Introduction
Prostate cancer (PCa) is the most commonly diagnosed
malignancy and the second leading cause of cancer-associated
mortality in men in America and England. (1) In Asia, the incidence and morbidity
rates of PCa have increased markedly in previous decades. However,
a large proportion of new cases are at an advanced stage at the
time of diagnosis. Androgen deprivation therapy (ADT) is the first
choice of treatment, however, a major obstacle is that the
sensitivity of PCa to ADT treatment is reduced over time and
eventually develops into castration-resistant prostate cancer
(CRPC). At present, the efficacy of other treatment options for
CRPC, including chemotherapy, radiotherapy and immunotherapy is
insufficient.
The mechanism causing CRPC to be refractory to
current treatment options remains to be fully elucidated. It has
been suggested that the possible mechanism is due to the
overexpression of survivin (2–4).
Survivin (baculoviral inhibitor of apoptosis protein repeat
containing 5) is a member of the inhibitor of apoptosis protein
(IAP) family, which has been implicated in inhibiting apoptosis and
controlling mitotic progression. Previous evidence that survivin is
overexpressed in the majority of types of human cancer (5–10),
but absent in normal adult tissues (11) has led to the proposal of targeting
survivin as a promising alternative treatment for cancer, including
CRPC (12,13).
MicroRNAs (miRNAs), a set of small, non-coding RNAs
of 21–23 nucleotides in length, can regulate target genes via the
degradation of mRNA or by suppressing translation and,
consequently, acting as tumor suppressor or promoter (oncomiR) to
regulate cancer initiation and progression. For example, miR-155
functions as an oncomiR in breast cancer by targeting suppressor of
cytokine signaling-1 (14).
miR-34a inhibits PCa stem cell metastasis by directly repressing
cluster of differentiation 44 (15). miR-494 acts as a tumor suppressor
in different types of cancers by targeting B cell lymphoma (Bcl)-2
interacting mediator of cell death (BIM) (16), KIT (17), and survivin (18) to inhibit cancer proliferation and
metastasis.
The function of miR-494 in PCa remains to fully
elucidated. In the present study investigated whether miR-494
targeted survivin and whether miR-494 and survivin shRNA in
combination had a synergistic effect on the suppression of PCa
growth.
Materials and methods
Cell culture
The present study was approved by the ethics
committee of the Second Affiliated Hospital of Soochow University
(Suzhou, China). The PC-3 human CRPC cell line and RWPE-1 human
normal prostate epithelial cell line were obtained from the Chinese
Academy of Science (Shanghai, China). The PC-3 cells were
maintained in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), containing penicillin (25 U/ml; Gibco;
Thermo Fisher Scientific, Inc.), streptomycin (25 g/ml; Thermo
Fisher Scientific, Inc.), 1% L-glutamate (Thermo Fisher Scientific,
Inc.), and 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.), at 37°C with 5% CO2 in a humidified atmosphere.
The RWPE-1 cells were maintained in complete
keratinocyte-serum-free medium (K-SFM), containing 50 µg/ml
BPE (Gibco; Thermo Fisher Scientific, Inc.) and 5 ng/ml EGF (Gibco;
Thermo Fisher Scientific, Inc.), with penicillin (25 U/ml) and
streptomycin (25 g/ml), at 37°C with 5% CO2 in a
humidified atmosphere.
Plasmids and adenovirus
Human genomic DNA (Promega Corporation, Madison, WI,
USA) was used as template DNA. The sequence of the hsa-miR-494
genomic clone was designed, according to the information in miRbase
(http://www.mirbase.org/). The region containing
the miR-494 stem-loop sequence was amplified using polymerase chain
reaction (PCR) and was inserted into the pDC315-EGFP plasmid for
the overexpression of miR-494. An adenovirus packaging system
(AdMax™) was used to produce the adenovirus. For RNA interference
(RNAi), a survivin shRNA recombined adenovirus vector was
constructed and preserved in the Department of Urology, The Second
Affiliated Hospital of Soochow University (Suzhou, China). When the
miR-494 was co-transfected with survivin shRNA (sequence, 5′-cac
cgg acc acc gca tct cta cat tca aga cgt gta gag atg cgg tgg tcc ttt
tttg-3′), the virus derived from each plasmid was reduced by half.
The sequences of the primers used are listed in Table I, they were obtained from Sangon
Biotech Co., Ltd., Shanghai, China. The cells were infected with
the adenovirus with 8 µg/µl polybrene (Sigma-Aldrich,
St. Louis, MO, USA) at a confluence of 60%.
 | Table IPrimers used for cloning, qPCR and
RT-qPCR. |
Table I
Primers used for cloning, qPCR and
RT-qPCR.
