Introduction
Hepatocellular carcinoma is one of the most common
types of hepatobiliar malignancy, and features high morbidity and
mortality (1). The incidence of
late-stage hepatocellular carcinoma has been reported to increase
by >1 million per year. Although chemotherapy and radiotherapy
are usually used to treat hepatocellular carcinoma, these methods
have limitations and are insufficient to abrogate its deleterious
effects (2). Therefore, novel
treatment strategies for hepatocellular carcinoma are urgently
required.
Resveratrol is a polyphenol which occurs naturally
in grapes, mulberries and peanuts (3). Resveratrol has several biological
activities, including anti-inflammatory, anti-oxidant and
vasorelaxant activities (4,5).
Resveratrol is often used as a cancer chemotherapeutic agent
(6) and has further biological
activities, including mimic effects of calorie restriction and
delay of aging (7). Delmas et
al (8) reported that
resveratrol inhibited cell proliferation at micromolar
concentrations in the HepG2 human hepatoblastoma cell line and the
Fao rat hepatoma cell line. In addition, Parekh et al
(9) reported that resveratrol
downregulates cyclin D1 through inhibiting the activities of p38
mitogen-activated protein kinase and Akt, and increasing
extracellular signal-regulated kinase activity to promote cell
apoptosis.
A large number of studies have shown that Akt can
promote cell proliferation and inhibit apoptosis, and has an
important role in the occurrence and progression of tumors
(10,11). Forkhead box O3a transcription
factor (FoxO3a) is a key downstream target of the
phosphoinositide-3 kinase (PI3K)/Akt pathway. FoxO3a has been
implicated in the regulation of diverse cellular functions,
including proliferation, apoptosis, protection against oxidative
stress and metabolism (12). Akt
can promote the phosphorylation of FoxO3a, which subsequently
translocates from the cell nucleus to the cytoplasm, thus
inhibiting apoptosis. By contrast, inhibition of the PI3K/Akt
pathway was shown to reduce the phosphorylation of FoxO3a and to
thereby promote the nuclear translocation of FoxO3a to cause cell
cycle arrest and apoptosis (13).
Polter et al (14) showed
that FoxO3a knockout mice presented with impaired development of
hematopoetic stem cells. Of note, low levels of FoxO3a have been
shown to be associated with resistance to chemotherapy in human
cancers (15). In addition,
purified vitexin compound 1 (16),
melatonin (17) and casticin
(18) were shown to induce
apoptosis in hepatocellular carcinoma via activation of FoxO3a.
Parekh et al (9) reported that resveratrol suppresses
the growth of human HepG2 liver cancer cells by causing apoptosis;
however, the mechanism of this pro-apoptotic response to
resveratrol has remained elusive. Based on this information, the
present study assessed the roles of the Akt/FoxO3a/Bim pathway in
resveratrol-induced HepG2 liver cancer-cell apoptosis.
Materials and methods
Materials
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), fibroblast growth factor 2 (FGF-2), Hoechst 33258
and resveratrol were purchased from Sigma-Aldrich (St Louis, MO,
USA). The enhanced chemiluminescence (ECL) solution,
paraformaldehyde, goat serum, Triton X, Tris-buffered saline with
0.1% Tween 20 (TBS-T), sodium dodecyl sulfate (SDS)-polyacrylamide
gel, non-fat milk and the polyvinylidene difluoride membrane (PVDF)
membrane were purchased from Beyotime Institute of Biotechnology
(Haimen, China). PI3K inhibitor LY294002 was purchased from Merck
Millipore (Billerica, MA, USA). All cell components of the culture
medium were purchased from Thermo Fisher Scientific (Waltham, MA,
USA) unless stated otherwise.
Cell culture
The HepG2 human hepatoma cell line was supplied by
Sun Yat-sen University Experimental Animal Center (Guangzhou,
China) and were cultured in a humidified atmosphere containing 5%
CO2 at 37°C. HepG2 cells were seeded on six-well plates
at a density of 2×106 cells/well in RPMI-1640 medium
with 10% fetal bovine serum, 100 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific) and 100 U/ml penicillin-streptomycin
(Gibco). HepG2 cells were passaged every two days.
MTT assay
The MTT assay was used to assess cell viability.
