Introduction
Ischemic heart disease (IHD) has gradually become
one of the major threats to quality of life (1). As a leading contributor to mortality
rates in cardiovascular disease (CVD), IHD accounts for 42% of the
mortality rates in patients with CVD (2,3).
Although the use of timely and appropriate reperfusion strategies,
including thrombolytic therapy, percutaneous coronary intervention
and coronary artery bypass grafting, have proved efficient and
beneficial, reperfusion itself causes further injury to the
ischemic cardiomyocytes, resulting in the induction of apoptosis or
even necrosis (4). Mitochondrial
dysfunction has been found to be critical, in that
ischemia-mediated damage to the mitochondrial electron transport
chain persists during reperfusion (5). This mitochondrial dysfunction not
only decreases energy production, but also increases the generation
of intracellular reactive oxygen species and activates the
executioner caspase cleavage pathway to trigger cell death during
reperfusion (6). The injured
myocardium undergoes an orchestrated remolding process, which is
associated with adverse clinical outcomes (7). Reducing the deleterious impact of
ischemia/reperfusion (I/R) injury is, therefore, important in the
treatment of IHD.
Mesenchymal stem cells (MSCs) expressing cluster of
differentiation (CD)105, CD73 and CD90, and rarely CD45, CD34 and
human leukocyte antigen-DR, can be isolated from bone marrow,
adipose tissue and the umbilical cord blood (8,9). Due
to their numerous advantages, including abundant sources, high
levels of activity, immune privilege, high proliferation and
differentiation potential and nutrient secretion, the use of MSCs
in cell therapies are considered among the most promising
approaches to repair damaged cardiac tissue in IHD, including
myocardial infarction and heart failure (10–12).
MSCs, as multipotent non-hematopoietic stem cells, typically
originating from the bone marrow, have been widely used in general
transplantation investigations. However, the overall effect of
regenerative stem cell therapy in the improvement of human heart
function is not considerable (13,14).
A number of animal studies have produced results demonstrating
significant cardioprotective effects. However, following prudent
review, the precise effects that MSCs have on myocardiocytes, and
the underlying mechanism remain controversial. Several studies
indicated that MSCs delivered to the heart successfully
differentiated into cardiomyocytes (15) and fibroblasts (16), which are the predominant cellular
constituents of the heart, whereas others demonstrated that they
did not (16) and suggested that
their therapeutic effects were paracrine in nature (17). Spees et al reported that
mitochondria can be actively transferred from stem cells to
recipient cells with nonfunctional mitochondria, resulting in a
significant amelioration of aerobic respiration (18). In vivo animal investigations
are inherently limited, due to the complication of whether the
observed therapeutic effect results from in situ cells being
'nourishedʼ by MSCs, or whether it is an artifact of MSCs, which
exhibit high activity and differentiation potential.
In the present study, the role of bone
marrow-derived MSCs (BM-MSCs) in the survival of H9c2
cardiomyocytes in a simulated I/R (SI/R) model was investigated. A
co-culture system was established to simulate the direct
cell-to-cell interactions. Consistently, the anti-apoptotic effects
and mitochondrial transfer by transient tunneling nanotube
(TNT)-like connections between cells were directly investigated
in vitro, through which the exact changes induced by BM-MSCs
in the H9c2 cells, and the possible mechanisms of direct
intercellular communication, were measured.
Materials and methods
Cell isolation and culture
MSCs were isolated from green fluorescent protein
(GFP)-expressing Sprague-Dawley rats (age, 3 months; weight,
250–300 g), which were obtained from the Second Military Medical
University Laboratory Animal Center (Shanghai, China). The rats
were housed in a specific pathogen-free facility (21±2°C and 50±15%
humidity) under a 12 h light/dark cycle with free access to food
and water. The present study was approved by the Ethics Committee
of the Second Military Medical University, Shanghai, China (no.
13071002114). Primary BM-MSCs were isolated and cultured, as