| Primer | Sequence | Use |
|---|
| hsa-miR-494 RT |
5′-gtcgtatccagtgcagggtccgaggtattcgcactggatacgacgaggtttc-3′ | qPCR |
| hsa-miR-494 Up |
5′-gcgcgcgctgaaacatac-3′ | qPCR |
| hsa-miR-494
Down |
5′-gggtccgaggtattcgcact-3′ | qPCR |
| u6 RT |
5′-aaaatatggaacgcttcacgaatttg-3′ | qPCR |
| u6 Up |
5′-ctcgcttcggcagcacatatact-3′ | qPCR |
| u6 Down |
5′-acgcttcacgaatttgcgtgtc-3′ | qPCR |
|
hsa-mir-494-EcoRI F |
5′-cggccgcgactctagttgattttttttgtttgttttttgatcagtgctaatcttcg-3′ | Cloning |
|
hsa-mir-494-EcoRI R |
5′-ataagcttgatatcggacgcatggcacgctgtc-3′ | Cloning |
| survivin shRNA
Up |
5′-caccggaccaccgcatctctaca ttcaagacg
tgtagagatgcggtggtccttttttg-3′ | Cloning |
| survivin shRNA
Down |
5′-agctcaaaaaaggaccaccgcatctctacacgtcttgaatgtagagatgcggtggtcc-3′ | Cloning |
| Survivin F |
5′-gcatgggtgccccgacgttg-3′ | RT-qPCR |
| Survivin R |
5′-gctccggccagaggctcaa-3′ | RT-qPCR |
| GAPDH F |
5′-tgatgacatcaagaaggtggtgaa-3′ | RT-qPCR |
| GAPDH R |
5′-tccttggaggccatgtgggcc-3′ | RT-qPCR |
| β-actin F |
5′-gtccaccgcaaatgcttcta-3′ | RT-PCR |
| β-actin R |
5′-tgctgtcaccttcaccgttc-3′ | RT-PCR |
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) for determination of miRNA
levels
RNA was extracted from the cells using an mirVana™
miRNA Isolation kit (Ambion; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. A total of 2 µg
RNA of each sample was used for the RT reaction. The subsequent
qPCR was performed using miRNA-specific primers to analyze the
miRNA expression levels, with U6 snRNA used as an internal control.
The SYBR FAST qPCR kit (Kapa Biosystems, Inc., Wilmington, MA, USA)
was used with 10 µl Master mix, 0.4 µl forward primer
(10 µM) and 0.4 µl reverse primer (10 µM) and
1 µl cDNA. Double distilled water (8.2 µl) was added
to bring the total volume to 20 µl. The thermocycling steps
were as follows: 95°C for 3 min; 45 cycles of 95°C for 3 sec, 60°C
for 20 sec and 72°C for 1 sec; and a final extension step at 72°C
for 5 min. The sequences of the primers used are listed in Table I.
RT-qPCR
The cells were collected at a confluence of 90% and
total RNA was extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and 2 µg total RNA from each
sample was converted into complementary DNA using a commercially
available RT-qPCR kit (Promega Corporation). The resultant
complementary DNAs were used in the qPCR reactions using
gene-specific primers, and the products were analyzed using gel
electrophoresis. The sequence of the primers used are listed in
Table I.
Western blot analysis
The cells were washed with phosphate-buffered saline
(PBS) and lysed in radioimmunoprecipitation assay buffer (Thermo
Fisher Scientific, Inc.). Proteins (30 µg) were separated by
10% SDS-PAGE (Sangon Biotech Co., Ltd.) and then transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% non-fat milk in PBS with
0.05% Tween 20 (Thermo Fisher Scientific, Inc.) for 1 h at room
temperature, and then incubated with diluted primary antibodies
overnight at 4°C. The antibodies were monoclonal rabbit anti-human
antibodies against survivin (Cell Signaling Technology, Inc.,
Danvers, MA, USA; cat. no. 2808; 1:2,000) or monoclonal mouse
anti-human antibodies against GAPDH (Abcam, Cambridge, MA, USA;
cat. no. ab8245; 1:5,000). The blots were then incubated with
horseradish peroxidase-conjugated secondary antibodies for 1 h at
room temperature, washed and developed using an enhanced
chemiluminescence system (Gel Doc XR+; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The secondary antibodies were horse anti-mouse
and goat anti-rabbit, diluted 1:5,000 (Cell Signaling Technology,
Inc.; cat. nos. 7076 and 7074, respectively). The expression levels
of survivin were compared with those of β-actin for further
quantitative analysis using Image J software, version 1.48
(National Institutes of Health, Bethesda, MD, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cells were seeded in a 96-well plate at a
concentration of 1×104 cells/well, and were maintained
at 37°C for 24, 48 and 72 h following transfection. The cells were
then treated with MTT (0.5 mg/ml) for 4 h at 37°C. The absorbance
at 570 nm was determined using a microplate reader (Model 550;
Bio-Rad Laboratories, Inc.).
Flow cytometric analysis
Cell cycle and apoptosis were determined using
either propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ,
USA) staining or an Annexin V-Allophycocyanin (APC) detection kit
from BD Pharmingen (San Diego, CA, USA). At 72 h post-transfection,
the cells were washed with cold PBS, and either stained with PI or
Annexin V-APC/PI double staining, and analyzed using flow
cytometry, according to the manufacturer's protocol.
Subcutaneous injection mouse model
All animal experiments were performed, according to
a protocol approved by the Ethics Committee of the Second Hospital
of Soochow University and in compliance with national and European
regulations. A total of 25 male nude mice (age, 6–8 weeks; weight,
25–30 g) were purchased from the Experimental Animal Center of
Soochow University. They were housed under standard conditions with
a 12-h light/dark cycle at 23±3°C, 55±5% relative humidity and
access to food and water ad libitum. The mice were randomly
assigned into the following groups, each containing five mice: PBS
group; scr1 group; oe494 group; shSur group and oe494+shSur group.
The pretreated PC-3 cells (107/mouse) were mixed with
Matrigel (BD Biosciences) were subcutaneously injected into the
right axillary space of each nude mice. When the tumors were
palpable, sliding calipers were used to measure the maximum
longitude diameter and transverse diameter of each tumor every 5
days. The tumor volumes were calculated according to the following
formula: Volume (cm3) = ab2 / 2. The mice
were then sacrificed 55 days following injection by cervical
dislocation, and tumor tissue samples were harvested and used for
western blot analysis and immunohistochemical staining.
