy.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81272755, 81201959 and
81001100), the Specialized Research Fund for Senior Personnel
Program of Jiangsu University (grant no. 11JDG114), the Natural
Science Foundation of Colleges and Universities of Jiangsu Province
(grant no. 12KJB310001, the Postdoctoral Research Funding Plan of
Jiangsu Province (grant no. 1401144C) and the China Postdoctoral
Science Foundation (grant no. 2014M561599). The authors would like
to thank Dr Gerry Boss and Dr Renate Pilz from the University of
California for providing the adenoviral constructs.
References
|
1
|
Ménard S, Casalini P, Campiglio M, Pupa SM
and Tagliabue E: Role of HER2/neu in tumor progression and therapy.
Cell Mol Life Sci. 61:2965–2978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim KC, Koh YW, Chang HM, Kim TH, Yook JH,
Kim BS, Jang SJ and Park YS: Evaluation of HER2 protein expression
in gastric carcinomas: Comparative analysis of 1,414 cases of
whole-tissue sections and 595 cases of tissue microarrays. Ann Surg
Oncol. 18:2833–2840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jørgensen JT: Role of human epidermal
growth factor receptor 2 in gastric cancer: Biological and
pharmacological aspects. World J Gastroenterol. 20:4526–4535. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olayioye MA: Update on HER-2 as a target
for cancer therapy: Intracellular signaling pathways of ErbB2/HER-2
and family members. Breast Cancer Res. 3:385–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olayioye MA, Graus-Porta D, Beerli RR,
Rohrer J, Gay B and Hynes NE: ErbB-1 and ErbB-2 acquire distinct
signaling properties dependent upon their dimerization partner. Mol
Cell Biol. 18:5042–5051. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roy V and Perez EA: Beyond trastuzumab:
Small molecule tyrosine kinase inhibitors in HER-2-positive breast
cancer. Oncologist. 14:1061–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Chen Y, Qu R, Lan T and Sang J: Type
II cGMP-dependent protein kinase inhibits EGF-triggered signal
transduction of the MAPK/ERK-mediated pathway in gastric cancer
cells. Oncol Rep. 27:553–558. 2012.
|
|
8
|
Lan T, Chen Y, Sang J, Wu Y, Wang Y, Jiang
L and Tao Y: Type II cGMP-dependent protein kinase inhibits
EGF-induced MAPK/JNK signal transduction in breast cancer cells.
Oncol Rep. 27:2039–2044. 2012.PubMed/NCBI
|
|
9
|
Taniyama K, Ishida K, Toda T, Motoshita J,
Kuraoka K, Saito A, Tani Y, Uike T, Teramoto S and Koseki M:
Tyrosine1248-phosphorylated HER2 expression and HER2 gene
amplification in female invasive ductal carcinomas. Breast Cancer.
15:231–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang L, Lan T, Chen Y, Sang J, Li Y, Wu
M, Tao Y, Wang Y, Qian H and Gu L: PKG II inhibits EGF/EGFR-induced
migration of gastric cancer cells. PLoS One. 8:e616742013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015 and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: Receptor heterodimerization in
development and cancer. Embo J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Birtwistle MR, Hatakeyama M, Yumoto N,
Ogunnaike BA, Hoek JB and Kholodenko BN: Ligand-dependent responses
of the ErbB signaling network: Experimental and modeling analyses.
Mol Syst Biol. 3:1442007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gulliford T, Ouyang X and Epstein RJ:
Intensification of growth factor receptor signalling by phorbol
treatment of ligand-primed cells implies a dimerstabilizing effect
of protein kinase C-dependent juxtamembrane domain phosphorylation.
Cell Signal. 11:245–252. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monje PV, Athauda G and Wood PM: Protein
kinase A-mediated gating of neuregulin-dependent ErbB2-ErbB3
activation underlies the synergistic action of cAMP on Schwann cell
proliferation. J Biol Chem. 283:34087–34100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fink BE, Norris D, Mastalerz H, Chen P,
Goyal B, Zhao Y, Kim SH, Vite GD, Lee FY, Zhang H, et al: Novel
pyrrolo[2,1-f] [1,2,4] triazin-4-amines: Dual inhibitors of EGFR
and HER2 protein tyrosine kinases. Bioorg Med Chem Lett.
21:781–785. 2011. View Article : Google Scholar
|
|
18
|
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H,
Rusnak DW, Owens G, Alligood KJ and Spector NL: Anti-tumor activity
of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation
of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene.
21:6255–6263. 2002. View Article : Google Scholar : PubMed/NCBI
|