described previously (19), at
37°C with 5% CO2. Cells were harvested when they reached
80–90% confluence. The harvested cells were centrifuged at 500 × g
for 15 min at 4°C and were subsequently resuspended in Dulbecco's
modified Eagle's medium (DMEM)/F-12 culture medium containing 10%
fetal bovine serum (FBS), 100 µg/ml streptomycin and 100
U/ml penicillin (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Cells at the third passage were subjected to flow
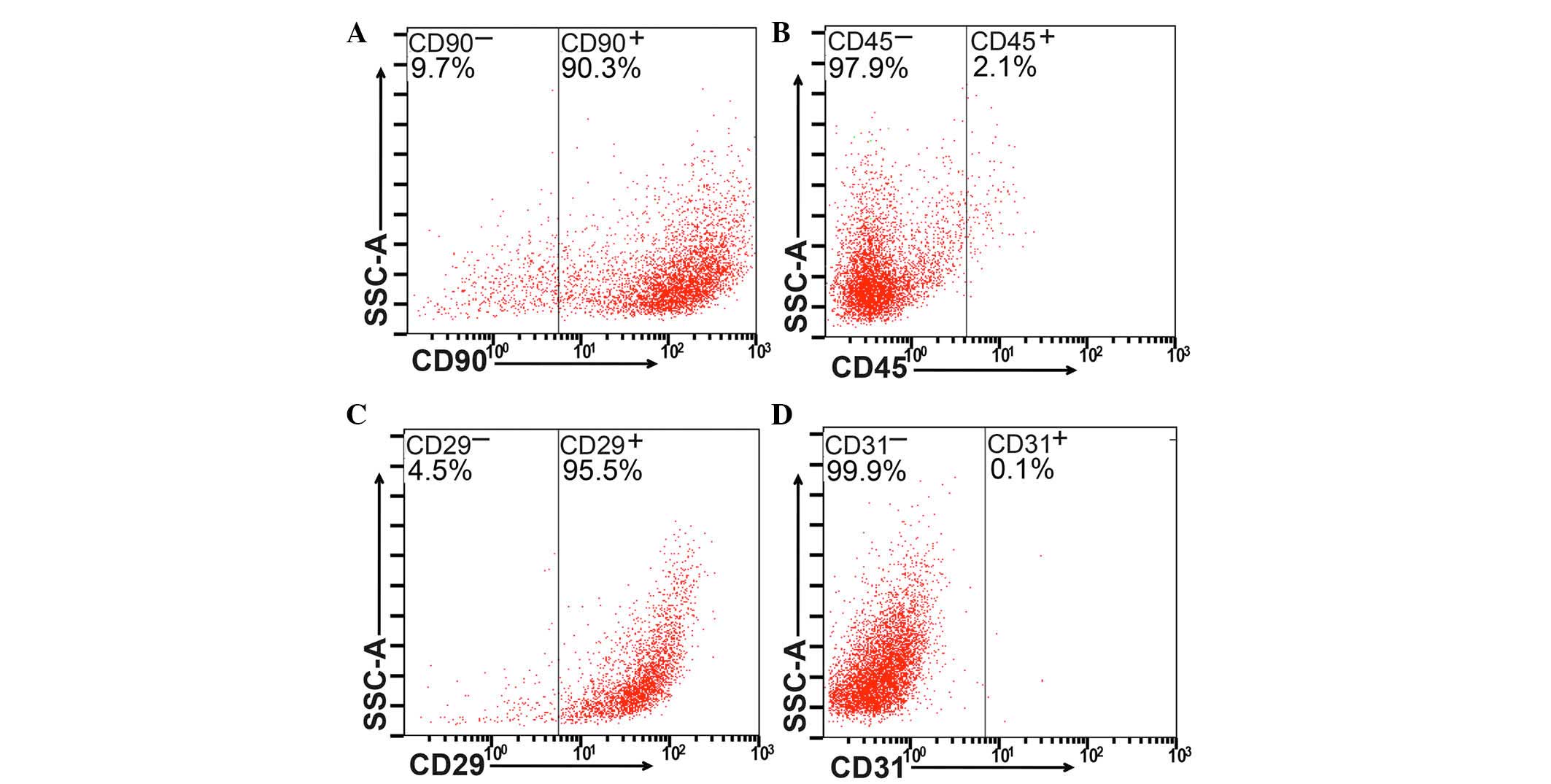
cytometric analysis to examine the expression levels of the CD29,
CD90, CD31 and CD45 surface markers (Fig. 1A–D).
Cells of the H9c2 rat ventricular cell line were
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were maintained in DMEM/F-12 culture medium
containing 10% FBS, 100 µg/ml streptomycin and 100 U/ml
penicillin (Gibco; Thermo Fisher Scientific, Inc.) in a
water-saturated atmosphere of 5% CO2 and 95% air at
37°C.
Establishment of a co-culture system and
cell treatment
To construct the co-culture system, the appropriate
density of H9c2 cells were plated into 6-well culture plates at a
density of 1×106 cells/well, and then divided into
different groups based on the specific treatments performed. An
in vitro model of simulated ischemia/reperfusion (SI/R) was
used, as previously described, with several modifications (20). Briefly, the medium was replaced by
serum- and glucose-deficient DMEM (21), and the cells were placed into a
hypoxic chamber (95% N2; 5% CO2) at 37°C for
12 h. The cell were then reoxygenated at 37°C for 6 h with DMEM
containing 10% FBS. The cells control group were treated with
complete medium throughout the experiment. The cells in the SI/R
group were subjected to SI/R, as described above. In the co-culture
group, BM-MSCs (1:1) were directly seeded into the co-culture
system during reoxygenation. In the remaining group, the
co-cultured BM-MSCs were pretreated with latrunculin-A (LatA; 10
nM; Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 4 h (pre-LatA
group).
Detection of apoptotic rates using flow
cytometry
The H9c2 cells were isolated from the co-cultures
using a MoFlo XDP high-speed flow cytometry sorter (Beckman Coulter
Brea, CA, USA). Cell apoptosis was measured using Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) with
an apoptosis detection kit (BD Pharmingen, San Diego, CA, USA),
according to the manufacturer's protocol. The samples were analyzed
on a fluorescence activated cell sorter (Cytomic FC500; Beckman
Coulter) within 1 h. The numbers of apoptotic cells, including
Annexin V-positive/PI-negative and double-positive cells, were
counted and expressed as a percentage of the total cell count.
Western blot analysis
H9c2 cells were washed twice with PBS, and lysed
using ProteoJET Mammalian Cell Lysis Reagent (Thermo Fisher
Scientific, Inc.) to extract cytoplasmic proteins. Protein
concentrations were determined using the bicinchoninic acid
(Beyotime Institute of Biotechnology, Haimen, China). Following
denaturation with loading buffer, 60 µg protein extracts
were subjected to 8% SDS-PAGE and were electrophoretically blotted
onto nitrocellulose membranes (Novex, San Diego, CA, USA). The
membranes were blocked with 5% non-fat milk in Tris-buffered saline
with Tween-20 (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and
were subsequently probed overnight at 4°C with rabbit polyclonal
anti-rat antibodies against B cell lymphoma-2 (Bcl-2; 1:1,000;
polyclonal; cat. no. 2876), Bcl-2-associated X protein (Bax;
1:1,000; polyclonal; cat. no. 2772) and GADPH (1:5,000; monoclonal;
cat. no. 5174). The membranes were then incubated with the
respective horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibodies (1:5,000; cat. no. 7074) at room temperature
for 1 h. Immunoreactive bands were detected using an enhanced
chemiluminescence system (EMD Millipore, Billerica, MA, USA) and
quantified using Image-Pro Plus 6.0 software(Media Cybernetics,
Inc., Rockville, MD, USA). All antibodies for western blotting were
purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Caspase-3 activity assay
Caspase-3 activity was determined using a Caspase-3
Activity kit (Beyotime Institute of Biotechnology), which is based
on the caspase-3-mediated conversion of acetyl-Asp-Glu-Val-Asp
p-nitroanilide into the yellow formazan product, p-nitroaniline.
