Introduction
Giant cell tumor (GCT) is a disease, which is
characterized by locally aggressive behavior, and designated as an
osteoclastoma due to the multi-nucleated, osteoclast-like giant
cells observed morphologically and histologically (1,2). GCT
accounts for 11% of all bone tumors in China, with the second
highest incidence after osteochondroma (3).
Runt-related transcription factor 2 (RunX2), also
termed core-binding factor α1, belongs to the transcription factors
of the Runt domain gene family. The expression of RunX2 is
regulated by multiple growth factors and hormones involved in bone
cell differentiation (3,4). Mak et al (5) reported that RunX2 is crucial in GCT
stromal cells through upregulation of matrix metalloproteinase-13.
Singh et al (6) confirmed
the significant role of fibroblast growth factor receptor-2
signaling in osteoblastic differentiation in GCT stromal cells via
inhibition of the extracellular signal-regulated kinases 1 and 2
(ERK1/2) signaling pathway.
MicroRNAs (miRs) are widely distributed in a variety
of organisms and regulate gene expression. They are involved in the
proliferation, differentiation and apoptosis of cells, in addition
to other important cell regulatory activities (7). The miR-30a genes exist in the genome
in a variety of forms. Previous studies have demonstrated that miRs
may produce similar effects to oncogenes or tumor suppressor genes
and various types of miR-30a are expressed abnormally in GCT
tissues (8–10). Furthermore, Huang et al
(11) suggested that miR-30a
inhibited GCT of bone by targeting RunX2.
Imatinib is a selective inhibitor of certain type
III tyrosine kinase receptor family members, including CD117,
platelet-derived growth factor receptor and the ABL family of
tyrosine kinases, which leads to inhibition of BCR-ABL protein
expression (12). Studies have
demonstrated that imatinib competes with ATP in binding to the
nucleotide-binding site catalyzed by the tyrosine kinase (13–15).
The catalytic activity of the kinase, therefore, cannot occur and
the phosphorylated substrate cannot interact with downstream
effector molecules, which leads to the inhibition of cell
proliferation and induces apoptosis (16). In the present study, the anticancer
effect of imatinib on GCT cell apoptosis was evaluated and the
signaling cascades that may mediate this effect were investigated.
The findings of the present study indicate that imatinib regulates
apoptosis of GCT cells by suppression of RunX2 and activation of
miR-30a, indicating that imatinib may serve as an important novel
molecular target for the treatment of GCT.
Materials and methods
Reagents
The chemical structure of imatinib (Sigma-Aldrich,
St. Louis, MO, USA; purity, ≥98%) is presented in Fig. 1. Hyclone RPMI-1640 was obtained
from GE Healthcare Life Sciences (Logan, UT, USA) and Gibco fetal
bovine serum (FBS) was obtained from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). A Vybrant® MTT Cell Proliferation
Assay kit was purchased from Molecular Probes (Thermo Fisher
Scientific, Inc.), and Caspase-3 and -9 Colorimetric Assay kits
were purchased from Beyotime Institute of Biotechnology (Jiangsu,
China). A Molecular Probes Annexin V-FITC/PI Apoptosis Detection
kit was obtained from Thermo Fisher Scientific, Inc. A BCA Protein
Assay kit was obtained from Beyotime Institute of Biotechnology
(Nanjing, China). TRIzol® and an miRNA qRT-PCR kit were
obtained from Invitrogen (Thermo Fisher Scientific, Inc.).
Ethics statement, tissue samples and cell
lines
The present study was approved by the regional
ethics committee of Second Xiangya Hospital, Central South
University (Changsha, China) and written informed consent was
obtained from the patients. GCT samples were collected from male
patients (age, 58 ±5 years) at the Second Xiangya Hospital, Central
South University between October 2013 and January 2014. The tumor
tissue samples were maintained in RPMI-1640 containing 10% FBS, 100
U/ml penicillin and 100 mg/ml streptomycin (both Amresco, LLC,
Solon, OH, USA). The small sections of tissue (500 mg) and the
resultant cell suspension were transferred to 25-cm2
flasks, which were incubated at 37°C in a humidified atmosphere of
5% CO2. Half of the culture medium was replaced with
fresh complete medium every 2 days. Primary cultures were
subcultured and stored in liquid nitrogen until reaching
confluence. The purified GCT samples then underwent bisphosphonate
treatment (Amresco, LLC) and evaluation (2). GCT cells (2.5×105
cells/well) were cultured in complete medium and treated with
imatinib at different concentrations (0, 0.625, 1.25, 2.5, 5 and 10
µM) for 3 days.
Cell viability assay
GCT cells (2.0×104 cells/well) were
seeded in 96-well plates and cultured with imatinib (0, 0.625,
1.25, 2.5, 5 and 10 µM) at a temperature of 37°C in a
humidified atmosphere of 5% CO2 for 0, 1, 2 and 3 days.
The cell viability was determined using the MTT assay. MTT (10
µl) was added to each well and incubated for 4 h at 37°C in
a humidified atmosphere of 5% CO2. The culture medium
was removed and 150 µl dimethyl sulfoxide (Invitrogen;
Thermo Fisher Scientific, Inc.) was added to each well and
incubated for 20 min at room temperature whilst being agitated. The
absorbance was determined with an ELISA reader
(Infinite® 200 PRO; Tecan, Männedorf, Switzerland) at a
wavelength of 570 nm.
Flow cytometric analysis of GCT cell
apoptosis
GCT cells (2.5×105 cells/well) were
seeded in 6-well plates and cultured with imatinib (1.25, 2.5 and 5
µM) for 48 h. GCT cells were washed twice with ice-cold
phosphate-buffered saline (Beyotime Institute of Biotechnology).
The cells were stained with 10 µl Annexin V-fluorescein
isothiocyanate and incubated for 10 min in the dark. Next, 10
µl propidium iodide was added to the cells and cell
apoptosis was immediately detected using an EPICS®
ALTRA™ flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Detection of caspase-3 and -9 activity
levels
GCT cells (2.5×105 cells/well) were
seeded in 6-well plates and cultured with imatinib (1.25, 2.5 and 5
µM) for 48 h. The caspase-3 and -9 activity level was
detected via fluorescence at a wavelength of 405 nm using the
caspase-3 and -9 colorimetric assay kits.