Immunohistochemistry
The tumor tissues were fixed in 4% neutral-buffered
paraformaldehyde (Sigma-Aldrich) and embedded in paraffin
(Sigma-Aldrich). The primary antibodies were: monoclonal rabbit
anti-human against survivin (dilution, 1:100); monoclonal rabbit
anti-human against Bcl-2-associated X protein (BAX; Cell Signaling
Technology, Inc.; cat. no. 5023; 1:100); monoclonal rabbit
anti-human against BCL2 (Cell Signaling Technology, Inc.; cat. no.
3796; 1:100); and monoclonal rabbit anti-human against caspase 3
(Biogot Technology Co., Ltd., Nanjing, China; cat. no. BS1518;
1:100) were used for staining. Primary antibodies were recognized
by goat anti-rabbit biotinylated secondary antibody (Vector
Laboratories, Inc., Burlingame, CA, USA; cat. no. BA-1000; 1:1,000)
and visualized using a VECTASTAIN ABC peroxidase system and
peroxidase substrate DAB kit (Vector Laboratories, Inc.).
Semi-quantification of the positive staining signals was performed
using ImageJ software.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Student's t-test and analysis of variance were
used to determine significant differences using SPSS 19.0 (IBM
SPSS, Armonk, NY, USA). P<0.05 (two-sided) was considered to
indicate a statistically significant difference.
Results
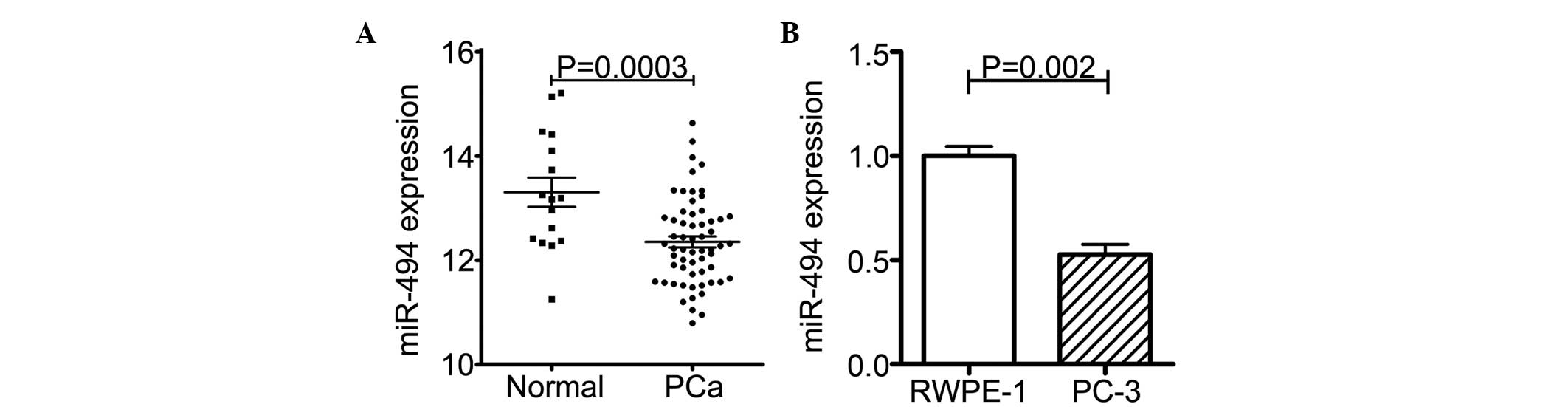
Expression levels of miR-494 are lower in
PCa samples and PC-3 cells
Previous studies have indicated that the expression
of miR-494 is decreased in different types of cancer and acts as a
tumor suppressor (19,20). Therefore, the present study aimed
to determine whether the expression of miR-494 in PCa is also lower
than that in normal prostate tissue.
An NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.giv/gds) dataset search
was performed and, in the GSE8126 dataset (20), 60 PCa clinical samples and 16
normal prostate samples were collected and subjected to an
miRNA-specific expression array. As expected, the expression of
miR-494 was markedly lower in the PCa samples, compared with the
normal prostate tissues (Fig.
1A).
The PC-3 CRPC cell line was then randomly selected,
and the expression levels of miR-494 in the PC-3 cells and in the
RWPE-1 normal prostate epithelial cell line were determined using
RT-qPCR. The results showed that the expression of miR-494 was
reduced in the PC-3 cells, compared with that in the RWPE-1 cells
(Fig. 1B).
Taken together, the results (shown in Fig. 1A and B) demonstrated lower
expression levels of miR-494 in PCa.