Prior to each experiment, HepG2 cells (5,000 cells/well) were
seeded in 96-well microtiter plates. HepG2 cells were treated with
various concentrations of resveratrol (0, 25, 50, 100 or 200
µM) for 48 h. Subsequently, 10 µl MTT solution
(5mg/ml) was added to each well, followed by further incubation for
4 h at 37°C. Following addition of dimethyl sulfoxide (150
µl, Beyotime Institute of Biotechnology), the absorbance was
measured at 470 nm with a SpectraMax 190 spectrophotometer
(Molecular Devices LLC, Sunnyvale, CA, USA) and used to calculate
the cell viability relative to that of the control group.
Hoechst 33258 nuclear staining for
assessment of apoptosis
Hoechst 33258 was used to assess cell apoptosis.
H9c2 cardiac myocytes were seeded at a density of 2×106
cells/well and incubated for 24 h. Following treatment with
resveratrol (0, 25, 50 or 100 µM for 48 h), HepG2 cells were
fixed in ice-cold 4% paraformaldehyde dissolved in
phosphate-buffered saline at 37°C for 15 min. 5% normal goat serum
in 0.01 M phosphate-buffered saline (PBS) containing 0.3% Triton
X-100 was used to block non-specific binding. The slides were
washed three times with PBS and then incubated with 10 µg/ml
Hoechst 33258 at 37°C for 15 min. The slides were visualized under
a fluorescence microscope (BX50-FLA; Olympus, Tokyo, Japan).
Apoptotic cells showed condensed, fractured or distorted nuclei,
while viable cells displayed a normal nuclear size and uniform
fluorescence.
Sub-cellular fractionation and western
blot analysis
HepG2 cells were incubated in 0.5% FBS DMEM for 24 h
(control group) or treated with resveratrol (100 µM) for 48
h (RES group), with 10 ng FGF-2 or 10 mol/l LY294002 for 30 min
prior to exposure to resveratrol (100 µM) for 48 h (FGF-2 +
RES group or LY + RES group), or with 10 ng FGF-2 or 10
µmol/l LY294002 for 30 min followed by culture for 24 h
(FGF-2 group or LY group). Cultured HepG2 cells were fractionated
into nuclear and cytoplasmic lysates using a PARIS kit (Ambion;
Thermo Fisher Scientific). HepG2 cells were directly homogenized in
cell lysis buffer (Cell Signaling Technology, Inc., Danvers, MA,
USA) with phosphatase inhibitor cocktail (Sigma-Aldrich), and
lysates were centrifuged at 14,000 × g for 10 min at 4°C. The
protein concentration was determined using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). The
extracted proteins (30 µg per lane) were separated in 10%
SDS-polyacrylamide electrophoresis gels and transferred onto a PVDF
membrane. Non-specific protein binding was blocked with 5% non-fat
dried milk in TBS-T for 2 h at room temperature with agitation. The
membranes were then incubated overnight at 4°C with the following
diluted primary antibodies: Rabbit anti-Akt polyclonal antibody
(cat. no. 9272; 1:1,000 dilution), rabbit anti-phosphorylated
(p)-Akt (Ser 473) monoclonal antibody (cat. no. 4060; 1:2,000
dilution), rabbit anti-FoxO3a polyclonal antibody (cat. no. 12829;
1:1,000 dilution), rabbit anti-p-FoxO3a (Ser 253) polyclonal
antibody (cat. no. 9466; 1:800 dilution) (all from Cell Signaling
Technology) and rabbit anti-Bim polyclonal antibody (cat. no.