Western blotting for RunX2 protein
expression levels
GCT cells (2.5×105 cells/well) were
seeded in 6-well plates and cultured with imatinib (1.25, 2.5 and 5
µM) for 48 h. The cells were incubated with ice-cold lysis
buffer (Beyotime Institue of Biotechnology) and maintained for 30
min on ice. The suspension was centrifuged at 12,000 × g for 10 min
at 4°C. The liquid supernatant was collected to measure the protein
concentration using the BCA protein assay. Proteins were separated
using 12% sodium dodecyl sulfate-polyacrylamide gels (Beyotime
Institute of Biotechnology) with Coomassie Brilliant Blue (Sangon
Biotech Co., Ltd., Shanghai, China) and transferred to
polyvinylidene fluoride membranes (0.22 mm; EMD Millipore,
Billerica, MA, USA). The blotting membrane was treated with
Tris-buffered saline (TBS) containing 5% non-fat milk to block
non-specific binding sites. The membranes were then incubated with
mouse monoclonal RunX2 (1:1,000; cat. no. sc-390715; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and rabbit polyclonal
anti-β-actin (1:500; cat. no. D110007; Sangon Biotech) antibodies
overnight at 4°C. The membrane was washed twice with TBS with
Tween-20 for 2 h and incubated with monoclonal anti-mouse
immunoglobulin G (1:1,000; cat. no. sc-51997; Santa Cruz
Biotechnology, Inc.) conjugated with horseradish peroxidase for 2
h. Enhanced chemiluminescence [GE Healthcare Life Sciences] was
conducted to detect the protein expression level.
Quantitative polymerase chain reaction
(qPCR) analysis of miR-30a expression levels
GCT cells (2.5×105 cells/well) were
seeded in 6-well plates and cultured with imatinib (1.25, 2.5 and 5
µM) for 48 h. Total RNA was extracted from the cells using
TRIzol® according to the manufacturer's instructions.
The expression of miR-30a was detected using a
Bulge-Loop® miRNA qRT-PCR kit (Sharp Bo Biological
Technology Co., Ltd., Guangzou, China) and observed by qPCR. The
primers used were as follows: Forward,
5′-GCAGTAAGTCACTTGCATGATTGT-3′ and revers,
5′-CGCTTGAAGCACTGTCTTATTTGT-3′ for miR-30a; and forward,
5′-GTTGACATCCGTAAAGACC-3′ and reverse, 5′-GGAGCCAGGGCAGTAA-3′ for
U6.
Transfection of miR-30a and
anti-miR-30a
The miR-30a precursor and anti-miR-30a (Ambion;
Thermo Fisher Scientific, Inc.) were synthesized by Sangon Biotech
Co., Ltd. and transfected using Invitrogen Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) at a final concentration of 50 nM.
GCT cells (2.5×105 cells/well) were seeded in 6-well
plates for 6 h. The transfection media was then replaced with
complete media without antibiotics in a humidified atmosphere at
37°C with 5% CO2 for 18 h.
Statistical analysis
Statistical analysis was performed with SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). All
experiments were performed at least three times and data are
presented as means ± standard deviation. Data were analyzed using
Student's t-test and P<0.05 was considered to indicate a
statistically significant difference.
Results
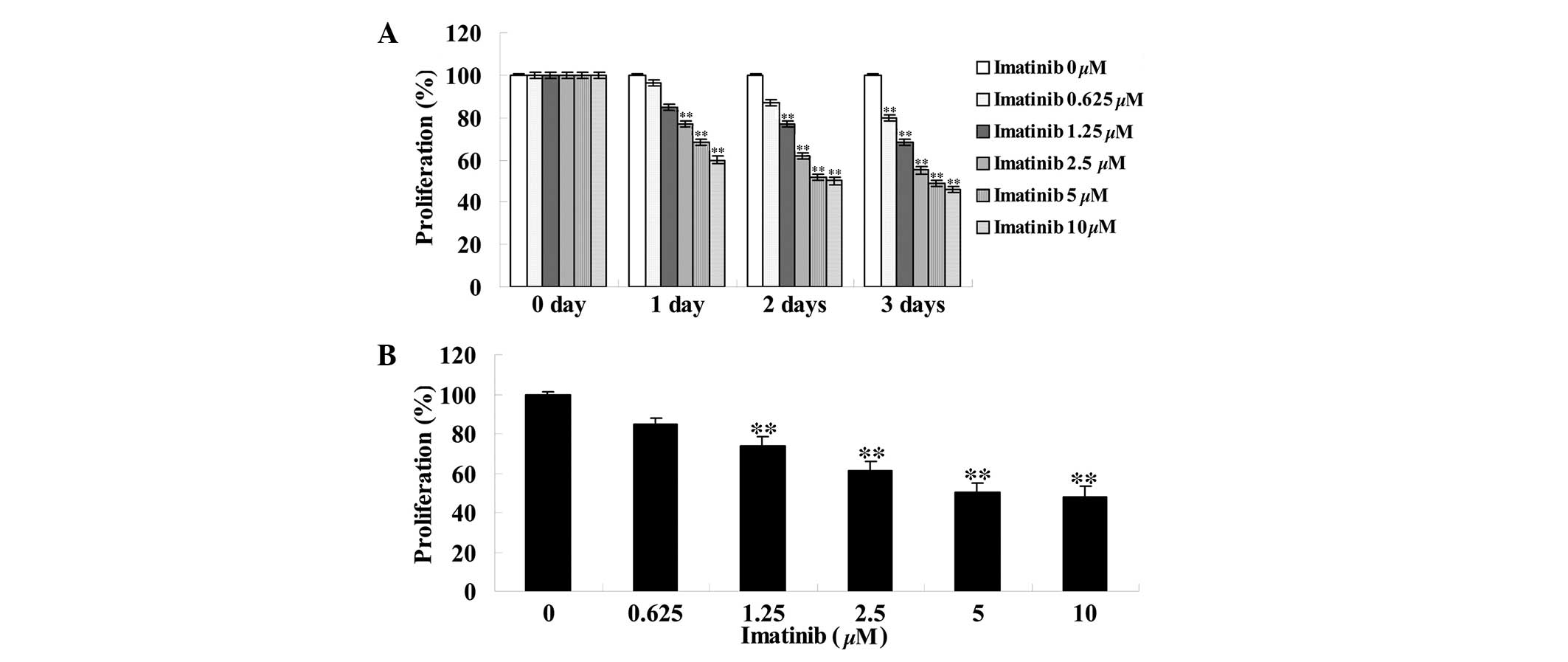
Imatinib inhibited cell viability of
GCT
To assess the effect of imatinib on cell viability,
different concentrations (0, 0.625, 1.25, 2.5, 5 and 10 µM)
of imatinib were incubated with GCT cells for 0, 1, 2 and 3 days
subsequent to which an MTT assay was performed. As indicated in
Fig. 2, imatinib inhibited the
viability of GCT cells in a dose- and time-dependent manner.
