Introduction
In addition to cancer, cardiovascular disease and
cerebrovascular disease, diabetes mellitus is the third leading
contributor to mortality rates, and produces significant pressure
on society and public health (1).
As previously reported, diabetes-associated mortality rates
represent almost 2.2% of the total morality rates worldwide, and
its prevalence in China has increased rapidly (2–4).
Complex metabolic disorders in three major nutrients, including
lipids, carbohydrates and proteins, are observed in patients with
diabetes (5). Insulin secretion
deficiency in diabetes further results in an increase of blood
glucose levels and organ damage (6). Additionally, various complications,
including nephropathy, neuropathy, retinopathy and hyperlipemia,
are observed in the majority of patients with diabetes (7).
As a widespread problem, traditional therapy for
diabetes has focused on blood glucose regulation, which does not
control the associated complications (8). At present, there remains no suitable
therapeutic regimen that can cure diabetes. Insulin injection and
commonly prescribed drugs, including metformin and pioglitzone,
have undesirable averse effects, including insulin resistance,
hypoglycemia and gastrointestinal disturbances (9). Therefore, the identification of
alternative treatment strategies for the treatment of diabetes is
in high demand.
Due to their reduced adverse effects and favorable
economic characteristics, herbal medicine is considered a valuable
reservoir of novel drugs (10). It
has been revealed that natural products exhibit anti-diabetic
activities and have auxiliary therapeutic effects on complications
(11). Our previous study
successfully demonstrated that Cordyceps militaris exhibits
anti-diabetic and anti-nephropathic activities (12). Paecilomyces tenuipes, a
well-known Chinese medicinal entomopathogenic fungi, has been
traditionally used in folk medicine in Japan, Korea and China for
years (13). Paecilomyces
tenuipes, containing polysaccharides, adenosine, cordycepin,
sterol and cyclopeptide, is increasingly notable for its
antidepressant, antitumor and immunomodulatory effects (14,15).
However, the regulatory effects of a polysaccharide-enriched
fraction of Paecilomyces tenuipes on diabetic mice has not
been reported previously. In our previous study, through chemical
mutagenesis, an improved mutant Paecilomyces tenuipes,
termed N45, was developed, which is preserved at the China Center
for Type Culture Collection (no. M2011145).
Based on our previous study, the present study
hypothesized that Paecilomyces tenuipes N45 may possess
anti-diabetic and hypolipidemic activities (12). The present study was designed to
confirm this hypothesis in an alloxan-induced diabetic animal
model. Following treatment with polysaccharide-enriched fractions
of Paecilomyces tenuipes N45, indices associated with
oxidation resistance, and hypoglycemic and hypolipidemic activities
were detected. These data aimed to provide experimental evidence to
support the clinical use of Paecilomyces tenuipes as an
effective agent for the treatment of diabetes.
Materials and methods
Submerged fermentation of Paecilomyces
tenuipes N45
The Paecilomyces tenuipes N45 mutant was
established from Paecilomyces tenuipes Pt196 (RCEF 4339;
Anhui Agricultural University, Anhui, China) via nitrosoguanidine
treatment. The wild strain (RCEF4339) was cultured at 30°C in a
potato dextrose agar (PDA) slant medium for 5 days and washed with
10 ml of sterilized normal saline. The cell concentration was
adjusted to 108/ml. The fungal suspension was treated
with nitrosoguanidine (1 mg/ml; Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China) for 15 min at room temperature. Then, the
fungal suspension was cultured in PDA medium, in a rotary shaker
incubator (10 l; Biostat B; Germany) at 150 rpm for 5 days. The
culture medium comprised glucose (40 g/l; Sinopharm Chemical
Reagent Co., Ltd.), peptone (10 g/l; Aoboxing Biotechnology Co.,
Ltd., Beijing, China) and yeast extract powder (10 g/l; Aoboxing
Biotechnology Co., Ltd.). The culture temperature was 26°C. The
mycelium pellets were harvested and lyophilized for further
use.
Paecilomyces tenuipes N45 extract
preparation
Similar to our previous study (12), water extract from Paecilomyces
tenuipes N45 (WE) was prepared, as follows: 100 g mycelial
powder was extracted twice in 5 liters of distilled water at 80°C
for 3 h. Following centrifugation at 3,550 × g for 10 min at 4°C,
the supernatant was evaporated under reduced pressure of 0.09 mPa
and 80°C using a rotary evaporator R1002B obtained from Shanghai
SENCO Technology Co., Ltd. (Shanghai, China) and was dissolved in
physiological saline prior to use. The concentration of the WE was
0.44 g/ml and the concentration for the alcohol extract (AE) was
0.35 g/ml. The AE was prepared using an alcohol
distillation-extractor, the heating mantle used was obtained from
Zhongxingweiye Instrument Co., Ltd, Beijing, China. Following
removal of the existing proteins, the extracts were lyophilized by
being dried using a Genesis Pilot Lyophilizer SQ 25ES (SP
Industries, Inc., Warminster, PA, USA) and stored at −20°C.
Chemical compositions analyzed
As reported previously (16), the present study examined the
levels of amino acids, polypeptides, proteins, sugars, phenolics,
tannin, alkaloids, sterols, terpenes, organic acid, essential oil,
anthraquinone, flavonoids, coumarin and lactone, were analyzed in
the WE and AE from Paecilomyces tenuipes N45.