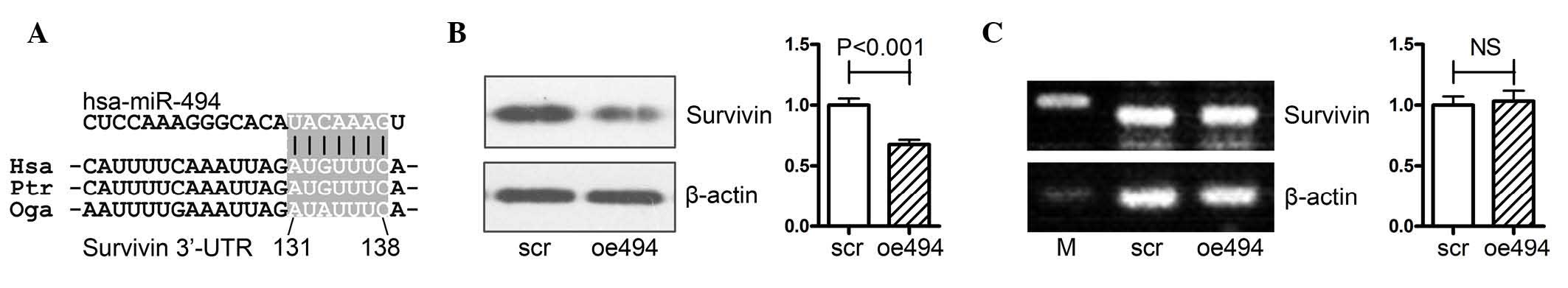
miR-494 targets survivin at the
translational level
The survivin gene is a well-known oncogene, which
belongs to the anti-apoptosis gene family; its expression is
correlated with tumor malignancy and progression in a variety of
types of cancer, as well as PCa (5–10).
It has been reported that miR-494 negatively regulates the gene
expression of survivin in acute myeloid leukemia cells (18), and the bioinformatics analysis
performed in the present study also indicated that miR-494
regulated survivin via binding to its 3′-untranslated region
(Fig. 2A). However, this effect
has not yet been clarified in prostate cancer, therefore, the
present study aimed to confirm this effect in PCa.
PCa PC-3 cells were transfected with either an
miR-494 overexpression (oe494) or negative control (scr)
adenovirus, following which the mRNA and protein expression levels
of survivin were determined using Western blot and RT-qPCR
analyses. As shown in Fig. 2B,
overexpression of miR-494 significantly decreased the protein
expression of survivin in PC-3 cells. However, the mRNA expression
of survivin was not affected by overexpression of miR-494 (Fig. 2C).
Taken together, the results (as shown in Fig. 2A–C) showed that miR-494 targeted
survivin at the translational level in PCa.
miR-494 combined with survivin shRNA has
synergistic effects on antiproliferation, inducing cell cycle
arrest and cell apoptosis in PC-3 cells
The fact that miR-494 is decreased in PCa indicated
that it may contribute to the progression of PCa. Therefore, the
present study examined the effect of miR-494 and survivin shRNA on
cell growth, cell cycle arrest and apoptosis in PCa PC-3 cells. The
PC-3 cells were transfected with either miR-494 (oe494) or survivin
shRNA (shSur) or the two together (oe494+shSur). Their negative
control groups were scr1, scr2 and scr1+scr2, respectively, and a
PBS group served as a blank control.
Cell proliferation was measured using an MTT assay.
The results indicated that all the three groups inhibited PC-3 cell
proliferation, compared with their control groups. Notably, the
oe494+shSur group had increased antiproliferation effects, compared
with the oe494 or shSur groups alone (Fig. 3A).
 | Figure 3Synergistic effects of miR-494
combined with survivin shRNA on antiproliferation, induction of
cell cycle arrest and apoptosis in PC-3 cells. The PC-3 cells were
transfected with an adenovirus carrying either miR-494 or survivin
shRNA, or both. (A) A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
was used to detect cell proliferation. Flow cytometry with PI
staining or Annexin-V/PI staining were used to detect (B and C)
cell cycle arrest and (D and E) apoptosis. Data are expressed as
the mean ± standard deviation. **P<0.01,
***P<0.001. miR, microRNA; shRNA, short hairpin RNA;
PI, propidium iodide; PBS, phosphate-buffered saline; OD, optical
density. |
Flow cytometry was performed to examine the cell
cycle and cell apoptosis. The results showed that there was
significant cell cycle arrest at the G2/M phase in the
oe494, shSur and oe494+shSur groups. The percentage of cells in the
G2/M phase in these groups were (8.19±3.23, 34.56±4.06
and 63.87±4.33%, respectively (Fig. 3B
and C). Similarly, cell apoptosis, detected using Annexin-V/PI
staining, showed that the percentages of cell apoptosis in these
groups were 23.79±4.36, 27.38±5.94 and 49.98±5.85%, respectively
(Fig. 3D and E).
Taken together, the results (as shown in Fig. 2A–E) indicated that miR-494 and
survivin shRNA were able to inhibit proliferation, and induce cell
cycle arrest and cell apoptosis in PCa PC-3 cells. Of note, miR-494
combined with survivin shRNA had a more marked effect. These
results indicated that targeting a single gene using two methods
simultaneously had synergetic effects.
miR-494+survivin shRNA has a synergistic
effect on inhibiting the protein expression of survivin in PCa
The present study hypothesized that the marked
effect of the combination of miR-494 and survivin shRNA is due to a
further decrease in the protein expression of survivin. The PC-3
cells were transfected with either miR-494 (oe494) or survivin
shRNA (shSur), or the two together (oe494+shSur). Although the mRNA
expression of survivin remained unchanged in the oe494+shSur group,
compared with the shSur group (Fig.
4A), the combination group had significantly lower protein
levels of survivin, compared with either the oe494 group or the
shSur group alone (Fig. 4B and C).
This indicated that simultaneously suppressing survivin gene
expression using different methods may have synergistic
effects.