Compared with the control group, the viability of GCT cells
significantly decreased in a dose-dependent manner following two
days of treatment with 1.25, 2.5, 5 and 10 µM imatinib
(P<0.01; Fig. 2B).
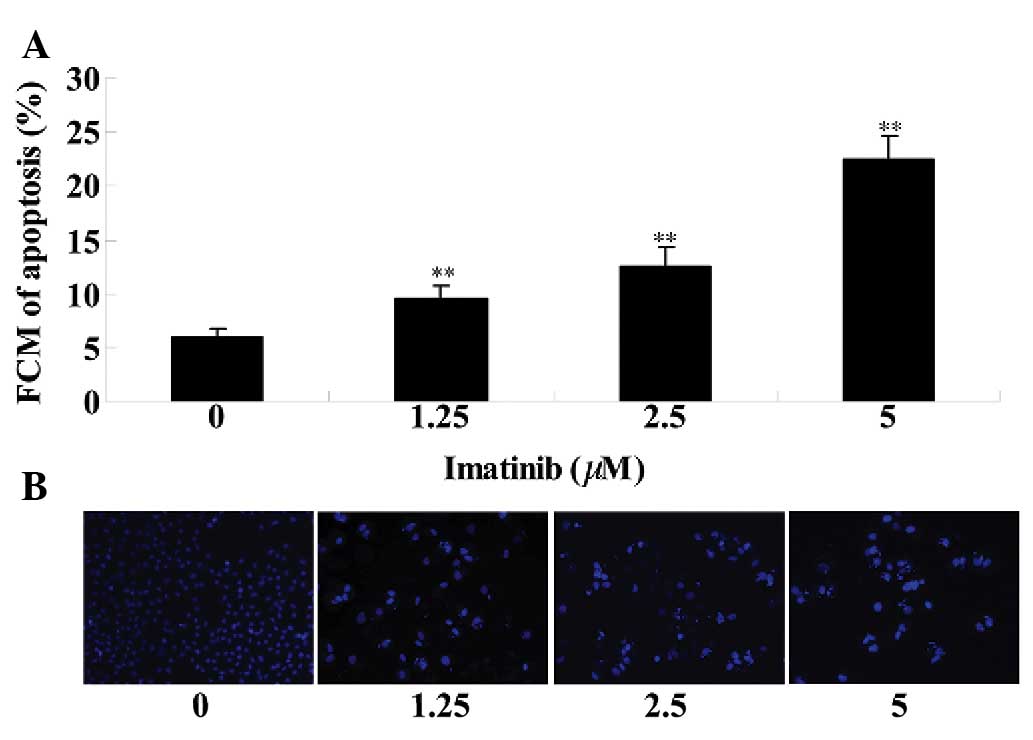
Imatinib induces cell apoptosis of
GCT
Flow cytometry and DAPI staining were used to
investigate the effects of imatinib on GCT apoptosis. As
demonstrated in Fig. 3A, imatinib
treatment (1.25, 2.5 or 5 µM) induced cell apoptosis of GCT
at 2 days, when compared with the control group. As presented in
Fig. 3B, the DAPI dye stained the
morphologically normal nuclei blue. Morphologic analysis of
apoptotic cells demonstrated shrunken and fragmented nuclei, as
well as condensed chromatin while the nuclei of normal cells were
round, sharp-edged and uniformly stained (Fig. 3B). GCT cell apoptosis was increased
in the imatinib-treated (1.25, 2.5 or 5 µM) group at 2 days
when compared with that of the control group (Fig. 3B).