The assay was performed according to the manufacturer's protocol.
Caspase-3 activity was quantified on a microplate spectrophotometer
(PowerWave HT Microplate spectrophotometer; Biotek Instruments,
Inc., Winooski, VT, USA) at 405 nm. Caspase-3 activity was
expressed as the fold-change in enzyme activity, compared with
synchronized cells.
Measurement of mitochondrial membrane
potential (Δψm)
Following the specific treatments, the Δψm of the
H9c2 cells was assessed using a lipophilic cationic probe,
5,5′,6,6′-tetra-chloro-1,1′,3,3′-tetraethylbenzimidazole-carbocyanide
iodine (JC-1; 5 µmol/l; Invitrogen; Thermo Fisher
Scientific, Inc.), which exhibits potential-dependent accumulation
in mitochondria. Briefly, the H9c2 cells (1×106
cells/ml) were incubated with JC-1 for 30 min at 37°C. The
fluorescence was detected using a microplate reader (Tecan Infinite
200; Tecan, Männedorf, Switzerland). The wavelengths of excitation
and emission were 514 and 529 nm to detect the monomeric form of
JC-1, and 585 and 590 nm to detect the aggregation of JC-1. The
ratio of mitochondrial JC-1 aggregates and monomers was considered
representative of the Δψm of H9c2 cells.
Staining of cells and confocal
microscopy
To trace the intercellular exchange of mitochondria,
GFP BM-MSCs were labeled with MitoTracker Deep Red (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Briefly, the cells (1×106 cells/ml) were
resuspended in pre-warmed (37°C) staining solution containing the
MitoTracker® probe (50 nM) for 30 min. Following
staining, the cells were washed three times in PBS, and resuspended
in fresh pre-warmed medium.
In the group of H9c2 cells subjected to 12 h
ischemia, labeled GFP BM-MSCs were directly seeded into the culture
system on glass slides. Following 6 h of mixture under conditions
of reoxygenation, the glass slides containing the two types of cell
were labeled with DAPI (10 µg/m; Beyotime Institute of
Biotechnology) for 5 min. Images were captured by confocal
microscopy (Leica Microsystems GmbH, Wetzlar, German) within 1 h,
and analyzed using Leica LAS AF Lite software (Leica Microsystems
GmbH).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using a paired or unpaired t-test, unless
otherwise stated. Differences between groups were analyzed using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference. Data were analyzed with the
use of GraphPad Prism 5 software (GraphPad Software Inc., San
Diego, CA, USA).
Results
Identification of isolated BM-MSCs
Flow cytometric analysis was used to detect the
expression of CD90, CD45, CD29 and CD31 (Fig. 1A–D, respectively). BM-MSCs
(>90%) were positive for CD90 or CD29, whereas <3% were
positive for CD31 or CD45.
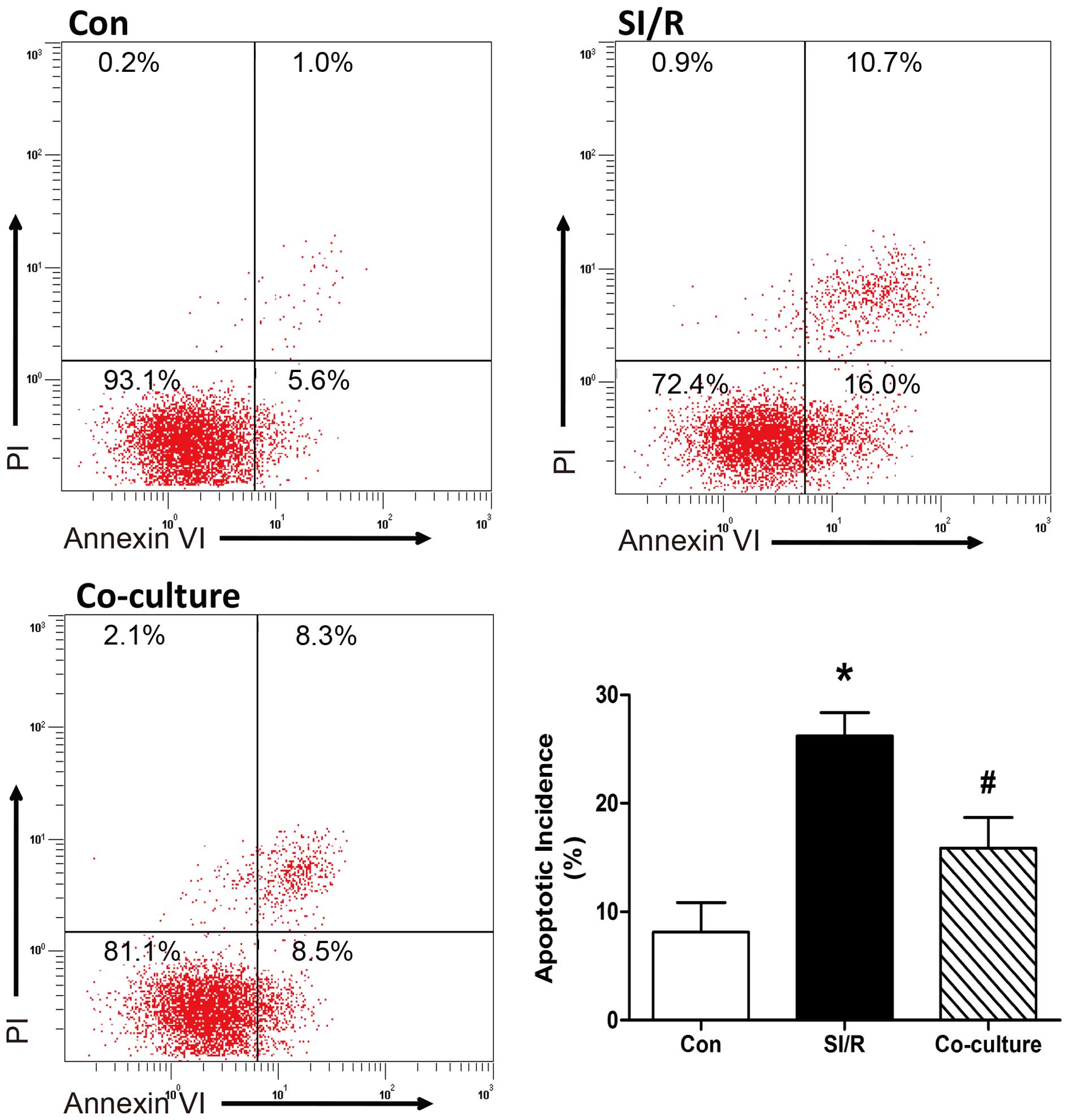
Co-culture with BM-MSCs decreases the
apoptosis of SI/R-stimulated H9c2 cells
The effects of the co-culture with BM-MSCs on
SI/R-stimulated phosphatidylserine exposure in H9c2 cells were
analyzed using flow cytometric analysis of annexin V-FITC/PI
staining. As shown in Fig. 2,
quantitative analysis indicated that the apoptotic rate was
significantly elevated in the H9c2 cells stimulated with SI/R,
compared with the cells in the control group; and direct
co-culturing of H9c2 cells with BM-MSCs decreased SI/R-induced
apoptosis (Con, 8.10±2.77%; SI/R, 26.23±2.14%; Co-culture,
15.87±2.81%).