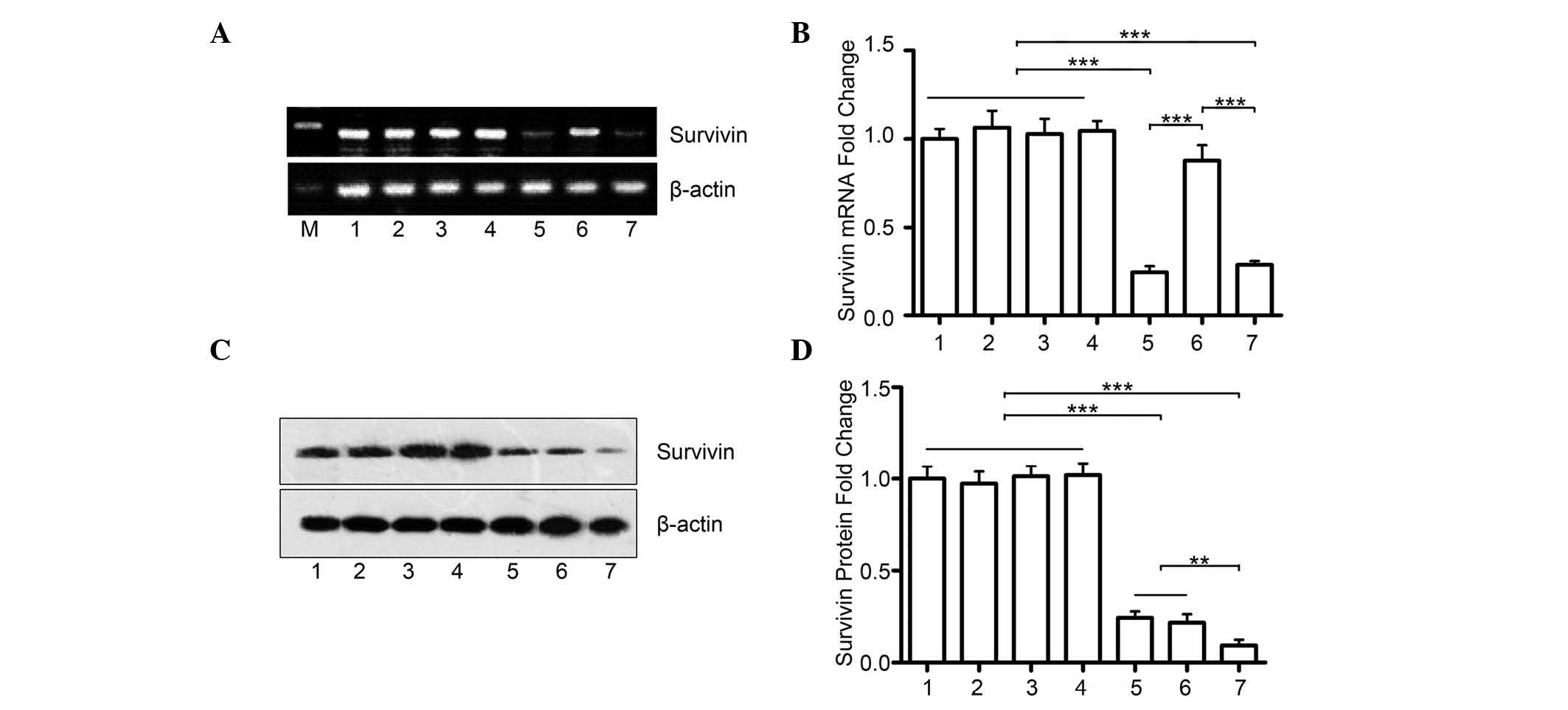 | Figure 4miR-494+survivin shRNA have a
synergistic effect on inhibiting the protein expression of survivin
in prostate cancer. (A) Reverse transcription-quantitative
polymerase chain reaction analyses revealed that co-transfection
with survivin shRNA and miR-494 did not further decrease the mRNA
levels of survivin mRNA, compared with the group transfected with
shRNA alone. (B) Quantitative data. (C) Transfection with miR-494
or survivin shRNA alone inhibited the protein expression of
survivin in PC-3 cells, which were detected using Western blot
analysis. However, the combination of the two had marked
synergistic effects on the protein expression of survivin. (D)
Quantitative data. 1, PBS; 2, scr1; 3, scr2; 4, scr1+scr2; 5,
shSur; 6, oe494; 7, shSur+oe494. Data are expressed as the mean ±
standard deviation. **P<0.01,
***P<0.001. miR, microRNA; shRNA, short hairpin RNA;
PBS, phosphate-buffered saline; M, marker. |
miR-494+survivin shRNA has a synergistic
effect on PCa growth in vivo
As the in vitro data showed that either
miR-494 or survivin shRNA effectively inhibited cell growth and
induced cell apoptosis in PC-3 cells, and the combination of the
two was more effective, the present study investigated whether the
same effects were observed in vivo. A total of 25 male nude
mice were divided into five groups: PBS, scr1, oe494, shSur and
oe494+shSur. A total of 107 treated cells were
subcutaneously injected into the right axillary space of each nude
mouse. Tumor size was monitored every 5 days and, 55 days
post-injection, the mice were sacrificed, tumors were collected and
tumor volume was calculated. The results demonstrated that tumors
in the miR-494, survivin shRNA or the combined group were smaller,
compared with those in the control (Fig. 5A and B). In addition, the combined
group had a more marked effect on cancer growth, compared with the
individual groups (Fig. 5A and B).
Although the expression levels of survivin in the tumor tissues of
all group were reduced, the expression was reduced most markedly in
the combined group, detected using Western blot analysis and
immunohistochemical staining (Fig 5C
and D), which further confirmed the results derived from the
in vitro experiments.