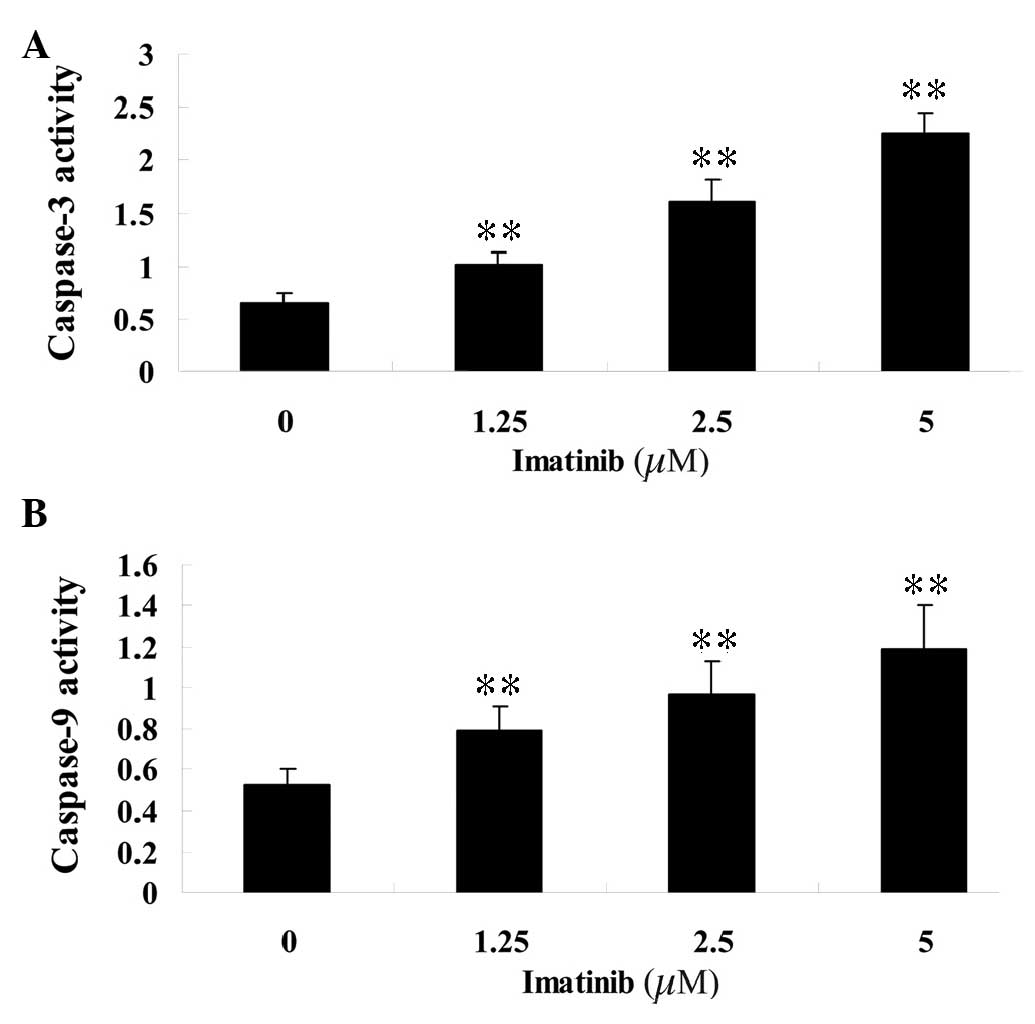
Imatinib induces caspase-3 and -9
activity of GCT cells
To determine the effect of imatinib on the activity
of caspase-3 and -9, varying concentrations (1.25, 2.5 and 5
µM) of imatinib were incubated with GCT cells for 2 days and
assayed with colorimetric kits. Following treatment, the activity
of caspase-3 and -9 in the GCT cells was significantly increased
compared with that of the control group (Fig. 4).
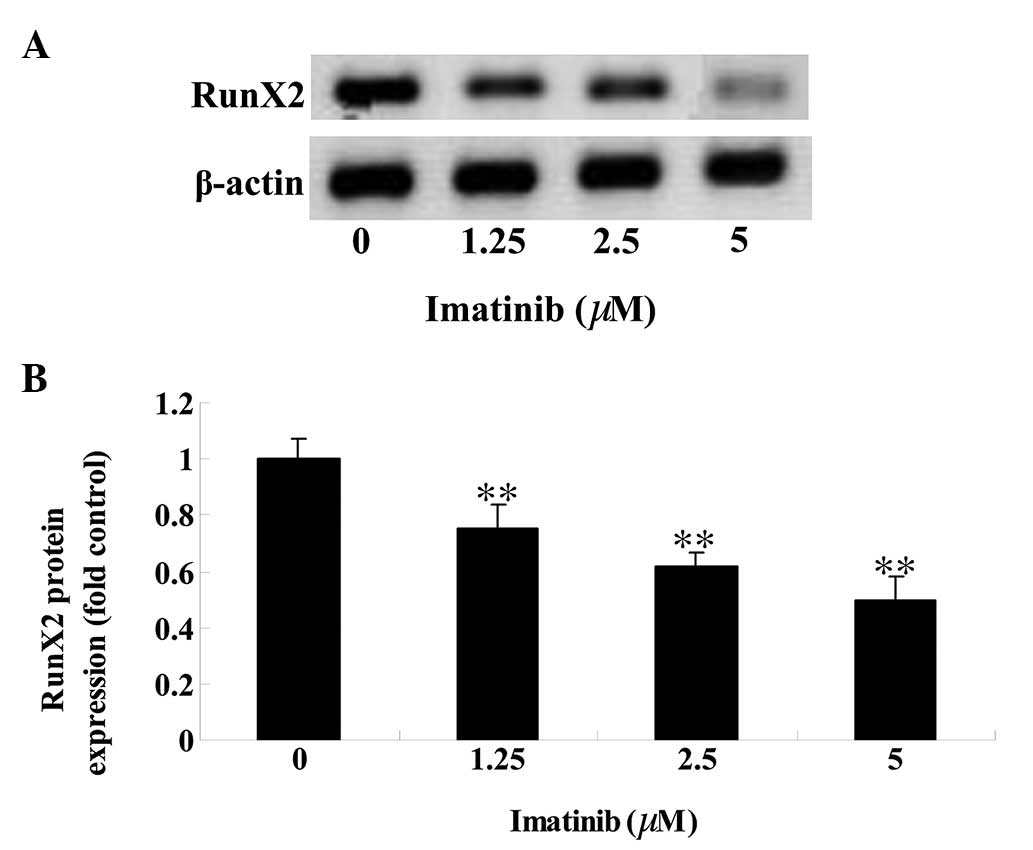
Imatinib downregulates RunX2 protein
expression in GCT
To determine the effect of imatinib on RunX2 protein
expression levels in GCT cells, western blot analysis was
performed. The RunX2 protein expression level in GCT cells was
reduced in a dose-dependent manner following 2 days of treatment
with 1.25, 2.5 and 5 µM imatinib (P<0.01; Fig. 5).