During the apoptotic process, the Bcl-2 protein
family, consisting of death agonists, including Bax, and death
antagonists, including Bcl-2, has emerged as a key regulator
(22,23). As shown in Fig. 3A, western blots from the present
study revealed a significant upregulation in the expression of Bax
and downregulation in the expression of Bcl-2 following SI/R. This
imbalance of Bax/Bcl-2 was partially reversed by co-culture of the
H9c2 cells with BM-MSCs.
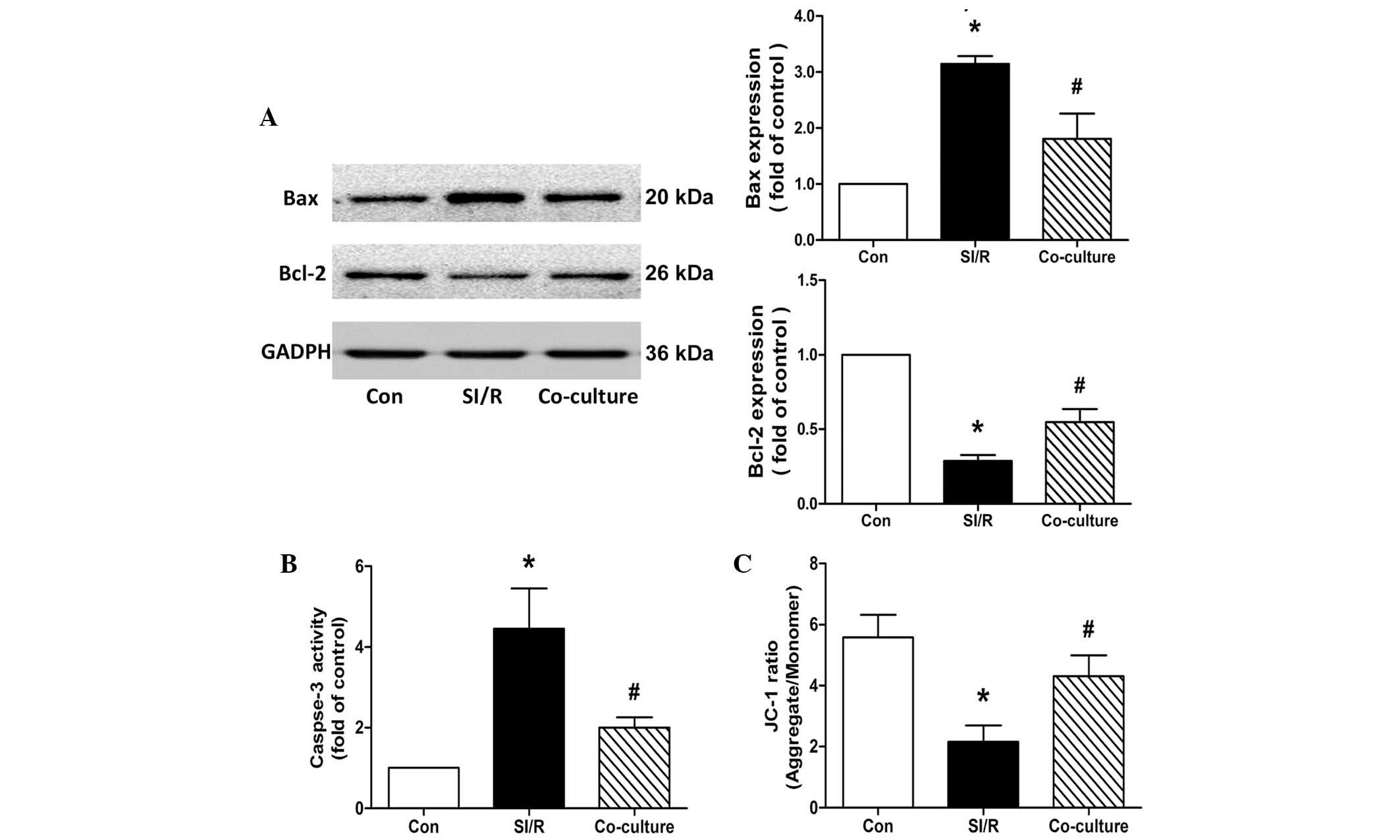 | Figure 3Effects of co-culture with BM-MSCs on
apoptosis and mitochondrial membrane potential in the control
(Con), SI/R and SI/R co-cultured with BM-MSCs (Co-culture) groups.