In vivo experiments using an animal model
of diabetes
The current study was approved by the ethics
committee of the Jilin University (Changchun, China). The
experimental protocol was approved by the Lab Animal Centre of
Jilin University. A total of 90 Kunming male mice (weight, 18–22 g;
age, 6 weeks; Norman Bethune University of Medical Science Jilin
University (Jilin, China) were maintained under a constant 12:12 h
light dark cycle (8:00 am–8:00 pm) at an environmental temperature
of 22±1°C and a humidity of 60±2%. The mice were fed standard chow
and had access to water ad libitum prior to and following
the experimental period, unless stated otherwise. All mice were fed
adaptively for 1 week prior to experiments.
As shown in Fig. 1,
the overnight fasted mice fed with 2 g/kg sucrose solution for 72 h
were used for diabetic mouse model establishment. Diabetes was
induced by intraperitoneal injection with a freshly prepared
solution of alloxan (Sigma-Aldrich, St Louis, MO, USA) in
physiological saline at a dose of 150 mg/kg bodyweight. After 4 h,
the mice were orally administered with 25% glucose solution
(0.3–0.4 ml) to prevent fetal hypoglycemia. The same procedure was
repeated on the second day. After 72 h, mice with persistent
fasting blood glucose levels >11.1 mmol/l were identified as a
severe diabetic group (17).
Another 10 mice were fed with normal water and injected with
physiological saline, and served as a control group (CTRL).
The alloxan-induced diabetic mice were separated
randomly into eight groups, as follows, and received drug
administration for 3 weeks (once a day):
Diabetic model group (model group; n=10):
administrated with physiological saline orally; metformin group (DH
group; n=10): administrated with 125 mg/kg metformin (Sino-American
Shanghai Squibb Pharmaceuticals Ltd, Shanghai, China) orally,
AE group: administrated orally with 2.5 g/kg (n=10;
equal to 5 g/kg of dried mycelial powder), 250 mg/kg (n=10; equal
to 0.5 g/kg of dried mycelial powder) and 50 mg/kg (n=10; equal to
0.1 g/kg of dried mycelial powder) of alcohol extract orally;
WE group: administrated with 2 g/kg (n=10; equal to
5 g/kg of dried mycelial powder), 200 mg/kg (n=10; equal to 0.5
g/kg of dried mycelial powder) and 40 mg/kg (n=10; equal to 0.1
g/kg of dried mycelial powder) of WE.
Bodyweight was recorded during the course of the
experiment. Fasting blood glucose levels were recorded on the 21st
day following 18 h of food deprivation. Blood samples (0.2 ml) were
collected from the caudal vein of the mice 60 min after the final
treatment, and the levels of superoxide dismutase (SOD),
glutathione peroxidase (GSH-Px), malondialdehyde (MDA), low-density
lipoprotein cholesterol (LDL-C) and high density lipoprotein choles
terol (HDL-C) were analyzed using commercial assay kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). Serum insulin
was measured using a mouse insulin enzyme-linked immunosorbent
assay kit (R&D Systems, Inc., Minneapolis, MN, USA). Following
an oral glucose tolerance test, the animals were sacrificed via
administration with 200 mg/kg pentobarbital (Sinopharm Chemical
Reagent Co., Ltd); following which organs (heart, liver, lungs,
spleen and kidneys) were excised out, washed with ice cold saline
and weighed immediately. The relative organ weights were calculated
by dividing the weight of each organ by the weight of the mouse.
The levels of SOD, GSH-Px and MDA in the liver were determined
using associated commercial kits (Nanjing Biotechnology Co. Ltd.,
Nanjing, China).
Oral glucose tolerance test
In order to investigate glucose homeostasis in the
experimental diabetic mice, an oral glucose tolerance test was
performed at the end of the experiment using a previously described
method with a modification (18).
Following overnight, but with provision of water ad libitum
for >12 h, the mice in all groups were administrated with the
relevant extracts. After 30 min, 2.0 g/kg glucose was administered
orally to all mice. Blood samples were collected 0, 0.5, 1 and 2 h
following the glucose load, and plasma glucose concentrations were
measured using a Glucose Assay kit (Nanjing Jiancheng
Bioengineering Institute). Calculation of the area under the blood
glucose curve (AUC) was made according to the following equation
(19): AUC = (basal glycemia +
glycemia at 0.5 h) × 0.25 + (glycaemia at 0.5 h + glycaemia at 1 h)
× 0.25 + (glycemia at 1 h + glycemia at 2 h) × 0.5.
Statistical analysis
All data are was expressed as the mean ± standard
deviation. Statistical significance was determined using one-way
analysis of variance, followed by post-hoc multiple comparisons
(Dunn's test) using SPSS 16.0 software (SPSS, Inc., Chicago, IL,
USA). P≤0.05 was considered to indicate a statistically significant
difference.