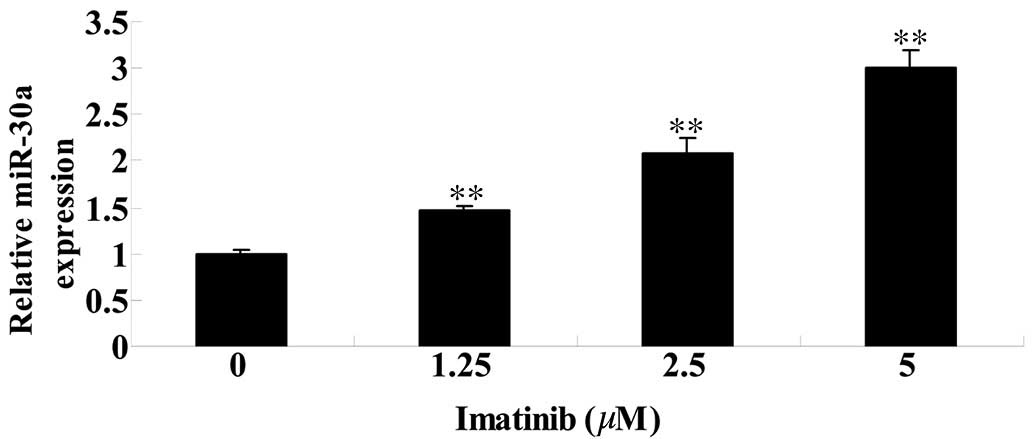
Imatinib activates miR-30a expression in
GCT cells
To further investigate the effect of imatinib on
miR-30a in GCT cels, the miR-30a expression level was analyzed
using qPCR. Compared with the control group, the level of miR-30a
expression was significantly increased following 2 days of imatinib
treatment at concentrations of 1.25, 2.5 and 5 µM
(P<0.01; Fig. 6).
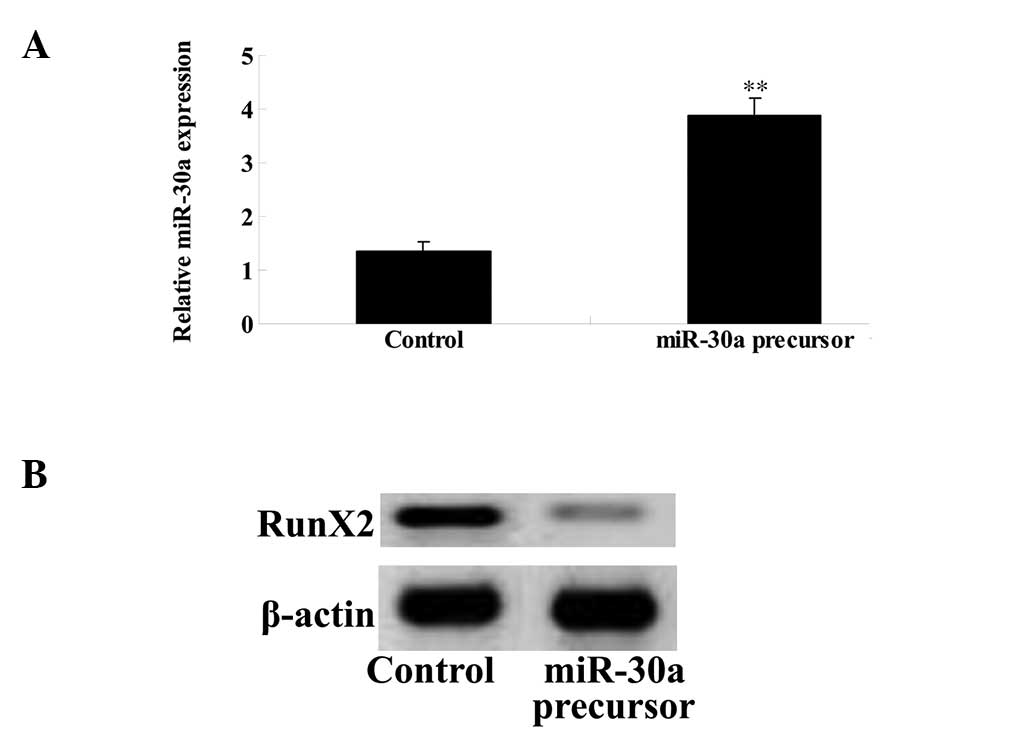
Overexpression of miR-30a inhibits RunX2
expression of GCT cells
To investigate the mechanism underlying the effect
of imatinib on GCT apoptosis, an miR-30a plasmid was transfected
into the GCT cells. The miR-30a plasmid markedly upregulated the
expression of miR-30a in the GCT cells (Fig. 7A), which reduced the level of RunX2
protein expression (Fig. 7B).