(A) Representative western blots showing the changes in the
expression Bax and Bcl-2 in H9c2 cells from the Con, SI/R and
Co-culture groups. (B) Changes in caspase-3 activity in H9c2 cells
from the Con, SI/R and Co-culture groups. (C) Changes in
mitochondrial membrane potential in H9c2 cells from the Con, SI/R
and Co-culture groups. Data are presented as the mean ± standard
deviation (*P<0.05, vs. Con; #P<0.05,
vs. SI/R). BM-MSCs, bone marrow-derived mesenchymal stem cells;
SI/R, simulated ischemia/reperfusion; Bcl-2, B cell lymphoma-2;
Bax, Bcl-2-associated X protein; JC-1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole-carbocyanide
iodine. |
Caspase-3 serves as an executioner of apoptosis, and
its activation is an early marker of apoptosis in H9c2 cells
(24). Compared with the control,
the data in the present study showed that co-culture with BM-MSCs
reduced the SI/R-induced elevation of caspase-3 in the H9c2 cells
(SI/R, 4.45±1.00-fold; Co-culture, 2.01±0.25-fold; Fig. 3B).
Co-culture with BM-MSCs increases Δψm in
SI/R-stimulated H9c2 cells
The mitochondrial function of H9c2 cells was
detected using the lipophilic and cationic JC-1 dye, with the
results expressed as the ratio between red (aggregated JC-1) and
green (monometric JC-1) fluorescence. Quantitative analysis showed
that Δψm decreased significantly during treatment with SI/R in the
H9c2 cells, compared with the control. Following co-culture with
the BM-MSCs, the SI/R-induced H9c2 cells exhibited a significantly
higher Δψm, compared with the cells exposed to SI/R treatment alone
(Con, 5.58±0.74; SI/R, 2.16±0.53; Co-culture, 4.30±0.68; Fig. 3C).
Transfer of mitochondria in direct
co-culture of H9c2 cells with BM-MSCs
As shown in Fig.
4A–D, TNT-like structures in (indicated by arrows) were
observed between the BM-MSCs and the H9c2 cells, on examination
using confocal microscopy. The mitochondria, visualized in the red
fluorescence channel, were found to transfer from the relabeled GFP
BM-MSCs to the SI/R-stimulated H9c2 cells by the TNT-like
structures.
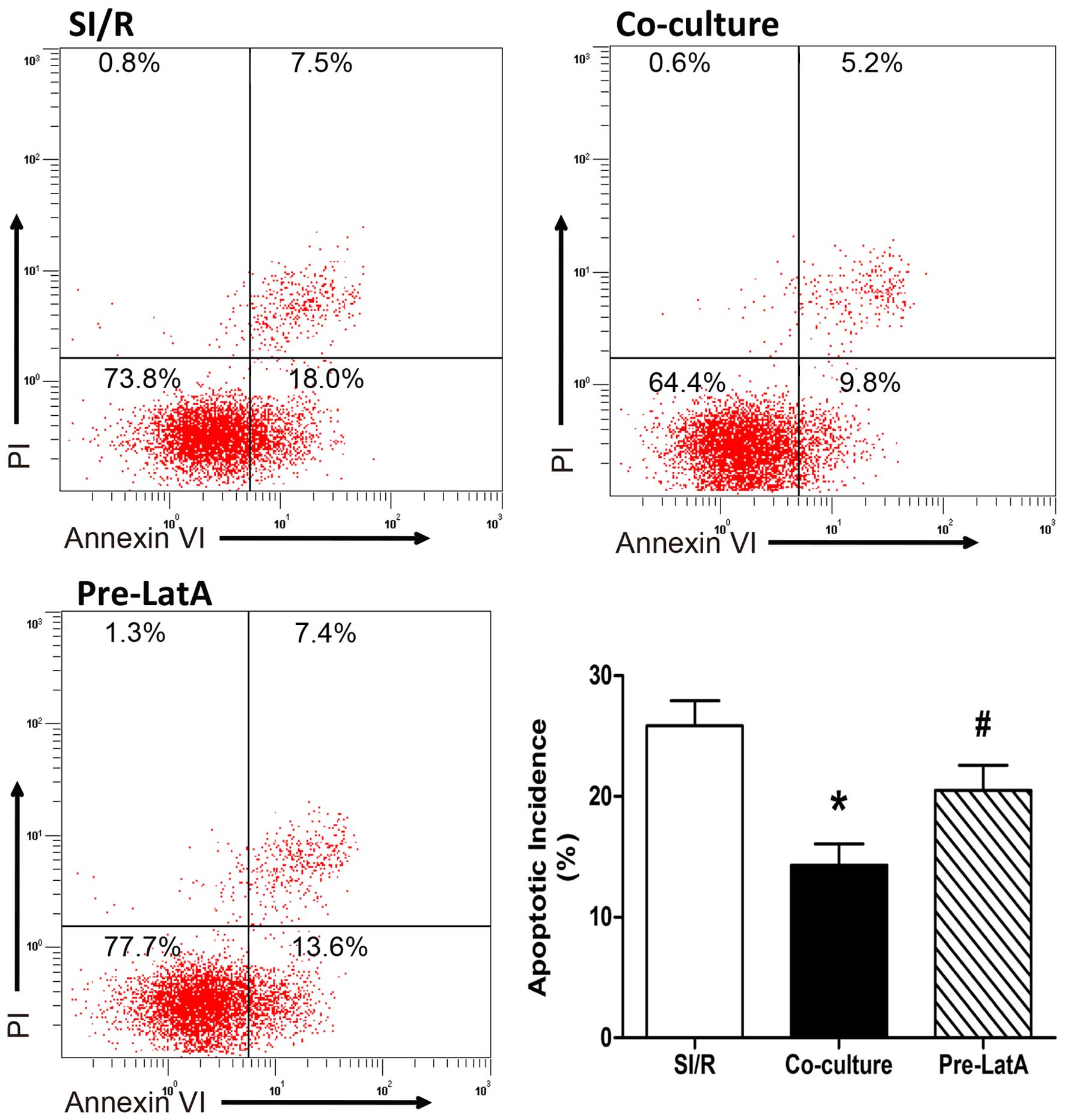
LatA pretreatment inhibits the protective
effects of the direct co-culture system
Consistent with results described above, co-culture
reduced the apoptotic incidence in the SI/R H9c2 cells. However,
when the BM-MSCs were pretreated with LatA, the increase in
apoptosis indicated that the protective effect of the co-culture
system was significantly undermined, as determined by annexin
V-FITC/PI staining (SI/R, 25.87±2.07%; Co-culture, 14.30±1.76%;
Pre-LatA, 20.50±2.10%; Fig.