Results
Identification of active ingredients
The screening assessment of the active ingredients
revealed that soluble reducing sugar, sugars and organic acid were
found in the WE and AE of Paecilomyces tenuipes N45
(Table I).
 | Table ISummary of the chemical constituents
in crude extracts of Paecilomyces tenuipes N45. |
Table I
Summary of the chemical constituents
in crude extracts of Paecilomyces tenuipes N45.
| Test | Reaction/test | AE | WE |
|---|
| Amino
acid/polypeptides/protein | Biuret
reaction/ninhydrin reaction | − | − |
| Soluble reducing
sugar | Fehling reagents
reaction | + | + |
| Sugars | Open-loop
reaction | + | + |
|
Phenolics/tannin | Ferric chloride
test | − | − |
| Alkaloids | Mercuric potassium
iodide/silicotungstic acid test | − | − |
| Sterols | Acetic
anhydride-concentrated sulfuric acid | − | − |
| Terpenes | Foam test | − | − |
| Organic acid | Bromophenol blue
test | + | + |
| Essential
oil/oil |
Phospho-molybdic-acid ethanol test | − | − |
| Anthraquinone | Magnesium acetate
test | − | − |
| Flavonoids | HCL-Mg powder
test/HCL-Zn powder test | − | − |
|
Coumarin/lactone | Open-loop
reaction | − | − |
Effect of Paecilomyces tenuipes N45
extracts on bodyweight and organs
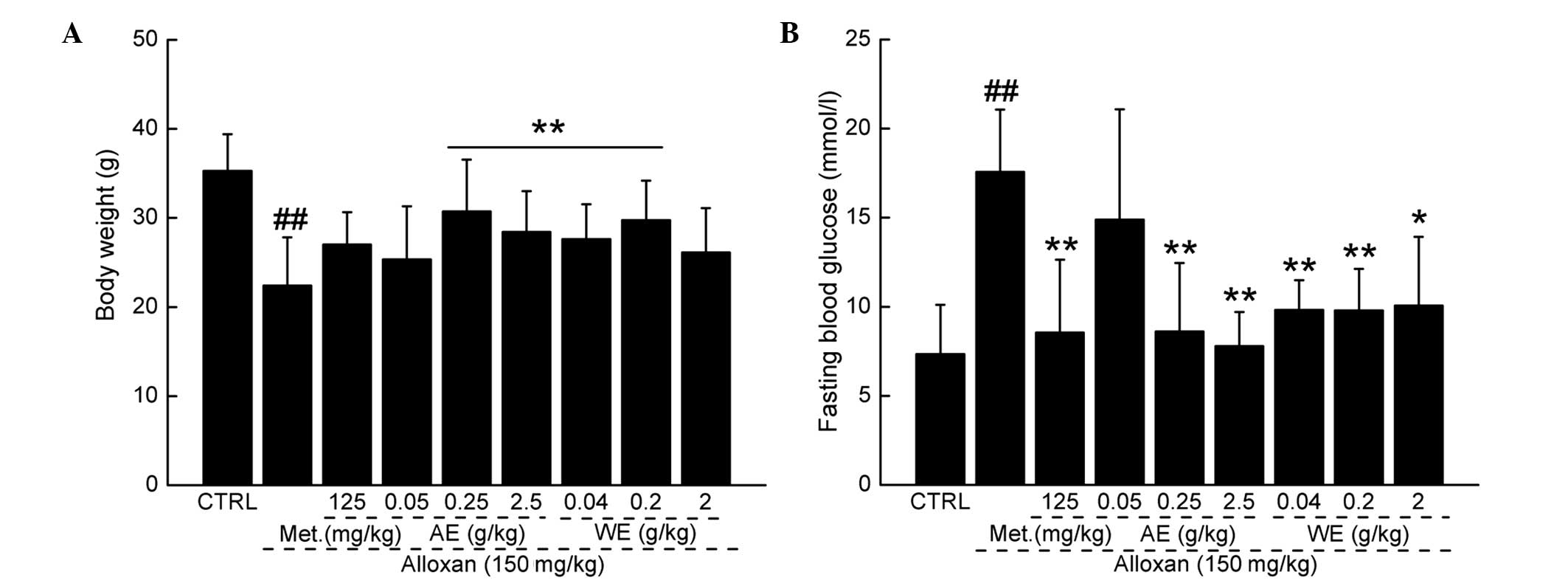
A decrease in bodyweight was found in the
alloxan-induced diabetic mice during the experimental period. The
suppressive effect of alloxan on bodyweight was reversed following
treatment with metformin hydrochloride, and with all doses of
Paecilomyces tenuipes N45 extract, with the exception of
0.05 g/kg AE and 2 g/kg WE (Fig.
2A; P<0.01).
In all drug treatment groups, no significant
differences were identified when relative organ weights of mice in
the control group were compared with mice in the exposure groups
(Table II). Compared with the
control mice, the lung index was significantly increased in the
alloxan-induced diabetic mice (P<0.05). Treatment for 21 days
with metformin hydrochloride, AE and WE suppressed lung hyperplasia
(Table II).
 | Table IIEffect of extracts on organ indices
in alloxan-induced diabetic mice. |
Table II
Effect of extracts on organ indices
in alloxan-induced diabetic mice.