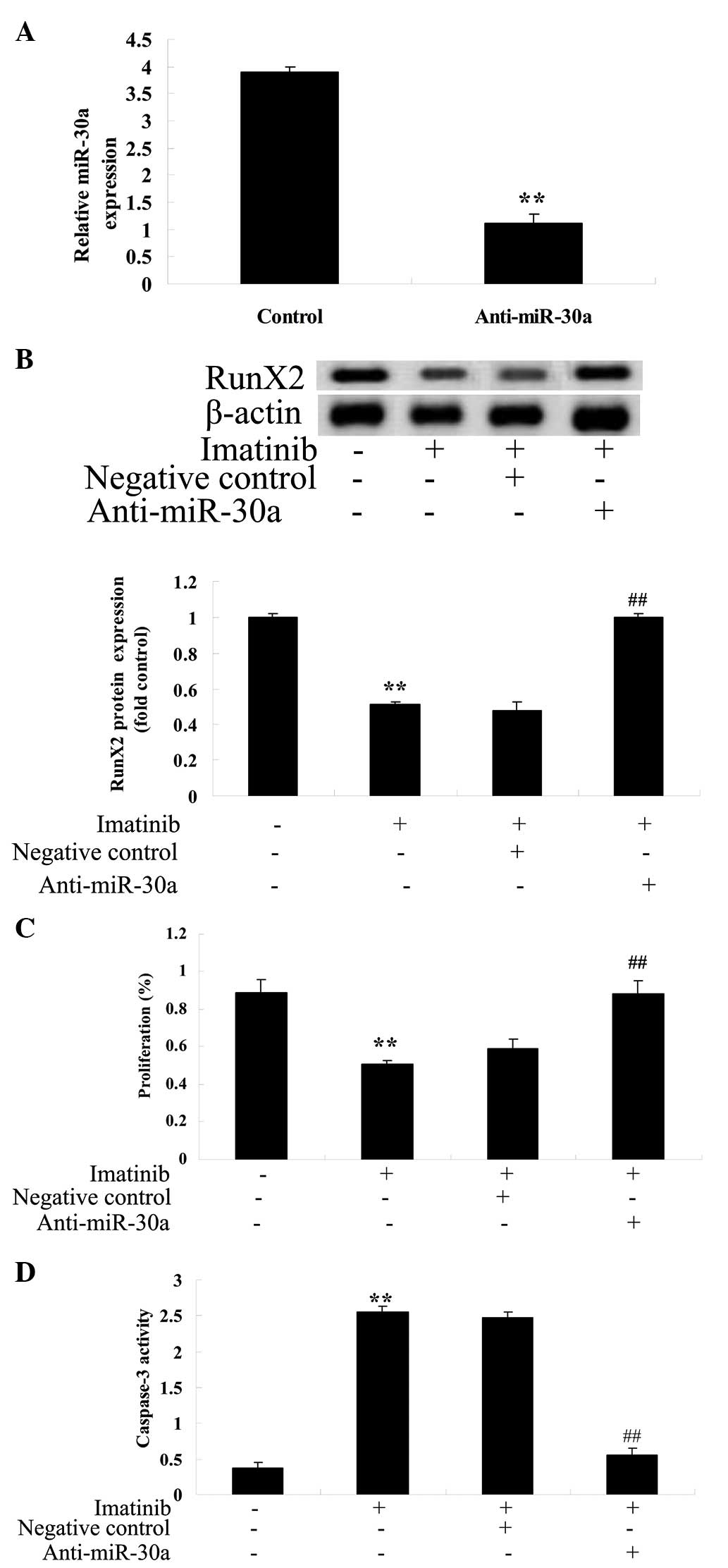
Anti-miR-30a reverses the effect of
imatinib on GCT cells
To further investigate the potential association
between miR-30a expression and the effect of imatinib on GCT cells,
analysis of the effect of imatinib on GCT was conducted following
transfection of an anti-miR-30a plasmid into GCT cells. The
anti-miR-30a plasmid reduced the expression of miR-30a (Fig. 8A) and increased RunX2 protein
expression (Fig. 8B). Furthermore,
the anti-miR-30a plasmid increased cell viability (Fig. 8C) and reduced caspase-3 activity in
GCT cells (Fig. 8D).
Discussion
GCT is a locally aggressive bone tumor, with
characteristic postoperative recurrence of ≤42% (17). GCT contains fibroblast-like,
spindle-shaped stromal cells, and multinucleated giant cells, which
exhibit resorption activity that is similar to osteoclasts
(18). The current study presented
evidence that imatinib inhibited cell viability and promoted cell
apoptosis in GCT in a dose-dependent manner. Maass et al
(19) reported that imatinib cured
patients with metastatic breast cancer, de Melo Campos et al
(20) suggested that imatinib
induced apoptosis of neoplastic mast cells and Halperin et
al (21) reported that
imatinib inhibited the viability of advanced endocrine cancer. In
the present study, exposure to imatinib significantly increased the
activity of caspase-3 and -9 in GCT cells. Zhang et al
(22) suggested that imatinib
increased the levels of cleaved caspase-3 and -9 in vitro
and in vivo, and Gamas et al (23) demonstrated that imatinib mediated
apoptosis of myelogenous leukemia cells by mediating caspase-3
activation.
ERK is an important signaling pathway for integrin
signaling to the nucleus (24).
Signaling molecules activate integrin, which promotes
RunX2-dependent transcription and, using U0126, rapidly inhibits
ERK phosphorylation and activation of the osteocalcin gene, upon
which ERK is dependent. In a previous study, ERK1-overexpressing
transfected cells were produced in which ERK1 elevated the level of
osteocalcin mRNA and significantly increased the phosphorylation of
RunX2, indicating that the phosphorylation cascade of
mitogen-activated protein kinase (MAPK) may regulate the activity
of RunX2 (25). Following
integration of the integrin ligand with the integrin on the surface
of osteoblasts, MAPK signaling pathways are activated to transfer
signals to the nucleus, promoting the expression of RunX2 and RunX2
protein phosphorylation (26,27).
Activated RunX2 promotes the differentiation of osteoblasts by
activating marker genes of osteoblast cells (including osteocalcin)
(28). In the present study, the
RunX2 protein level in GCT cells was significantly decreased in a
dose-dependent manner following two days of imatinib treatment.
Jönsson et al (29)
reported that imatinib inhibited the proliferation of human
mesenchymal stem cells via modulation of RunX2 expression levels
and Tibullo et al (30)
demonstrated that imatinib potentially favored osteoblastogenesis
by mRNA expression of RunX2.
miRs are closely associated with various types of
cancer. Previous studies have demonstrated that breast (31), ovarian (32), gastric (33) and lung (34) cancer all demonstrate abnormal
expression of miRs (35).
miRNA-30a is closely associated with the occurrence and development
of GCT, as well as other types of tumor (such as breast and renal
cell cancers) (36,37). The study of miRs is important for
early clinical diagnosis, treatment and prognosis of GCT. Specific
miR-30a expression profiling of GCT, and detection and monitoring
of miR-30a in the peripheral blood may serve as an effective marker
for GCT diagnosis, screening and to produce effective, novel
treatments (38). Data obtained in
the present study suggest that imatinib treatment significantly
increased the miR-30a expression in GCT cells. Yu et al
(39) demonstrated that the
administration of imatinib against human chronic myeloid leukemia
cells functioned by targeting miR-30a-mediated processes.