5).
Similarly, western blot analysis of the expression
levels of Bax and Bcl-2, and for the assessment of caspase-3
activity suggested that BM-MSCs pretreated with LatA resulted in
decreased anti-apoptotic potential in the co-culture system
(Fig. 6A and B).
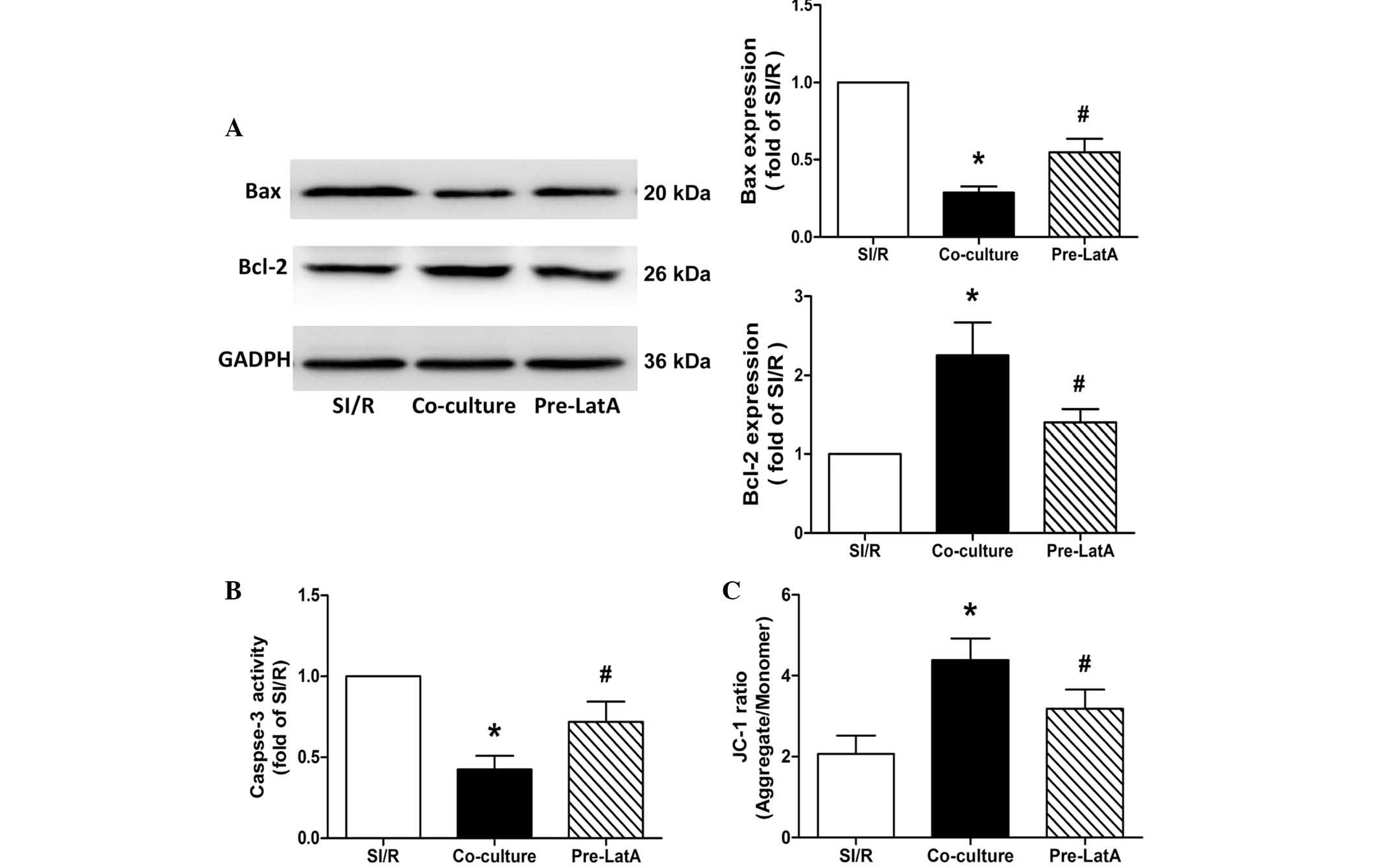 | Figure 6Effects of co-culture with BM-MSCs on
apoptosis and mitochondrial membrane potential in the SI/R, Co
culture and Pre LatA groups. (A) Representative western blotting of
the changes in the expression levels of Bax and Bcl-2 in H9c2
cells. (B) Changes in caspase-3 activity in H9c2 cells from the
different groups. (C) Changes in mitochondrial membrane potential
in H9c2 cells from the different groups. Data are presented as the
mean ± standard deviation (*P<0.05, vs. SI/R;
#P<0.05, vs. Co-culture). BM-MSCs, bone
marrow-derived mesenchymal stem cells; SI/R, simulated
ischemia/reperfusion; Co-culture, SI/R co-cultured with BM-MSCs;
LatA, latrunculin-A; Pre-LatA, pretreated with LatA; Bcl-2, B cell
lymphoma-2; Bax, Bcl-2-associated X protein; JC-1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole-carbocyanide
iodine. |
Pretreatment of the BM-MSCs with LatA also
significantly impaired the ability of BM-MSCs to protect the Δψm
against the decrease induced by SI/R in the SI/R-stimulated H9c2
(SI/R, 2.07±0.46; Co-culture; 4.39±0.53; Pre-LatA, 3.18±0.47;
Fig. 6C).