| Group | Dose (g/kg) | Heart index
(g/g) | Liver index
(g/g) | Spleen index
(g/g) | Lung index
(g/g) | Kidney index
(g/g) |
|---|
| CTRL | – | 5.70±0.74 | 52.68±5.34 | 7.27±1.66 | 7.21±0.58 | 7.06±0.60 |
| Model | – | 6.12±0.79 | 53.62±7.74 | 7.51±2.10 | 9.06±2.36a | 7.37±1.24 |
| DH | 0.125 | 7.20±2.80 | 49.65±7.61 | 8.52±1.03 | 7.14±1.38b | 7.69±0.71 |
| 0.05 | 5.89±0.44 | 52.43±8.85 | 6.67±2.26 | 8.40±1.44 | 8.07±1.36 |
| AE | 0.25 | 5.69±1.51 | 50.27±11.13 | 6.67±2.46 | 7.36±3.42b | 7.41±0.95 |
| 2.50 | 5.37±0.66 | 51.69±4.32 | 7.18±2.13 | 7.42±1.08b | 7.57±0.61 |
| 0.04 | 6.40±1.07 | 53.42±14.05 | 8.23±1.05 | 7.84±2.32 | 7.90±2.09 |
| WE | 0.20 | 6.20±1.01 | 48.84±3.84 | 7.09±1.27 | 7.56±1.14b | 7.24±0.98 |
| 2.00 | 6.23±1.06 | 49.98±11.23 | 8.30±1.92 | 8.61±1.66 | 7.84±1.04 |
Paecilomyces tenuipes N45 extracts exert
hypoglycemic effects
Fasting blood glucose levels were measured to
evaluate the hypoglycemic effects of Paecilomyces tenuipes
N45 extracts. The fasting blood glucose concentration in the
alloxan-induced diabetic mice was 11.1 mmol/l higher than that of
the normal control animal group; whereas treatment with 125 mg/kg
metformin hydrochloride resulted in a 51.24% reduction in fasting
blood glucose concentration (P<0.01; Fig. 2B). The administration of 0.25 and
2.5 g/kg AE reduced fasting blood glucose levels by almost 44.29
and 50.53%, respectively (P<0.01; Fig. 2B), compared with the model group.
Similarly, administration of 0.04, 0.2 and 2 g/kg WE suppressed
fasting blood glucose levels by 40.70, 56.72 and 37.31%,
respectively, compared with the model group (P<0.01; Fig. 2B).
The oral glucose tolerance test served as a second
diagnostic indices to further confirm the hypoglycemic effects of
Paecilomyces tenuipes N45 extracts (20). Significantly higher fasting blood
glucose levels were observed in the alloxan-induced diabetic mice
between 0.5 and 2 h, compared with the normal mice (P<0.05;
Fig. 3A). Similar to the results
following exposure to metformin hydrochloride, AE and WE
significantly prevented the rapid increase in blood glucose levels,
particularly at 60 min (P<0.05; Fig. 3A). High AUC values in the model
group revealed a state of impaired glucose tolerance in the
diabetic mice (P<0.01; Fig.
3B). Treatment with metformin hydrochloride, AE and WE
significantly reduced the AUC during the oral glucose tolerance
test (P<0.05; Fig. 3B).
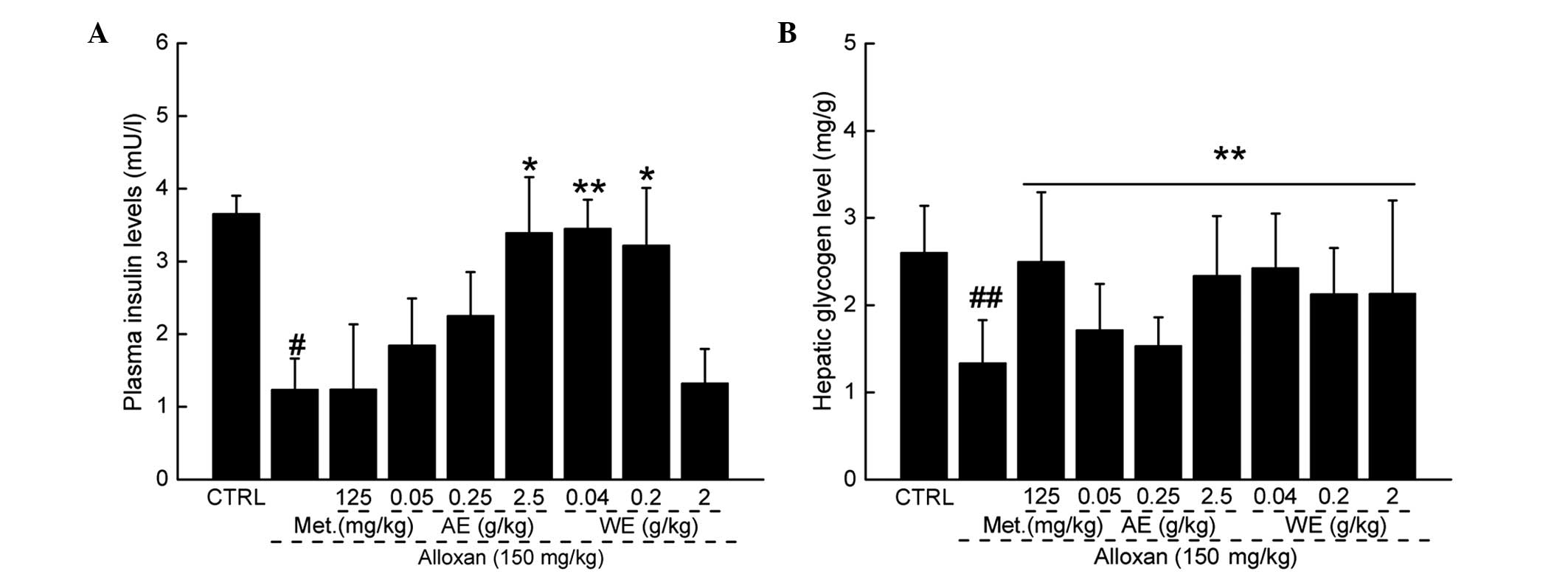
Paecilomyces tenuipes N45 extracts
increase plasma levels of insulin and hepatic glycogen
Compared with the untreated group, a significant
decrease in plasma insulin levels was observed following alloxan
treatment. AE and WE treatment resulted in the elevation of plasma
insulin levels (Fig. 4A;
P<0.01). The synthesis and degradation of glycogen in the liver
are important mechanisms in the control of blood glucose
homeostasis (21). Hepatic
glycogen levels were measured to determine whether elevation of
hepatic glycogen is involved in the hypoglycemic effects of the
extracts. The levels of hepatic glycogen measured at the
termination of the various treatments are shown in Fig. 4B. Significant decreases in hepatic
glycogen were observed in the diabetic mice. As expected, repeated
oral treatment with metformin hydrochloride, and all extract doses
caused a marked increase in the levels of hepatic glycogen,
compared with the diabetic model group (Fig. 4B; P<0.01).