ab32158; Abcam, Cambridge, MA, USA; 1:800 dilution). The membranes
were then washed three times with TBS-T and incubated with
horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G
(cat. no. A0208; Beyotime Institute of Biotechnology; 1:1,000
dilution) for 2 h at 37°C. Protein bands were analyzed using
visualized using an enhanced chemiluminescence reagent kit
(Beyotime Institute of Biotechnology) and a western blotting
detection system (Tanon-5500; Tanon Science & Technology,
Shanghai, China) and the Quantity One Software 4.6.2 Package
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for
analysis.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analysis was performed using one-way analysis
of variance with SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Resveratrol reduces viability of HepG2
cells
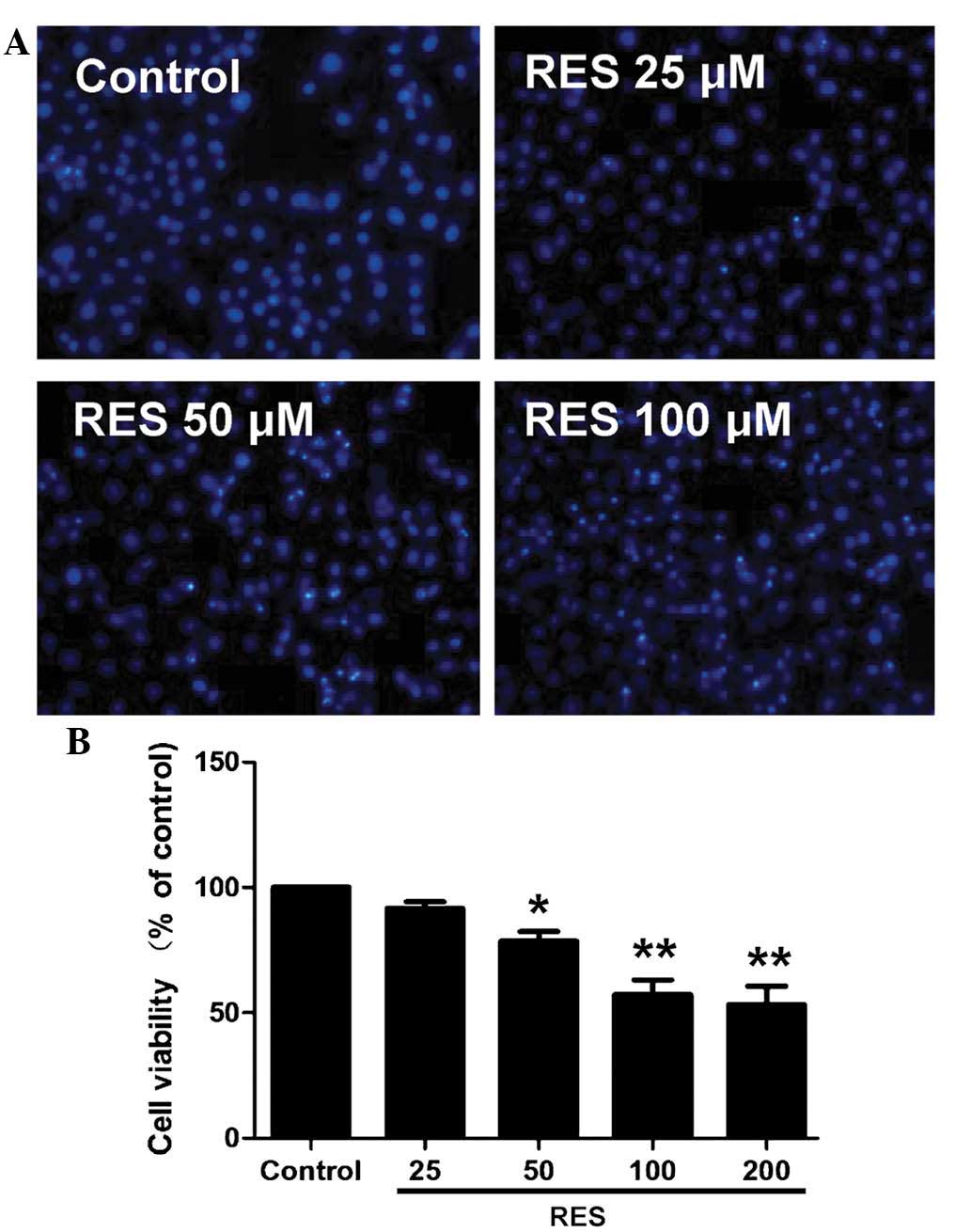
HepG2 cells were treated with increasing doses of
resveratrol for 48 h and subjected to an MTT assay. The results
showed that resveratrol at 50–200 µM decreased the cell
viability in a concentration-dependent manner (Fig. 1A). Hoechst 33258 staining further
confirmed the apoptosis-inducing potential of resveratrol (Fig. 1B). Resveratrol treatment resulted
in a significant increase in the apoptotic rate of the
cardiomyocytes. As 100 µM resveratrol induced a significant
reduction in cell viability and promoted apoptosis in HepG2 cells,
this concentration was selected for use in the subsequent
experiments.