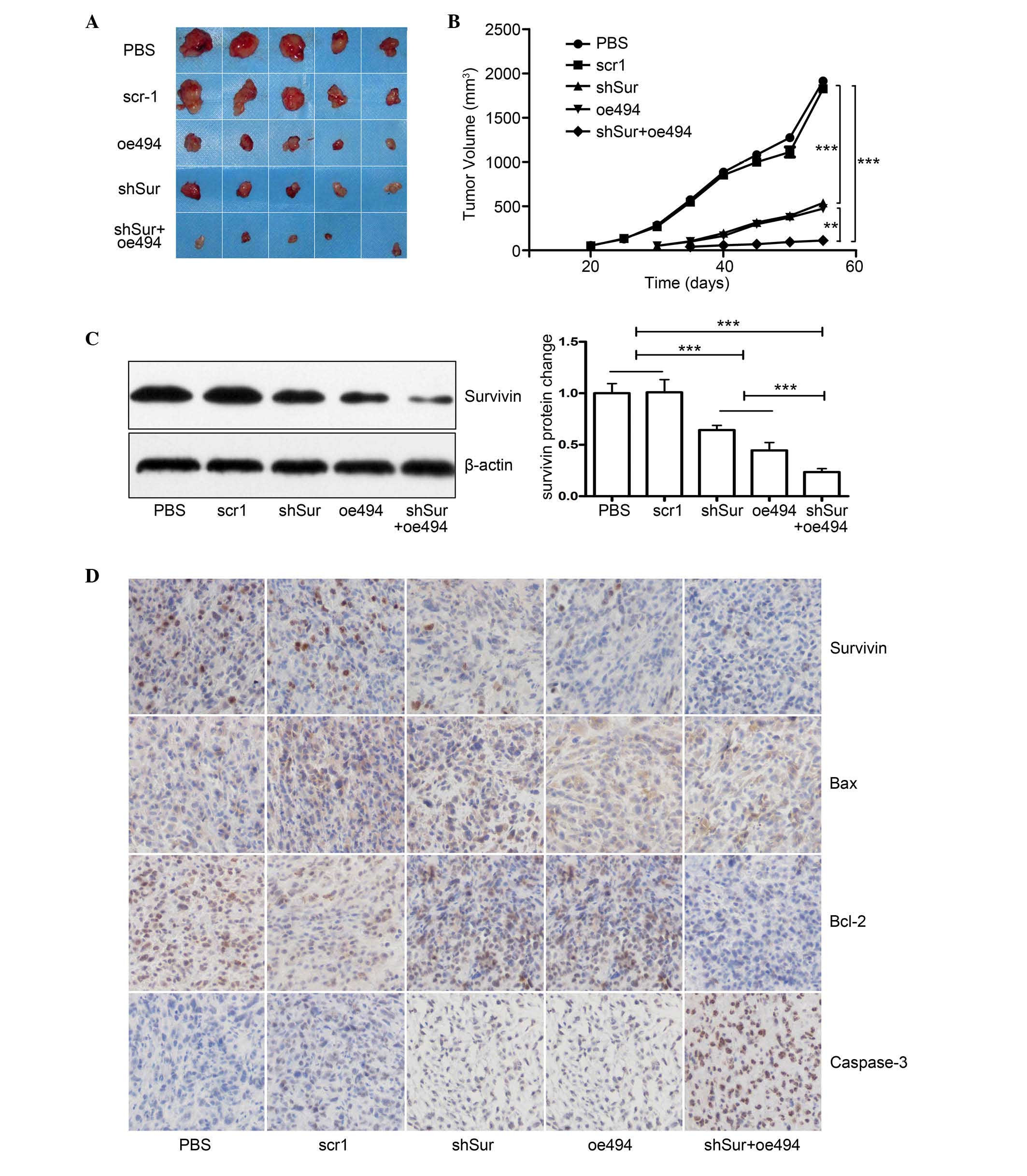 | Figure 5Synergistic effect of
miR-494+survivin shRNA on PCa growth in vivo. PCa PC-3 cells
were transfected with either miR-494 overexpression or survivin
shRNA adenovirus or both, and were subcutaneously injected into
6–8-week-old male nude mice. Tumor size was monitored every 5 days
and, 55 days post-injection, the mice were sacrificed and the
tumors were harvested for evaluation. (A) Gross morphology of
tumors in each group. (B) Tumor growth curve shows that, although
miR-494 or survivin shRNA alone inhibited the growth of xenograft
tumors, co-transfection with miR-494 and survivin shRNA had a
synergistic effect, compared with either miR-494 or survivin shRNA
alone (P<0.05). Expression levels of survivin in xenograft
tumors were analyzed using (C) Western blot analysis and (D)
immunohistochemical staining (magnification, ×200) demonstrating
that survivin and Bcl-2 expression levels were decreased and Bax
and caspase-3 increased in the shSur and oe494 groups. Combination
of shSur + oe494 had synergistic effect on expression levels of
these proteins. Cell nuclei are stained blue and proteins are
stained brown. The results showed that miR-494, survivin shRNA or
both inhibited the protein expression of survivin, and the combined
group had synergetic effects (P<0.05). Data are expressed as the
mean ± standard deviation. **P<0.01,
***P<0.001. PCa, prostate cancer; miR, microRNA;
shRNA, short hairpin RNA; PBS, phosphate-buffered saline; M,
marker; Bcl-2, B-cell lymphoma 2; Bax, BCL2-associated X
protein. |
Taken together, these data indicated that miR-494
and survivin shRNA inhibited the expression of survivin and
suppressed PCa cell growth in vitro and in vivo.