Paecilomyces tenuipes N45 extracts exert
hypolipidemic effects
In diabetic mice, significantly high levels of
low-density lipoprotein cholesterol (LDL-C) were observed. AE and
WE administration reduced the concentrations of LDL-C to normal
levels (Fig. 5A; P<0.05). By
contrast, compared with the untreated mice, alloxan injection
reduced the concentrations of high density lipoprotein cholesterol
(HDL-C). AE administration at 0.05 g/kg, and WE at all doses,
increased the low level of HDL after 4-week treatment (Fig. 5B; P<0.05). The therapeutic
effect on HDL was noted in metformin hydrochloride-treated mice
(Fig. 5B; P<0.05).
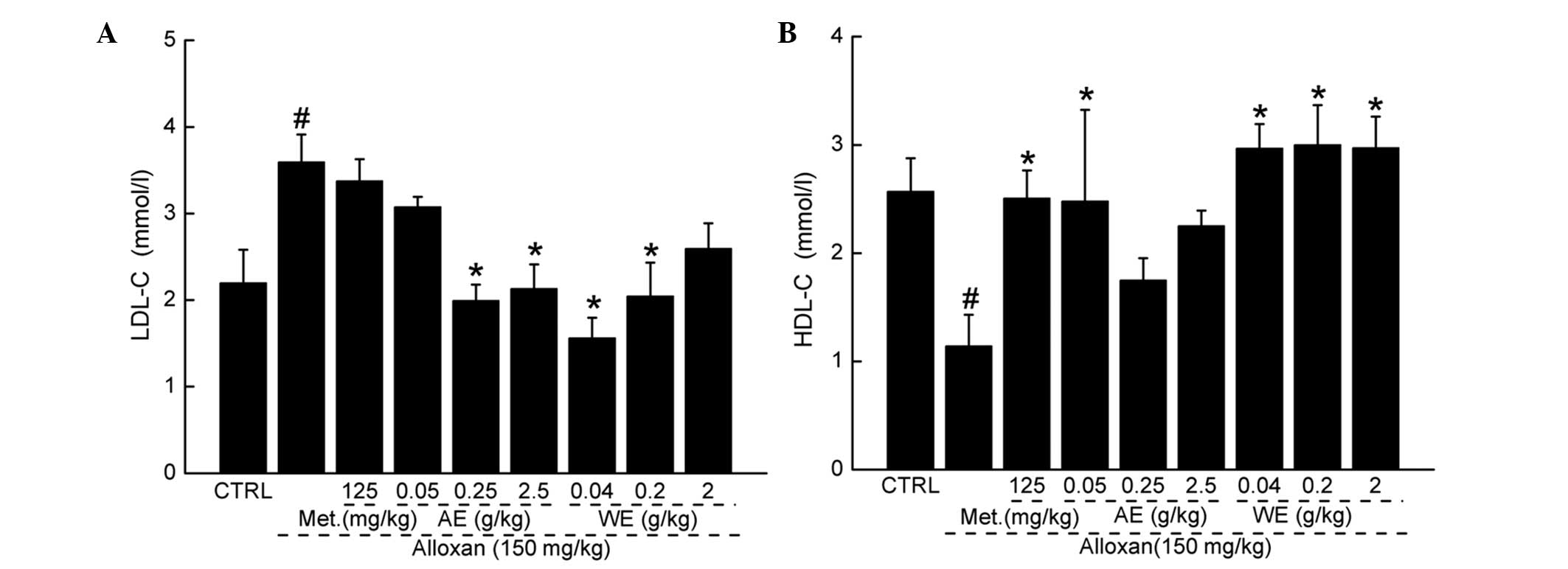 | Figure 5Hypolipidemic effects of
Paecilomyces tenuipes N45 extracts on alloxan-induced
diabetic mice. Administration of AE and WE for 3 weeks resulted in
a reduction in (A) LDL-C (A) and an increase in (B) HDL-C in the
serum, compared with the diabetic model mice. Data are expressed as
the mean ± standard deviation (n=10) and were analyzed using
one-way analysis of variance, followed by Dunn's test.