Discussion
In the present study, a model of BM-MSC intervention
in SI/R-induced H9c2 cells was established to assess changes in
apoptosis. Direct co-culture with BM-MSCs was found to
significantly restore the impaired mitochondrial function in
SI/R-stimulated H9c2 cells. TNT-like structures and directed
mitochondria transfer were also found between these two types of
cells. However, this benefit was partially offset if the formation
of TNTs was inhibited by pretreatment of the BM-MSCs with LatA, an
inhibitor of the cytoskeleton.
Mitochondria are essential organelles, and are key
in processes, including oxidative phosphorylation, aerobic
metabolism of glucose and fat, calcium signaling and apoptosis
(18). Among the complex
mechanisms underlying I/R, mitochondrial dysfunction appears to
contribute significantly to these procedures (25,26).
During the apoptosis process, reduced Δψm is one of the early
events, triggering the uncoupling of the respiratory chain, release
of cytochrome c, and caspase cascade activation. The present
study hypothesized that direct cell-to-cell communications is one
of the key mechanisms for the observed amelioration of
mitochondrial function in co-cultures. Despite the formation of
TNT-like structures between MSCs and other cell types having been
reported in several studies (27–30),
limited experimental data are available on the effects of MSCs on
injured mitochondria in H9c2 cells (31). The formation of TNTs, which are
directly induced by stress, may be considered as a defense
mechanism of injured cells. In the present study, only GFP BM-MSCs
were pre-labeled using MitoTracker Deep Red. Following direct
co-culture, however, the SI/R H9c2 cells were also labeled red.
Consistently, the Δψm assay confirmed the reversion of
mitochondrial dysfunction by co-culture. These data demonstrated
that BM-MSCs had transferred their own intact mitochondria to the
cells with compromised mitochondrial function.
TNTs are fine, long, non-adherent, actin-based
cytoplasmic extensions, which were first described in a rat
pheochromocytoma cell line (32).
TNTs, predominantly generated by actin-driven protrusions of the
cytoplasmic membrane, are open-ended tubes, the lumen of which
establishes a direct connection between the cytoplasm of the
connected cells (33). Thus, TNT
formation facilitates the exchange of cellular components and
signals (34). It may be that this
novel inter-cellular communication is important in the rescue
effects of BM-MSCs. To further investigate the effects of the
observed bridging structures in the present study, LatA was used to
inhibit the formation of TNTs in the co-cultures. As an F-actin
depolymerizing drug, LatA is efficient in reducing the potential of
MSCs to produce TNTs with neighboring cells (28,35).
In the in vitro co-culture system, pretreatment with LatA
resulted in a marked decline in mitochondrial recovery in the SI/R
H9c2 cells, suggesting that TNTs are, at least partially, involved
in the therapeutic effects of direct co-culture with BM-MSCs.
In conclusion, the present study demonstrated that
co-culture with BM-MSCs protected H9c2 cells against the apoptosis
induced by SI/R. During this process, co-cultured BM-MSCs bridged
with the injured H9c2 cells via TNTs, through which intact
mitochondria were transferred. This rescue by the BM-MSCs
efficiently assisted in the recovery of the injured cells from
mitochondrial dysfunction. Further investigation of the protective
effects of stem cells through TNT-mediated mitochondrial transfer
may provide novel insights into the therapeutics of IHD.
Acknowledgments
This study was supported, in part, by grants from
the National Natural Science Foundation of China (grant nos.
81300178 and 81370401).
References
|
1
|
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ,
Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et
al: Heart disease and stroke statistics-2011 update: A report from
the American heart association. Circulation. 123:e18–e209. 2011.
View Article : Google Scholar
|
|
2
|
Forouzanfar MH, Moran AE, Flaxman AD, Roth
G, Mensah GA, Ezzati M, Naghavi M and Murray CJ: Assessing the
global burden of ischemic heart disease, part 2: analytic methods
and estimates of the global epidemiology of ischemic heart disease
in 2010. Glob Heart. 7:331–342. 2012. View Article : Google Scholar
|
|
3
|
Yu D, Li M, Tian Y, Liu J and Shang J:
Luteolin inhibits ROS-activated MAPK pathway in myocardial
ischemia/reperfusion injury. Life Sci. 122:15–25. 2015. View Article : Google Scholar
|
|
4
|
Chen C, Feng Y, Zou L, Wang L, Chen HH,
Cai JY, Xu JM, Sosnovik DE and Chao W: Role of extracellular RNA
and TLR3-Trif signaling in myocardial ischemia-reperfusion injury.
J Am Heart Assoc. 3:e0006832014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka-Esposito C, Chen Q and Lesnefsky
EJ: Blockade of electron transport before ischemia protects
mitochondria and decreases myocardial injury during reperfusion in
aged rat hearts. Transl Res. 160:207–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gustafsson AB and Gottlieb RA: Bcl-2
family members and apoptosis, taken to heart. Am J Physiol Cell
Physiol. 292:C45–C51. 2007. View Article : Google Scholar
|
|
7
|
Pfeffer MA and Braunwald E: Ventricular
remodeling after myocardial infarction. Experimental observations
and clinical implications. Circulation. 81:1161–1172. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Nbaheen M, Vishnubalaji R, Ali D,
Bouslimi A, Al-Jassir F, Megges M, Prigione A, Adjaye J, Kassem M
and Aldahmash A: Human stromal (mesenchymal) stem cells from bone
marrow, adipose tissue and skin exhibit differences in molecular
phenotype and differentiation potential. Stem Cell Rev. 9:32–43.
2013. View Article : Google Scholar :
|
|
10
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jameel MN and Zhang J: Stem cell therapy
for ischemic heart disease. Antioxid Redox Signal. 13:1879–1897.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karpov AA, Uspenskaya YK, Minasian SM,
Puzanov MV, Dmitrieva RI, Bilibina AA, Anisimov SV and Galagudza
MM: The effect of bone marrow- and adipose tissue-derived
mesenchymal stem cell transplantation on myocardial remodelling in
the rat model of ischaemic heart failure. Int J Exp Pathol.