Resveratrol treatment causes nuclear
translocation of FoxO3a
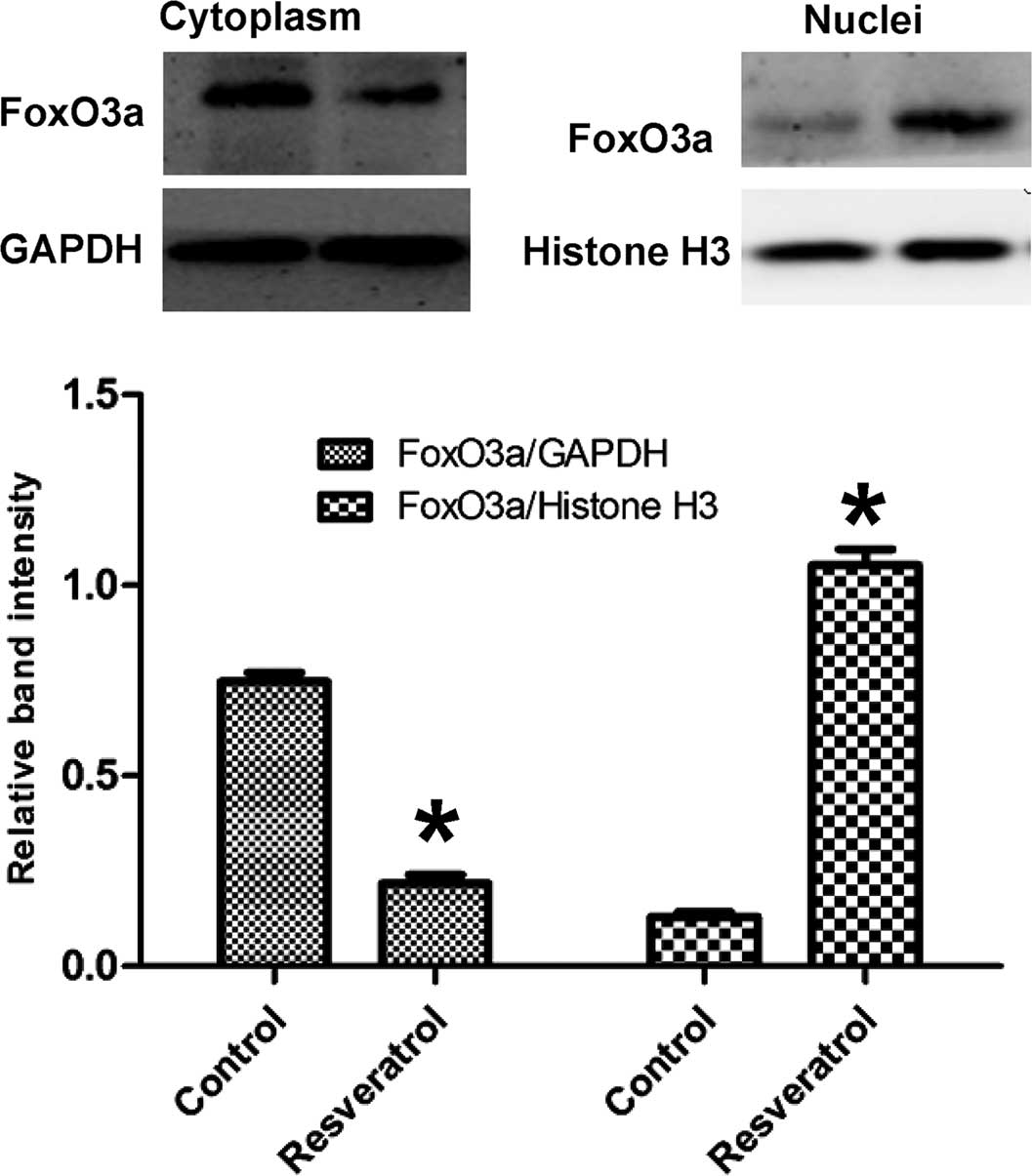
To investigate the role of resveratrol on the
sub-cellular location of FoxO3a, nuclear and cytosolic protein
fractions of HepG2 cells were obtained and subjected to western
blot analysis of FoxO3a (Fig. 2).
Following treatment with resveratrol, the protein levels of FoxO3a
were increased in the nucleus, but decreased in the cytoplasm,
indicating that resveratrol caused nuclear translocation of
FoxO3a.
Resveratrol decreases the phosphorylation
of Akt and FoxO3a in HepG2 cells
To investigate the roles of the PI3K/Akt/FoxO3a
pathway in resveratrol-induced apoptosis, the phosphorylation
levels of Akt and FoxO3a were determined in HepG2 cells. Fig. 3 shows that resveratrol decreased
the phosphorylation of Akt and FoxO3a in a time-dependent manner.
The levels of phosphorylated Akt and FoxO3a were significantly
decreased at 24 h, and were almost completely abolished following
48 h of resveratrol treatment.