#P<0.05, vs. CTRL; *P<0.05, vs.
alloxin-induced model group. CTRL, untreated control; AE, alcohol
extract; WE, water extract; Met, metformin; HDL-C, high density
lipoprotein cholesterol; LDL-C, low density lipoprotein
cholesterol. |
Paecilomyces tenuipes N45 extracts exert
antioxidant effects
Hyperglycemia-induced oxidative stress leads to
excessive production of reactive oxygen species (ROS), which may be
responsible for the pathogenesis of diabetes-associated
complications (22). SOD and
GSH-Px, important antioxidant enzymes in mammalian cells, have
critical roles against oxidative stress-induced cell damage
(23). In the present study,
following alloxan injection, the levels of SOD and GSH-Px in the
plasma and liver were reduced significantly; whereas the
concentration of MDA was significantly enhanced (Fig. 6A–F; P<0.05). Similar to
metformin hydrochloride, AE and WE administration normalized the
levels of SOD, GSH-Px and MDA to healthy levels in the plasma and
liver (Fig. 6; P<0.05).
Discussion
Due to various pathologic changes, diabetes has
become the third leading contributor to mortality rates worldwide
(24). As reported previously,
natural products, which possess antidiabetic activity offer a
valuable reservoir of potential diabetes medications (25). Fungi have received increasing
attention, as they have comprehensive hypoglycemic effects
(25,26). Paecilomyces tenuipes has
been considered to be a valuable source of medicinal remedies and
health promotion. (27,28). The immune-stimulating and
antifatigue activities of Paecilomyces tenuipes have been
investigated in animal models (14,29).
In the present study, based on fermentation culture, the
hypoglycemic effects associated with fasting blood glucose levels
of Paecilomyces tenuipes N45 extracts were successfully
investigated in an alloxan-induced diabetic mouse model. In
addition, the hypolipidemic and antioxidative activities of
Paecilomyces tenuipes N45 extracts were confirmed. Through
bodyweight and monitoring of visceral indices, Paecilomyces
tenuipes N45 was confirmed as a safe pharmacological agent.
Various methods of chemical analysis were applied to
detect the composition of Paecilomyces tenuipes N45 crude
extracts. Soluble reducing sugar, sugars and organic acids were
found in WEs and AEs. Although natural products are generally
accepted as safe and efficacious for clinical use, pharmacological
and toxicological evaluations are necessary (30). In our previous investigations,
through histopathological detection, and hematological and
biochemical analyses, Paecilomyces tenuipes N45 was found to
have no adverse effects in terms of acute oral toxicity or 90-day
subchronic inhalation toxicity (31). In the present study, compared with
the untreated mice, minimal change in organ indices were noted,
which further confirmed the safety of Paecilomyces tenuipes
N45.
The pancreas is responsible for the regulation of
glucose concentrations in the plasma. Alloxan, an uncommon
substance used for diabetes mellitus establishment, possesses a
destructive activity on the β-cells of the pancreas (32,33).
Through damage of β-cells, alloxan causes a reduction in insulin
release, thereby inducing hyperglycemia (34). As reported, the antihyperglycemic
activity of natural products is predominantly due to their activity
in restoring pancreatic function by increasing insulin output
(11). Similarly, the data in the
present study confirmed that the WEs and AEs of Paecilomyces
tenuipes N45 suppressed the alloxan-elevated levels of serum
insulin. However, whether Paecilomyces tenuipes N45 reverses
increasing insulin secretion through the regeneration of damaged
β-cells requires further investigation.
Due to the increase in free fatty acid mobilization,
abnormally high serum lipid concentrations are were observed in the
diabetic animals. Compared with the alloxan-induced diabetic mice,
Paecilomyces tenuipes N45 extracts reduced serum LDL-C
concentrations, whereas the serum levels of HDL-C were markedly
enhanced 21 days following extract administration. The above
effects may be beneficial in preventing various complications,
including coronary heart diseases (35) and atherosclerosis (36).
The levels of liver glycogen, an important reserve
of glucose, were also measured in the present study. Administration
of the extracts, at all doses, enhanced the abnormally low levels
of liver glycogen in the diabetic mice, indicating that it may be a
target involved in Paecilomyces tenuipes N45-mediated
hypoglycemic activities.
ROS accumulate in diabetic patients due to
disequilibrium in the production and the scavenging effects on free
radicals (37,38). Abnormal high glucose concentrations
causes the autooxidation and autooxidative glycosylation of
proteins (20), which leads to
damage of proteins, lipids and nucleic acids (39,40).
The use of antioxidant compounds are considered an effective method
to prevent or inhibit pancreatic β-cell destruction caused by
alloxan (41). The data obtained
in the present study confirmed that the antioxidant activity of SOD
and GSH-Px in diabetes mellitus is associated with higher
concentrations of peroxide (42).
Paecilomyces tenuipes N45-mediated hypoglycemic activities
may be associated with its normalization effects on the levels of
SOD, MDA and GSH-Px in the plasma and liver.
In conclusion, the results of the present study
indicated that the extracts of Paecilomyces tenuipes N45
prevented the increased fasting plasma glucose levels induced by
alloxan. The plasma glucose lowering effects of the extracts may be
explained by improving blood glucose and insulin homeostasis,
enhancing the levels of liver glycogen and improving antioxidant
levels. This primary investigation on the antihyperglycemic,
antihyperlipidemic and antioxidant efficacy of Paecilomyces
tenuipes N45 extracts may assist in isolating the active
principles responsible for antidiabetic effects.
Acknowledgments
This study was supported by the National Science and
Technology Support Program of China (grant no. 2012BAL29B05), the
National Natural Science Foundation of China (grant no. 81402955)
and the Science and Technology Key Project (grant no.
20130201006ZY).