Combining miR-494 with survivin shRNA had more significant effects
on the suppressing the gene expression of survivin and PCa growth,
compared with either the mir-494 or survivin shRNA treatment
groups. The in vitro and in vivo data obtained in the
present study confirmed that simultaneously suppressing the gene
expression of survivin using different methods may have synergistic
effects.
Discussion
The mechanisms involved in the carcinogenesis,
progression and metastasis of PCa are complex. Substantial
evidences has indicated that oncogenes, anti-oncogenes, microRNAs
and long non-coding RNA are involved in PCa. However, their
individual roles have been considered less important, than androgen
receptor (AR), as AR target therapy, which constitutes the ADT
strategy, is the mainstay for the treatment of advanced PCa. At
present, no single oncogene or anti-oncogene target therapy has
been found to be as effective as ADT for used to treat PCa in
clinical settings. The primary reason for this is that the
gene-based regulatory pathways are complex. For example, one gene
can regulate the function of several downstream genes, and the gene
itself is also controlled by multiple upstream genes. However, it
is difficult to determine which oncogene or anti-oncogene is key in
PCa, particularly in the progression of CRPC.
The survivin gene, a member of the IAP family, has
been confirmed to be overexpressed in almost all types of cancer
cell, which include radiation resistant (10,21,22)
and drug resistant (23–25) cancer cells, as well as CRPC cells
(26,27). Inhibiting the gene expression of
survivin suppresses PCa cell growth, induces apoptosis and enhances
radiation and drug sensitivity in PCa cells, as well as in other
types of cancer cell (28). These
findings indicate that survivin may be a potential useful target
for anticancer intervention. A number of anti-survivin strategies,
including the use of the antisense oligonucleotide, LY2181308
(27), small interfering (si)RNA
(29) and locked nucleic acid
siRNA-based strategies (30) have
been reported to successfully reduce the expression of survivin,
inducing cell apoptosis and enhancing chemosensitivity in various
types of cancer in vitro, which include CRPC (31). However, these strategies have only
yielded a partial positive response in clinical trials, which may
be due to the inhibition of survivin by a single agent being
insufficient Although the reduction in the protein expression of
survivin has been reported to be at least 30% upon the treatment
with LY2181308, to achieve a more marked effect, >30% inhibition
from baseline is required (27).
This suggests that directly knocking down survivin via one method
has only a limited effect in suppressing cancer proliferation.
As cancer cell proliferation is regulated by
multiple genes, whether knocking down two oncogenes simultaneously
or knocking down one oncogene combined with the overexpression of
another anti-oncogene has more marked effects has been
investigated. For example, simultaneously knocking down survivin
and vascular endothelial growth factor has shown synergistic
effects on inhibiting in vitro cell proliferation and in
vivo tumor growth in pancreatic cancer cell (32). In addition, survivin knockdown
combined with apoptin over-expression inhibits cell growth
significantly in HeLa cells and HepG2 cells (33). The co-expression of
survivin-specific siRNA and wild-type p53 have also been observed
to significantly inhibit PCa cell proliferation in vitro and
in vivo (34).
These previous reports indicate that the effects of
simultaneously controlling the expression of two genes are more
marked, compared with the effect of controlling one individual gene
for suppressing cancer cell growth. As one target gene is
controlled by multiple mechanisms, including DNA amplification,
mRNA translation and protein modification (35), whether inhibiting one gene via two
methods has more advanced effects remains to be fully
elucidated.
Our bioinformatics analysis and experimental results
confirmed that miR-494 targets survivin in PCa. This is consistent
with a previous report that miR-494 induces cell apoptosis by
suppressing the gene expression of survivin in AML cells (18). Various reports have also shown that
miR-494 is downregulated in multiple types of cancer, including
liver cancer (36) and pancreatic
cancer, as well as in PCa (13).
Furthermore, miR-494 inhibits cell proliferation and induces cell
apoptosis by regulating the expression of multiple genes, including
KIT (17), BIM (16), C-X-C chemokine receptor type 4
(37) and survivin (18). The present study investigated the
role of miR-494 and its interaction with survivin in PCa growth.
The results indicated that miR-494 was decreased in PCa tissues and
in the PC-3 cell line. Overexpression of miR-494 was found to
inhibit cell proliferation and induce cell apoptosis in PC-3 cells
by inhibiting the expression of survivin, and its activity is
similar to that of survivin shRNA. Notably, simultaneous
transfection with miR-494 and survivin shRNA had synergistic
effects on the expression of survivin and on the growth of the PC-3
cells in vitro and in vivo.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472776) for
Professor D Yang, Dr J Zhu and Professor Y Shan, and the Natural
Science Foundation of Jiangsu Province (grant no. BK2008167) for
Professor D Yang and Professor Y Shan. It was also funded by the
Priority Academic Program Development of Jiangsu Higher Education
Institutes for Dr J Zhu, Professor Y Shan and Professor D Yang, the
Specialized Research Fund for the Doctoral Program of Higher
Education (grant no. 20133201110016) for Professor Y Shan,
Professor D Yang, Dr J Zhu and Dr Y Zang, and the Preponderant
Discipline Construction Funding of the Second Affiliated Hospital
of Soochow University.
References
|
1
|
Oliver SE, Gunnell D and Donovan JL:
Comparison of trends in prostate cancer mortality in England and
Wales and the USA. Lancet. 355:1788–1789. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang M, Latham DE, Delaney MA and
Chakravarti A: Survivin mediates resistance to antiandrogen therapy
in prostate cancer. Oncogene. 24:2474–2482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krajewska M, Krajewski S, Banares S, Huang
X, Turner B, Bubendorf L, Kallioniemi OP, Shabaik A, Vitiello A,
Peehl D, et al: Elevated expression of inhibitor of apoptosis
proteins in prostate cancer. Clin Cancer Res. 9:4914–4925.