Effects of resveratrol treatment on the
PI3K/Akt/FoxO3a pathway
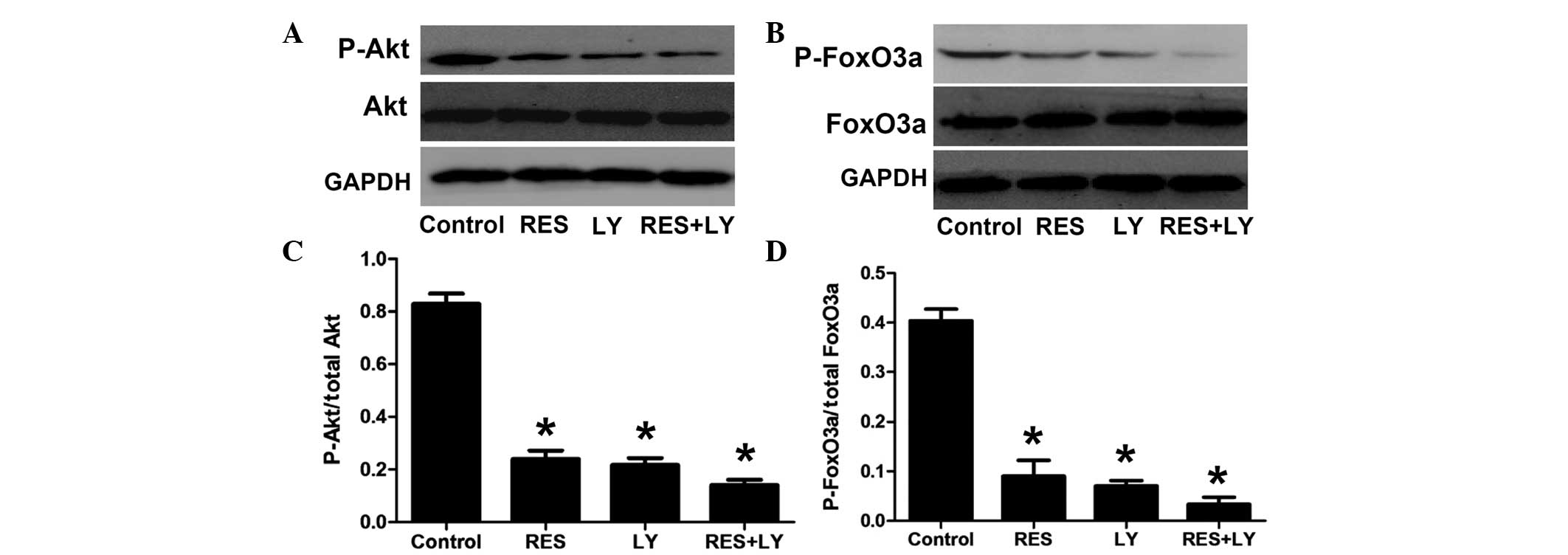
To further confirm the involvement of the
PI3K/Akt/FoxO3a pathway in resveratrol-induced apoptosis, the PI3K
inhibitor LY294002 was used (Fig.
4). Treatment with resveratrol or LY294002 alone significantly
decreased the phosphorylation of Akt and FoxO3a, which was further
decreased by the combination of LY294002 and resveratrol.