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu D, Fu P, Xie J, Chen CS, Yu D, Whelton
PK, He J and Gu D; MS for the InterASIA Collaborative Group:
Increasing prevalence and low awareness, treatment and control of
diabetes mellitus among Chinese adults: The InterASIA study.
Diabetes Res Clin Pract. 81:250–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Fu CW, Pan CY, Chen W, Zhan S,
Luan R, Tan A, Liu Z and Xu B: How do type 2 diabetes
mellitus-related chronic complications impact direct medical cost
in four major cities of urban China? Value Health. 12:923–929.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Qiu Q, Tan LL, Liu T, Deng XQ,
Chen YM, Chen W, Yu XQ, Hu BJ and Chen WQ: Prevalence and
determinants of diabetes and impaired fasting glucose among urban
community dwelling adults in Guangzhou, China. Diabetes Metab.
35:378–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kerner W and Brückel J; German Diabetes
Association: Definition, classification and diagnosis of diabetes
mellitus. Exp Clin Endocrinol Diabetes. 122:384–386. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You Q, Chen F, Wang X, Luo PG and Jiang Y:
Inhibitory effects of muscadine anthocyanins on α-glucosidase and
pancreatic lipase activities. J Agric Food Chem. 59:9506–9511.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winkler G, Hidvegi T, Vandorfi G, Balogh S
and Jermendy G: Risk-stratified screening for type 2 diabetes in
adult subjects: Results from Hungary. Diabetologia. 54:S119–S120.
2011.
|
|
8
|
Kania DS, Gonzalvo JD and Weber ZA:
Saxagliptin: A clinical review in the treatment of type 2 diabetes
mellitus. Clin Ther. 33:1005–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scheen AJ: Antidiabetic agents in subjects
with mild dysglycaemia: Prevention or early treatment of type 2
diabetes? Diabetes Metab. 33:3–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Novak M and Vetvicka V: Beta-glucans,
history and the present: Immunomodulatory aspects and mechanisms of
action. J Immunotoxicol. 5:47–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malviya N, Jain S and Malviya S:
Antidiabetic potential of medicinal plants. Acta Pol Pharm.
67:113–118. 2010.PubMed/NCBI
|
|
12
|
Dong Y, Jing T, Meng Q, Liu C, Hu S, Ma Y,
Liu Y, Lu J, Cheng Y, Wang D and Teng L: Studies on the
antidiabetic activities of Cordyceps militaris extract in
diet-streptozotocin-induced diabetic Sprague-Dawley rats. Biomed
Res Int. 2014:1609802014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukatsu T, Sato H and Kuriyama H:
Isolation, inoculation to insect host and molecular phylogeny of an
entomogenous fungus Paecilomyces tenuipes. J Invertebr Pathol.
70:203–208. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Lu J, Zhang Y, He J, Guo X, Tian G
and Jin L: Studies of macrophage immune-modulating activity of
polysaccharides isolated from Paecilomyces tenuipes. Int J Biol
Macromol. 43:252–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahn MY, Jung YS, Jee SD, Kim CS, Lee SH,
Moon CH, Cho SI, Lee BM and Ryu KS: Anti-hypertensive effect of the
Dongchunghacho, Isaria sinclairii, in the spontaneously
hypertensive rats. Arch Pharm Res. 30:493–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong IP, Nam SH, Sung GB, Chung IM, Hur H,
Lee MW, Kim MK and Guo SX: Chemical Components of Paecilomyces
tenuipes (Peck) Samson. Mycobiology. 35:215–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Federiuk IF, Casey HM, Quinn MJ, Wood MD
and Ward WK: Induction of type-1 diabetes mellitus in laboratory
rats by use of alloxan: Route of administration, pitfalls and
insulin treatment. Comp Med. 54:252–257. 2004.PubMed/NCBI
|
|
18
|
Park S, Kim da S, Kim JH, Kim JS and Kim
HJ: Glyceollin-containing fermented soybeans improve glucose
homeostasis in diabetic mice. Nutrition. 28:204–211. 2012.
View Article : Google Scholar
|
|
19
|
Subramanian R, Asmawi MZ and Sadikun A: In
vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects
of Andrographis paniculata extract and andrographolide. Acta
Biochim Pol. 55:391–398. 2008.PubMed/NCBI
|
|
20
|
Park JH, Park NS, Lee SM and Park E:
Effect of Dongchunghacho rice on blood glucose level, lipid profile
and antioxidant metabolism in streptozotocin-induced diabetic rats.
Food Science and Biotechnology. 20:933–940. 2011. View Article : Google Scholar
|
|
21
|
Wu SY, Wang GF, Liu ZQ, Rao JJ, Lü L, Xu
W, Wu SG and Zhang JJ: Effect of geniposide, a hypoglycemic
glucoside, on hepatic regulating enzymes in diabetic mice induced
by a high-fat diet and streptozotocin. Acta Pharmacol Sin.
30:202–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rubattu S, Pagliaro B, Pierelli G,
Santolamazza C, Castro SD, Mennuni S and Volpe M: Pathogenesis of
target organ damage in hypertension: Role of mitochondrial
oxidative stress. Int J Mol Sci. 16:823–839. 2014. View Article : Google Scholar
|
|
23
|
Baynes JW: Role of oxidative stress in
development of complications in diabetes. Diabetes. 40:405–412.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi LW, Liu EH, Chu C, Peng YB, Cai HX and
Li P: Anti-diabetic agents from natural products-an update from
2004 to 2009. Curr Top Med Chem. 10:434–457. 2010. View Article : Google Scholar
|
|
25
|
Yang BK, Jung YS and Song CH: Hypoglycemic
effects of Ganoderma applanatum and Collybia confluens exo-polymers
in streptozotocin-induced diabetic rats. Phytother Res.