94:169–177. 2013.PubMed/NCBI
|
|
13
|
Janssens S, Dubois C, Bogaert J,
Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve
P, Dens J, et al: Autologous bone marrow-derived stem-cell transfer
in patients with ST-segment elevation myocardial infarction:
Double-blind, randomised controlled trial. Lancet. 367:113–121.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lunde K, Fjeld JG, Smith HJ, Solheim S,
Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A,
et al: Intracoronary injection of mononuclear bone marrow cells in
acute myocardial infarction. N Engl J Med. 355:1199–1209. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murry CE, Soonpaa MH, Reinecke H, Nakajima
H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH,
Poppa V, et al: Haematopoietic stem cells do not transdifferentiate
into cardiac myocytes in myocardial infarcts. Nature. 428:664–668.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawada H, Fujita J, Kinjo K, Matsuzaki Y,
Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, et
al: Nonhematopoietic mesenchymal stem cells can be mobilized and
differentiate into cardiomyocytes after myocardial infarction.
Blood. 104:3581–3587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spees JL, Olson SD, Whitney MJ and Prockop
DJ: Mitochondrial transfer between cells can rescue aerobic
respiration. Proc Natl Acad Sci USA. 103:1283–1288. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crevensten G, Walsh AJ, Ananthakrishnan D,
Page P, Wahba GM, Lotz JC and Berven S: Intervertebral disc cell
therapy for regeneration: Mesenchymal stem cell implantation in rat
intervertebral discs. Ann Biomed Eng. 32:430–434. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li B, Li R, Zhang C, Bian HJ, Wang F, Xiao
J, Liu SW, Yi W, Zhang MX, Wang SX, et al: MicroRNA-7a/b protects
against cardiac myocyte injury in ischemia/reperfusion by targeting
poly(ADP-ribose) polymerase. PLoS One. 9:e900962014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ekhterae D, Lin Z, Lundberg MS, Crow MT,
Brosius FC III and Núñez G: ARC inhibits cytochrome c release from
mitochondria and protects against hypoxia-induced apoptosis in
heart-derived H9c2 cells. Circ Res. 85:e70–e77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Z, Liu Y, Deng W, Dai J, Li F, Yuan
Y, Wu Q, Zhou H, Bian Z and Tang Q: Hesperetin attenuates
mitochondria-dependent apoptosis in lipopolysaccharide-induced H9C2
cardiomyocytes. Mol Med Rep. 9:1941–1946. 2014.PubMed/NCBI
|
|
23
|
Han H, Zhu J, Zhu Z, Ni J, Du R, Dai Y,
Chen Y, Wu Z, Lu L and Zhang R: p-Cresyl sulfate aggravates cardiac
dysfunction associated with chronic kidney disease by enhancing
apoptosis of cardiomyocytes. J Am Heart Assoc. 4:e0018522015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao X, Zhang H, Zhuang W, Yuan G, Sun T,
Jiang X, Zhou Z, Yuan H, Zhang Z and Dong H: PEDF and PEDF-derived
peptide 44mer protect cardiomyocytes against hypoxia-induced
apoptosis and necroptosis via anti-oxidative effect. Sci Rep.
4:56372014.PubMed/NCBI
|
|
25
|
Loor G, Kondapalli J, Iwase H, Chandel NS,
Waypa GB, Guzy RD, Vanden Hoek TL and Schumacker PT: Mitochondrial
oxidant stress triggers cell death in simulated
ischemia-reperfusion. Biochim Biophys Acta. 1813:1382–1394. 2011.
View Article : Google Scholar :
|
|
26
|
Galluzzi L, Kepp O, Trojel-Hansen C and
Kroemer G: Mitochondrial control of cellular life, stress and
death. Circ Res. 111:1198–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plotnikov EY, Khryapenkova TG, Galkina SI,
Sukhikh GT and Zorov DB: Cytoplasm and organelle transfer between
mesenchymal multipotent stromal cells and renal tubular cells in
co-culture. Exp Cell Res. 316:2447–2455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu K, Ji K, Guo L, Wu W, Lu H, Shan P and
Yan C: Mesenchymal stem cells rescue injured endothelial cells in
an in vitro ischemia-reperfusion model via tunneling nanotube like
structure-mediated mitochondrial transfer. Microvasc Res. 92:10–18.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Cui J, Sun X and Zhang Y:
Tunneling-nanotube development in astrocytes depends on p53
activation. Cell Death Differ. 18:732–742. 2011. View Article : Google Scholar :
|
|
30
|
Pasquier J, Guerrouahen BS, Al Thawadi H,
Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L,
Rafii S, et al: Preferential transfer of mitochondria from
endothelial to cancer cells through tunneling nanotubes modulates
chemoresistance. J Transl Med. 11:942013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cselenyák A, Benko Z, Szepes M, Kiss L and
Lacza Z: Stem cell transplantation in an in vitro simulated
ischemia/reperfusion model. J Vis Exp. 57:e35752011.PubMed/NCBI
|
|
32
|
Rustom A, Saffrich R, Markovic I, Walther
P and Gerdes HH: Nanotubular highways for intercellular organelle
transport. Science. 303:1007–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lou E, Fujisawa S, Morozov A, Barlas A,
Romin Y, Dogan Y, Gholami S, Moreira AL, Manova-Todorova K and
Moore MA: Tunneling nanotubes provide a unique conduit for
intercellular transfer of cellular contents in human malignant
pleural mesothelioma. PLoS One. 7:e330932012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Plotnikov EY, Khryapenkova TG, Vasileva
AK, Marey MV, Galkina SI, Isaev NK, Sheval EV, Polyakov VY, Sukhikh
GT and Zorov DB: Cell-to-cell cross-talk between mesenchymal stem
cells and cardiomyocytes in co-culture. J Cell Mol Med.
12:1622–1631. 2008. View Article : Google Scholar
|
|
35
|
Coué M, Brenner SL, Spector I and Korn ED:
Inhibition of actin polymerization by latrunculin A. FEBS Lett.
213:316–318. 1987. View Article : Google Scholar : PubMed/NCBI
|