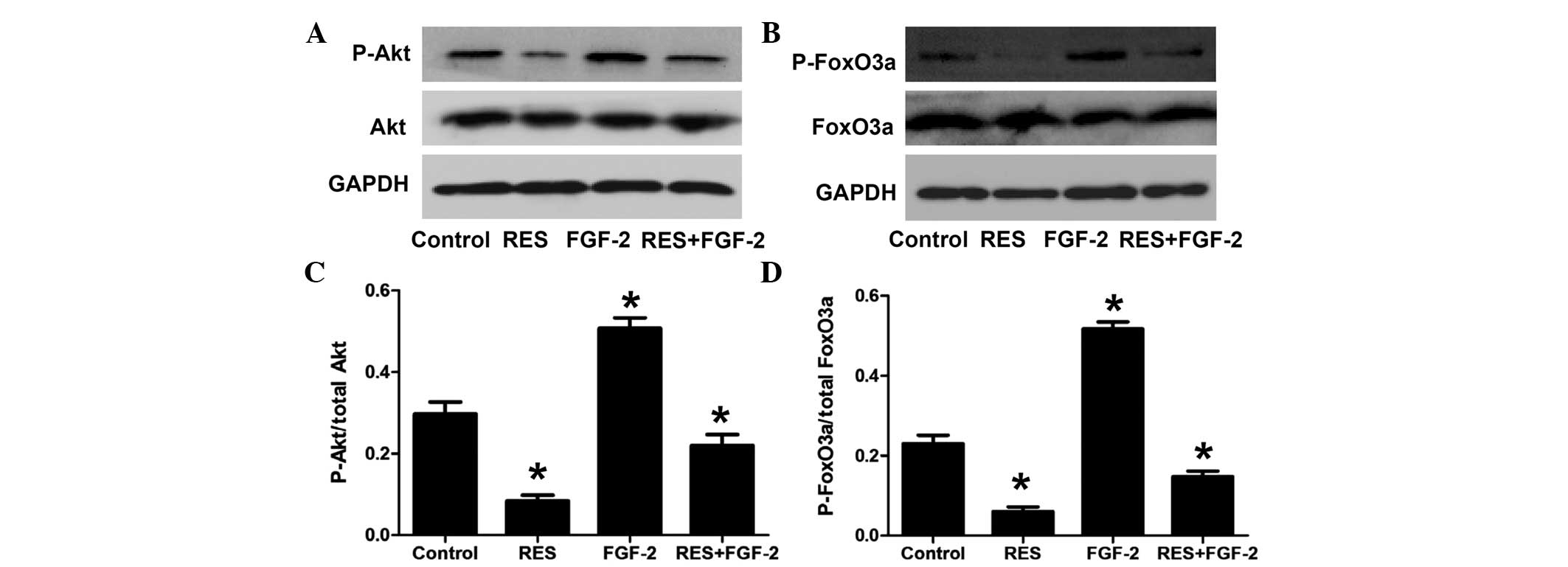
In addition, the effect of resveratrol on the
PI3K/Akt/FoxO3a pathway was assessed under stimulation with FGF-2.
As shown in Fig. 5, FGF-2
significantly increased the phosphorylation levels of Akt and
FoxO3a. Furthermore, compared with FGF-2 treatment alone, combined
treatment with FGF-2 and resveratrol significantly decreased the
levels of phosphorylated Akt and FoxO3a.
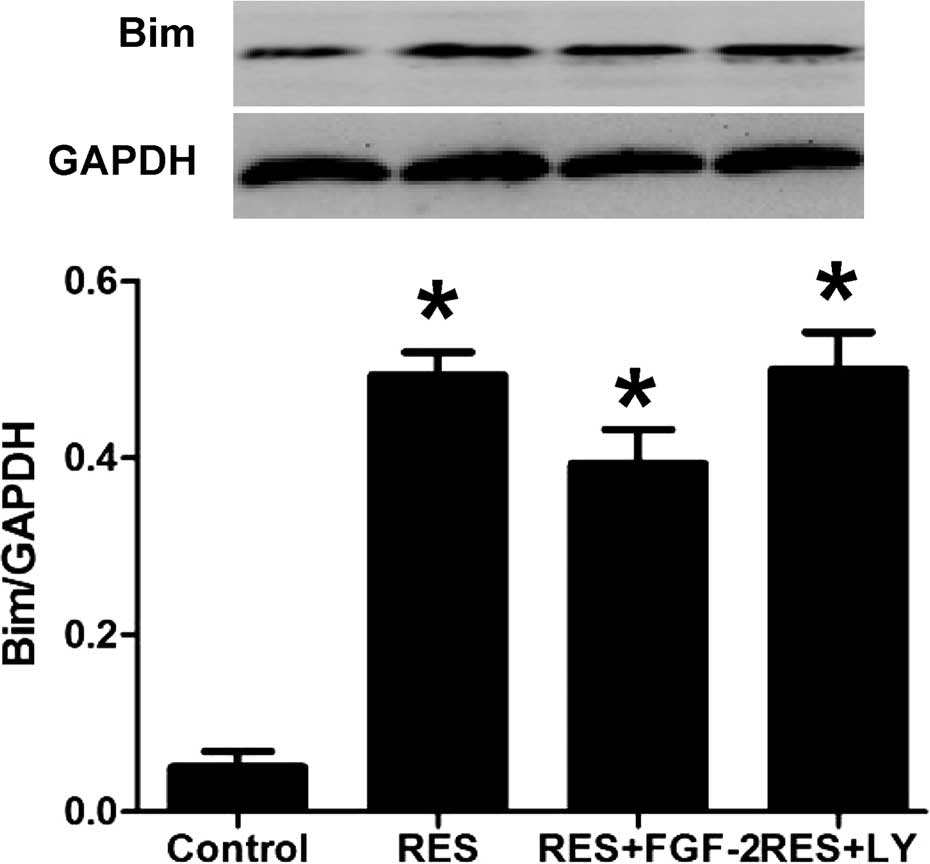
Resveratrol treatment increases Bim
expression in HepG2 cells
Western blot analysis showed that resveratrol
treatment significantly induced the expression of Bim protein. In
addition, as shown in Fig. 6,
resveratrol-induced Bim expression was further enhanced by
co-treatment with FGF-2 or LY294002.