21:1066–1069. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang G, Huang Y, Bian Y, Wong JH, Ng TB
and Wang H: Hypoglycemic activity of the fungi Cordyceps militaris,
Cordyceps sinensis, Tricholoma mongolicum and Omphalia lapidescens
in streptozotocin-induced diabetic rats. Appl Microbiol Biotechnol.
72:1152–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nam SH, Li CR, Li ZZ, Fan MZ, Kang SW, Lee
KG, Yeo JH, Hwang JS, Choi JY, Han SM and Lee KM: Long-term
preservation, regeneration and cultivation of Paecilomyces tenuipes
(Peck) Samson (Ascomycetes), an entomopathogenic fungus inoculated
into the silkworm larva of Bombyx mori. Int J Med Mushrooms.
13:83–91. 2011. View Article : Google Scholar
|
|
28
|
Chung EJ, Choi K, Kim HW and Lee DH:
Analysis of cell cycle gene expression responding to
acetoxyscirpendiol isolated from Paecilomyces tenuipes. Biol Pharm
Bull. 26:32–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takata T, Tanaka T, Yahagi N, Yahagi R,
Tsuchida H, Ishigaki Y, Tomosugi N, Fushiya S, Takano F and Ohta T:
The liquid culture filtrates of entomogenous fungus Paecilomyces
tenuipes and its glycoprotein constituent protects against anemia
in mice treated with 5-fluorouracil. Biol Pharm Bull. 31:1565–1573.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
lbarrola DA, Hellión-lbarrola MC,
Montalbetti Y, Heinichen O, Alvarenga N, Figueredo A and Ferro EA:
Isolation of hypotensive compounds from Solanum sisymbriifolium
Lam. J Ethnopharmacol. 70:301–307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du L, Liu Y, Liu C, Meng Q, Song J, Wang
D, Lu J and Teng L, Zhou Y and Teng L: Acute and subchronic
toxicity studies on safety assessment of Paecilomyces tenuipes N45
extracts. Comb Chem High Throughput Screen. 18:809–818. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Balamurugan K, Nishanthini A and Mohan VR:
Antidiabetic and antihyperlipidaemic activity of ethanol extract of
Melastoma malabathricum Linn. Leaf in alloxan induced diabetic
rats. Asian Pac J Trop Biomed. 4(Suppl 1): S442–S448. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakahara Y, Ozaki K, Sano T, Kodama Y and
Matsuura T: Assessment of alloxan-induced diabetic rats as a
periodontal disease model using a selective cyclooxygenase (COX)-2
inhibitor. J Toxicol Pathol. 27:123–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar V, Mahdi F, Khanna AK, Singh R,
Chander R, Saxena JK, Mahdi AA and Singh RK: Antidyslipidemic and
antioxidant activities of Hibiscus rosa sinensis root extract in
Alloxan induced diabetic rats. Indian J Clin Biochem. 28:46–50.
2013. View Article : Google Scholar :
|
|
35
|
Ripley DP, Motwani M, Plein S and
Greenwood JP: Established and emerging cardiovascular magnetic
resonance techniques for the assessment of stable coronary heart
disease and acute coronary syndromes. Quant Imaging Med Surg.
4:330–344. 2014.PubMed/NCBI
|
|
36
|
Chinetti-Gbaguidi G, Colin S and Staels B:
Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 12:10–17.
2015. View Article : Google Scholar
|
|
37
|
Yao Y, Sang W, Zhou M and Ren G:
Antioxidant and alpha-glucosidase inhibitory activity of colored
grains in China. J Agric Food Chem. 58:770–774. 2010. View Article : Google Scholar
|
|
38
|
Adisa RA, Choudhary MI and Olorunsogo OO:
Hypoglycemic activity of Buchholzia coriacea (Capparaceae) seeds in
streptozotocin-induced diabetic rats and mice. Exp Toxicol Pathol.
63:619–625. 2011. View Article : Google Scholar
|
|
39
|
Sharma M, Akhtar N, Sambhav K, Shete G,
Bansal AK and Sharma SS: Emerging potential of citrus flavanones as
an antioxidant in diabetes and its complications. Curr Top Med
Chem. 15:187–195. 2015. View Article : Google Scholar
|
|
40
|
Monea A, Mezei T, Popsor S and Monea M:
Oxidative stress: A link between diabetes mellitus and periodontal
disease. Int J Endocrinol. 2014:9176312014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sebai H, Selmi S, Rtibi K, Souli A, Gharbi
N and Sakly M: Lavender (Lavandula stoechas L.) essential oils
attenuate hyperglycemia and protect against oxidative stress in
alloxan–induced diabetic rats. Lipids Health Dis. 12:1892013.
View Article : Google Scholar
|
|
42
|
Suryawanshi NP, Bhutey AK, Nagdeote AN,
Jadhav AA and Manoorkar GS: Study of lipid peroxide and lipid
profile in diabetes mellitus. Indian J Clin Biochem. 21:126–130.
2006. View Article : Google Scholar : PubMed/NCBI
|