Furthermore, Yu et al (40)
reported that imatinib promoted autophagy in response to cancer
therapy via miR-30a-mediated processes. In the present study,
overexpression of miR-30a was identified to inhibit RunX2 protein
expression in GCT cells. Thus, downregulation of miR-30a may
reverse the anticancer effect of imatinib and increase the level of
RunX2 protein expression in GCT cells. Recent studies reported that
miR-30a inhibited osteolysis by targeting miR-30a-mediated
processes in GCTs of the bone (11,41).
In conclusion, the present study demonstrated that
the anticancer effect of imatinib increases apoptosis of GCT cells
by downregulating RunX2 protein expression and upregulating the
expression of miR-30a. These data indicate that there may be
potential benefits of administering imatinib in clinical practice
in the future.
References
|
1
|
Wang T, Hou Y, Ding X, Song B, Wang F and
Hou W: Overexpression, purification, molecular characterization and
pharmacological evaluation for anticancer activity of ribosomal
protein S23 from the giant panda (Ailuropoda melanoleuca). Mol Med
Rep. 7:1875–1882. 2013.PubMed/NCBI
|
|
2
|
Yang T, Zheng XF, Li M, Lin X and Yin QS:
Stimulation of osteogenic differentiation in stromal cells of giant
cell tumour of bone by zoledronic acid. Asian Pac J Cancer Prev.
14:5379–5383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang N, Qin CH, Tan CX, Wen SF, Ma YF,
Dong F, Diao XC, Zhang P and Yu B: A retrospective analysis of 140
patients with giant cell tumor in the extremity: a multicenter
study based on four hospitals in South China. Cancer Epidemiol.
37:294–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Komori T: Mechanism of transcriptional
regulation by Runx2 in osteoblasts. Clin Calcium. 16:801–807.
2006.In Japanese. PubMed/NCBI
|
|
5
|
Phimphilai M, Zhao Z, Boules H, Roca H and
Franceschi RT: BMP signaling is required for RUNX2-dependent
induction of the osteoblast phenotype. J Bone Miner Res.
21:637–646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mak IW, Cowan RW, Popovic S, Colterjohn N,
Singh G and Ghert M: Upregulation of MMP-13 via Runx2 in the
stromal cell of Giant Cell Tumor of bone. Bone. 45:377–386. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh S, Singh M, Mak IW, Turcotte R and
Ghert M: Investigation of FGFR2-IIIC signaling via FGF-2 ligand for
advancing GCT stromal cell differentiation. PLoS One. 7:e467692012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumarswamy R, Mudduluru G, Ceppi P,
Muppala S, Kozlowski M, Niklinski J, Papotti M and Allgayer H:
MicroRNA-30a inhibits epithelial-to-mesenchymal transition by
targeting Snai1 and is downregulated in non-small cell lung cancer.
Int J Cancer. 130:2044–2053. 2012. View Article : Google Scholar
|
|
9
|
Liu J, Shi W, Wu C, Ju J and Jiang J:
miR-181b as a key regulator of the oncogenic process and its
clinical implications in cancer (Review). Biomed Rep. 2:7–11.
2014.PubMed/NCBI
|
|
10
|
Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen
X, Chen X, Zhang CY, Zhang Q and Zen K: MicroRNA-30a sensitizes
tumor cells to cis-platinum via suppressing beclin 1-mediated
autophagy. J Biol Chem. 287:4148–4156. 2012. View Article : Google Scholar :
|
|
11
|
Huang Q, Jiang Z, Meng T, Yin H, Wang J,
Wan W, Cheng M, Yan W, Liu T, Song D, et al: MiR-30a inhibits
osteolysis by targeting RunX2 in giant cell tumor of bone. Biochem
Biophys Res Commun. 453:160–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roth DB, Akbari S and Rothstein A: Macular
ischemia associated with imatinib mesylate therapy for chronic
myeloid leukemia. Retin Cases Brief Rep. 3:161–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weisberg E, Deng X, Choi HG, Barrett R,
Adamia S, Ray A, Moreno D, Kung AL, Gray N and Griffin JD:
Beneficial effects of combining a type II ATP competitive inhibitor
with an allosteric competitive inhibitor of BCR-ABL for the
treatment of imatinib-sensitive and imatinib-resistant CML.
Leukemia. 24:1375–1378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dohse M, Scharenberg C, Shukla S, et al:
Comparison of ATP-binding cassette transporter interactions with
the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib.
Drug Metab Dispos. 38:1371–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sarszegi Z1, Bognar E, Gaszner B, Kónyi A,
Gallyas F Jr, Sumegi B and Berente Z: BGP-15, a PARP-inhibitor,
prevents imatinib-induced cardiotoxicity by activating Akt and
suppressing JNK and p38 MAP kinases. Mol Cell Biochem. 365:129–137.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grunwald L, Mehta S, Hogarty MD and Liu
GT: Chronic myelogenous leukemia and retinopathy treated with
imatinib. Retin Cases Brief Rep. 5:366–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park YS, Lee JK, Baek SW and Park CK: The
rare case of giant cell tumor occuring in the axial skeleton after
15 years of follow-up: Case report. Oncol Lett. 2:1323–1326.
2011.
|
|
18
|
Fu S, Bai R, Zhao Z, Zhang Z, Zhang G,
Wang Y, Wang Y, Jiang D and Zhu D: Overexpression of
hypoxia-inducible factor-1α and vascular endothelial growth factor
in sacral giant cell tumors and the correlation with tumor
microvessel density. Exp Ther Med. 8:1453–1458. 2014.PubMed/NCBI
|
|
19
|
Maass N, Schem C, Bauerschlag DO, Tiemann
K, Schaefer FW, Hanson S, Muth M, Baier M, Weigel MT, Wenners AS,
et al: Final safety and efficacy analysis of a phase I/II trial
with imatinib and vinorelbine for patients with metastatic breast
cancer. Oncology. 87:300–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Melo Campos P, Machado-Neto JA,
Scopim-Ribeiro R, Visconte V, Tabarroki A, Duarte AS, Barra FF,
Vassalo J, Rogers HJ, Lorand-Metze I, et al: Familial systemic
mastocytosis with germline KIT K509I mutation is sensitive to
treatment with imatinib, dasatinib and PKC412. Leuk Res.