Discussion
Hepatocellular carcinoma is the most common type of
liver cancer and to date, treatments have remained ineffective
(2). Resveratrol is a nutrient
compound with anti-cancer activity, and has been demonstrated to
inhibit the initiation and development of cancer (6). The ability to cause cell-growth
arrest is an important feature and a basic characteristic of cancer
chemopreventive agents (19).
Resveratrol is able to inhibit the proliferation of tumor cells via
interfering with the progression of the cell cycle (9) and inhibiting the synthesis of DNA
(20). However, the underlying
molecular mechanism of resveratrol-induced apoptosis in
hepatocellular carcinoma has remained to be fully elucidated.
FoxO3a is an important transcription factor and
tumor suppressor; it is activated by stress signaling, which
results in cell apoptosis (12,13).
Akt can promote FoxO3a phosphorylation, leading to FoxO3a
translocation from the nucleus to the cytoplasm, which de-activates
FoxO3a; conversely, inhibition of Akt promotes de-phosphorylation
of FoxO3a, resulting in nuclear translocation of FoxO3a (13). The present study provided clear
evidence of resveratrol-induced FoxO3a translocation to the
nucleus. In addition, the present study showed that treatment of
HepG2 cells with resveratrol significantly reduced the
de-phosphorylated forms of Akt and FoxO3a. These results indicated
that resveratrol inhibited the Akt survival pathway, leading to the
promotion of FoxO3a translocation.
In order to further investigate the role of Akt in
resveratrol-induced HepG2-cell apoptosis, HepG2 cells were treated
with the PI3K/Akt inhibitor LY294002, or with FGF-2, which is able
to activate Akt and promote cancer-cell viability. LY294002
significantly decreased the phosphorylation of Akt and FoxO3a,
while FGF-2 significantly increased the phosphorylation of Akt and
FoxO3a. In addition, the phosphorylation of Akt and FoxO3a was
inhibited or enhanced to an even greater extent in the resveratrol
+ LY294002 and resveratrol + FGF-2 groups, respectively. This
result further confirmed that resveratrol induced FoxO3a
translocation via Akt signaling.
Tzivion et al (21) reported that FoxO3a was able to
promote the expression of pro-apoptotic gene Bim. While the role of
FoxO3a has been extensively studied in certain types of cancer,
including thyroid cancer cell lines (22), little is known with regard to the
effect of resveratrol on hepatocellular carcinoma and the possible
implication of the FoxO3a. Roy et al (23) has reported that resveratrol
inhibited the phosphorylation of FoxO3a and with simultaneous
upregulation of the expression of Bim in in vitro cancer
models. The present study was the first, to the best of our
knowledge, to show that resveratrol-induced HepG2-cell apoptosis
was mediated via the FoxO3a/Bim pathway.
In conclusion, the results of the present study
indicated that resveratrol induces apoptosis in HepG2 liver cancer
cells through modulation of the Akt/FoxO3a/Bim pathway. The study
provided evidence that resveratrol is a promising drug against
hepatocellular carcinoma.
Acknowledgments
This study was supported by a grants from the
Graduate Student Research Innovation Project of Hunan province (no.
CX2013B397).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao Q, Shi Y, Wang X, Zhou J, Qiu S and
Fan J: Translational medicine in hepatocellular carcinoma. Front
Med. 6:122–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pervaiz S and Holme AL: Resveratrol: Its
biologic targets and functional activity. Antioxid Redox Signal.
11:2851–2897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foti Cuzzola V, Ciurleo R, Giacoppo S,
Marino S and Bramanti P: Role of resveratrol and its analogues in
the treatment of neurodegenerative diseases: Focus on recent
discoveries. CNS Neurol Disord Drug Targets. 10:849–862. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zordoky BN, Robertson IM and Dyck JR:
Preclinical and clinical evidence for the role of resveratrol in
the treatment of cardiovascular diseases. Biochim Biophys Acta.
1852:1155–1177. 2015. View Article : Google Scholar
|
|
6
|
Signorelli P and Ghidoni R: Resveratrol as
an anticancer nutrient: Molecular basis, open questions and
promises. J Nutr Biochem. 16:449–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wood JG, Rogina B, Lavu S, Howitz K,
Helfand SL, Tatar M and Sinclair D: Sirtuin activators mimic
caloric restriction and delay ageing in metazoans. Nature.
430:686–689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delmas D, Jannin B, Cherkaoui Malki M and
Latruffe N: Inhibitory effect of resveratrol on the proliferation
of human and rat hepatic derived cell lines. Oncol Rep. 7:847–852.
2000.PubMed/NCBI
|
|
9
|
Parekh P, Motiwale L, Naik N and Rao KV:
Downregulation of cyclin D1 is associated with decreased levels of
p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of
resveratrol in liver cancer cells. Exp Toxicol Pathol. 63:167–173.
2011. View Article : Google Scholar
|
|
10
|
Weng SC, Kashida Y, Kulp SK, Wang D,
Brueggemeier RW, Shapiro CL and Chen CS: Sensitizing estrogen
receptor-negative breast cancer cells to tamoxifen with OSU-03012,
a novel celecoxib-derived phosphoinositide-dependent protein
kinase-1/Akt signaling inhibitor. Mol Cancer Ther. 7:800–808. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan A, Aljarbou AN, Aldebasi YH, Faisal
SM and Khan MA: Resveratrol suppresses the proliferation of breast
cancer cells by inhibiting fatty acid synthase signaling pathway.
Cancer Epidemiol. 38:765–772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nho RS and Hergert P: FoxO3a and disease
progression. World J Biol Chem. 5:346–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu MH, Yuan C, He J, Tan TP, Wu SJ, Fu
HY, Liu J, Yu S, Chen YD, Le QF, et al: Resveratrol Protects PC12
cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a
pathway. Cell Mol Neurobiol. 35:513–522. 2015. View Article : Google Scholar
|
|
14
|
Polter A, Yang S, Zmijewska AA, van Groen
T, Paik JH, Depinho RA, Peng SL, Jope RS and Li X: Forkhead box,
class O transcription factors in brain: Regulation and behavioral
manifestation. Biol Psychiatry. 65:150–159. 2009. View Article : Google Scholar :
|
|
15
|
Su JL, Cheng X, Yamaguchi H, Chang YW, Hou
CF, Lee DF, Ko HW, Hua KT, Wang YN, Hsiao M, et al:
FOXO3a-dependent mechanism of E1A-Induced chemosensitization.
Cancer Res. 71:6878–6887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang JG, Zheng XX, Zeng GY, Zhou YJ and
Yuan H: Purified vitexin compound 1 induces apoptosis through
activation of FOXO3a in hepatocellular carcinoma. Oncol Rep.
31:488–496. 2014.
|
|
17
|
Carbajo-Pescador S, Steinmetz C, Kashyap
A, Lorenz S, Mauriz JL, Heise M, Galle PR, González-Gallego J and
Strand S: Melatonin induces transcriptional regulation of Bim by
FoxO3a in HepG2 cells. Br J Cancer. 108:442–449. 2013. View Article : Google Scholar :
|
|
18
|
He L, Yang X, Cao X, Liu F, Quan M and Cao
J: Casticin induces growth suppression and cell cycle arrest
through activation of FOXO3a in hepatocellular carcinoma. Oncol
Rep. 29:103–108. 2013.
|
|
19
|
Izzotti A: Molecular medicine and the
development of cancer chemopreventive agents. Ann N Y Acad Sci.
1259:26–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pirola L and Fröjdö S: Resveratrol: One
molecule, many targets. IUBMB Life. 60:323–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tzivion G, Dobson M and Ramakrishnan G:
FoxO transcription factors; Regulation by AKT and 14-3-3 proteins.
Biochim Biophys Acta. 1813:1938–1945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong ZY, Lee HJ, Shin DY, Kim SK, Seo M
and Lee EJ: Inhibition of Akt/FOXO3a signaling by constitutively
active FOXO3a suppresses growth of follicular thyroid cancer cell
lines. Cancer Lett. 314:34–40. 2012. View Article : Google Scholar
|
|
23
|
Roy SK, Chen Q, Fu J, Shankar S and
Srivastava RK: Resveratrol inhibits growth of orthotopic pancreatic
tumors through activation of FOXO transcription factors. PLoS One.
6:e251662011. View Article : Google Scholar : PubMed/NCBI
|