38:1245–1251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halperin DM, Phan AT, Hoff AO, Aaron M,
Yao JC and Hoff PM: A phase I study of imatinib, dacarbazine, and
capecitabine in advanced endocrine cancers. BMC Cancer. 14:5612014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YF, Xu GB, Gan YC, Xu XH and Xu RZ:
Inhibitory effect of 4-chlorobenzoyl berbamine on
imatinib-resistant K562 cells in vitro and in vivo. Nan Fang Yi Ke
Da Xue Xue Bao. 31:1997–2001. 2011.In Chinese. PubMed/NCBI
|
|
23
|
Gamas P, Marchetti S, Puissant A, Grosso
S, Jacquel A, Colosetti P, Pasquet JM, Mahon FX, Cassuto JP and
Auberger P: Inhibition of imatinib-mediated apoptosis by the
caspase-cleaved form of the tyrosine kinase Lyn in chronic
myelogenous leukemia cells. Leukemia. 23:1500–1506. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tatematsu T, Sasaki H, Shimizu S, Okuda K,
Shitara M, Hikosaka Y, Moriyama S, Yano M, Brown J and Fujii Y:
Investigation of neurotrophic tyrosine kinase receptor 1 fusions
and neurotrophic tyrosine kinase receptor family expression in
non-small-cell lung cancer and sensitivity to AZD7451. Mol Clin
Oncol. 2:725–730. 2014.PubMed/NCBI
|
|
25
|
Xiao ZS, Hjelmeland AB and Quarles LD:
Selective deficiency of the bone-related Runx2-II unexpectedly
preserves osteoblast-mediated skeletogenesis. J Biol Chem.
279:20307–20313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu N, Feng C, Jiang Y, Miao Q and Liu H:
Regulative effect of mir-205 on osteogenic differentiation of bone
mesenchymal stem cells (BMSCs): Possible role of SATB2/Runx2 and
ERK/MAPK pathway. Int J Mol Sci. 16:10491–10506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang RL, Yuan Y, Tu J, Zou GM and Li Q:
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways
converge on Runx2 to regulate BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 5:e11872014. View Article : Google Scholar
|
|
28
|
Glynn ER, Londono AS, Zinn SA, Hoagland TA
and Govoni KE: Culture conditions for equine bone marrow
mesenchymal stem cells and expression of key transcription factors
during their differentiation into osteoblasts. J Anim Sci
Biotechnol. 4:402013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jönsson S, Hjorth-Hansen H, Olsson B,
Wadenvik H, Sundan A and Standal T: Imatinib inhibits proliferation
of human mesenchymal stem cells and promotes early but not late
osteoblast differentiation in vitro. J Bone Miner Metab.
30:119–123. 2012. View Article : Google Scholar
|
|
30
|
Tibullo D, Giallongo C, La Cava P, Beretta
S, Stagno F, Chiarenza A, Conticello C, Palumbo GA and Di Raimondo
F: Effects of imatinib mesylate in osteoblastogenesis. Exp Hematol.
37:461–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heneghan HM, Miller N, Lowery AJ, Sweeney
KJ, Newell J and Kerin MJ: Circulating microRNAs as novel minimally
invasive biomarkers for breast cancer. Ann Surg. 251:499–505. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Corney DC, Hwang CI, Matoso A, Vogt M,
Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH,
Hermeking H and Nikitin AY: Frequent downregulation of miR-34
family in human ovarian cancers. Clin Cancer Res. 16:1119–1128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsukamoto Y, Nakada C, Noguchi T, Tanigawa
M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M and
Moriyama M: MicroRNA-375 is downregulated in gastric carcinomas and
regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer
Res. 70:2339–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chacon-Cabrera A, Fermoselle C, Salmela I,
Yelamos J and Barreiro E: MicroRNA expression and protein
acetylation pattern in respiratory and limb muscles of
Parp-1−/− and Parp-2−/− mice with lung cancer
cachexia. Biochim Biophys Acta. 1850:2530–2543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kodahl AR, Lyng MB, Binder H, Cold S,
Gravgaard K, Knoop AS and Ditzel HJ: Novel circulating microRNA
signature as a potential non-invasive multi-marker test in
ER-positive early-stage breast cancer: A case control study. Mol
Oncol. 8:874–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng RC, Zhang W, Yan XQ, Ye ZQ, Chen ED,
Huang DP, Zhang XH and Huang GL: Down-regulation of miRNA-30a in
human plasma is a novel marker for breast cancer. Med Oncol.
30:4772013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng B, Zhu H, Gu D, Pan X2 Qian L, Xue
B, Yang D, Zhou J and Shan Y: MiRNA-30a-mediated autophagy
inhibition sensitizes renal cell carcinoma cells to sorafenib.
Biochem Biophys Res Commun. 459:234–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mao C, Zhang J, Lin S, Jing L, Xiang J,
Wang M, Wang B, Xu P, Liu W, Song X and Changjun L: MiRNA-30a
inhibits AECs-II apoptosis by blocking mitochondrial fission
dependent on Drp-1. J Cell Mol Med. 18:2404–2416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu Y, Yang L, Zhao M, Zhu S, Kang R,
Vernon P, Tang D and Cao L: Targeting microRNA-30a-mediated
autophagy enhances imatinib activity against human chronic myeloid
leukemia cells. Leukemia. 26:1752–1760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu Y, Cao L, Yang L, Kang R, Lotze M and
Tang D: microRNA 30A promotes autophagy in response to cancer
therapy. Autophagy. 8:853–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eguchi T, Watanabe K, Hara ES, Ono M,
Kuboki T and Calderwood SK: OstemiR: A novel panel of microRNA
biomarkers in osteoblastic and osteocytic differentiation from
mesencymal stem cells. PLoS One. 8:e587962013. View Article : Google Scholar : PubMed/NCBI
|