2003.PubMed/NCBI
|
|
4
|
Shariat SF, Lotan Y, Saboorian H, Khoddami
SM, Roehrborn CG, Slawin KM and Ashfaq R: Survivin expression is
associated with features of biologically aggressive prostate
carcinoma. Cancer. 100:751–757. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ito T, Shiraki K, Sugimoto K, Yamanaka T,
Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, et
al: Survivin promotes cell proliferation in human hepatocellular
carcinoma. Hepatology. 31:1080–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sui L, Dong Y, Ohno M, Watanabe Y,
Sugimoto K and Tokuda M: Survivin expression and its correlation
with cell proliferation and prognosis in epithelial ovarian tumors.
Int J Oncol. 21:315–320. 2002.PubMed/NCBI
|
|
8
|
Zaffaroni N, Pennati M, Colella G, Perego
P, Supino R, Gatti L, Pilotti S, Zunino F and Daidone MG:
Expression of the anti-apoptotic gene survivin correlates with
taxol resistance in human ovarian cancer. Cell Mol Life Sci.
59:1406–1412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu B, Mu Y, Cao C, Zeng F, Schneider S,
Tan J, Price J, Chen J, Freeman M and Hallahan DE: Survivin as a
therapeutic target for radiation sensitization in lung cancer.
Cancer Res. 64:2840–2845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kennedy SM, O'Driscoll L, Purcell R,
Fitz-Simons N, McDermott EW, Hill AD, O'Higgins NJ, Parkinson M,
Linehan R and Clynes M: Prognostic importance of survivin in breast
cancer. Br J Cancer. 88:1077–1083. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kishi H, Igawa M, Kikuno N, Yoshino T,
Urakami S and Shiina H: Expression of the survivin gene in prostate
cancer: Correlation with clinicopathological characteristics,
proliferative activity and apoptosis. J Urol. 171:1855–1860. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang M, Mukherjee N, Bermudez RS, Latham
DE, Delaney MA, Zietman AL, Shipley WU and Chakravarti A:
Adenovirus-mediated inhibition of survivin expression sensitizes
human prostate cancer cells to paclitaxel in vitro and in vivo.
Prostate. 64:293–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayashi N, Asano K, Suzuki H, Yamamoto T,
Tanigawa N, Egawa S and Manome Y: Adenoviral infection of survivin
antisense sensitizes prostate cancer cells to etoposide in vivo.
Prostate. 65:10–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu
H, Liu MF and Wang ED: MicroRNA-155 functions as an OncomiR in
breast cancer by targeting the suppressor of cytokine signaling 1
gene. Cancer Res. 70:3119–3127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romano G, Acunzo M, Garofalo M, Di Leva G,
Cascione L, Zanca C, Bolon B, Condorelli G and Croce CM: MiR-494 is
regulated by ERK1/2 and modulates TRAIL-induced apoptosis in
non-small-cell lung cancer through BIM down-regulation. Proc Natl
Acad Sci USA. 109:16570–16575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim WK, Park M, Kim YK, Tae YK, Yang HK,
Lee JM and Kim H: MicroRNA-494 downregulates KIT and inhibits
gastrointestinal stromal tumor cell proliferation. Clin Cancer Res.
17:7584–7594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diakos C, Zhong S, Xiao Y, Zhou M,
Vasconcelos GM, Krapf G, Yeh RF, Zheng S, Kang M and Wiencke JK:
TEL-AML1 regulation of survivin and apoptosis via miRNA-494 and
miRNA-320a. Blood. 116:4885–4893. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang SS, Jiang WW, Smith I, Poeta LM,
Begum S, Glazer C, Shan S, Westra W, Sidransky D and Califano JA:
MicroRNA alterations in head and neck squamous cell carcinoma. Int
J Cancer. 123:2791–2797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe
TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, et al:
Genomic profiling of microRNA and messenger RNA reveals deregulated
microRNA expression in prostate cancer. Cancer Res. 68:6162–6170.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao C, Mu Y, Hallahan DE and Lu B: XIAP
and survivin as therapeutic targets for radiation sensitization in
preclinical models of lung cancer. Oncogene. 23:7047–7052. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chakravarti A, Zhai GG, Zhang M, Malhotra
R, Latham DE, Delaney MA, Robe P, Nestler U, Song Q and Loeffler J:
Survivin enhances radiation resistance in primary human
glioblastoma cells via caspase-independent mechanisms. Oncogene.
23:7494–7506. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olie RA, Simões-Wüst AP, Baumann B, Leech
SH, Fabbro D, Stahel RA and Zangemeister-Wittke U: A novel
antisense oligonucleotide targeting survivin expression induces
apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer
Res. 60:2805–2809. 2000.PubMed/NCBI
|
|
24
|
Kato J, Kuwabara Y, Mitani M, Shinoda N,
Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J and
Fujii Y: Expression of survivin in esophageal cancer: Correlation
with the prognosis and response to chemotherapy. Int J Cancer.
95:92–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Als AB, Dyrskjøt L, von der Maase H, Koed
K, Mansilla F, Toldbod HE, Jensen JL, Ulhøi BP, Sengeløv L, Jensen
KM and Orntoft TF: Emmprin and survivin predict response and
survival following cisplatin-containing chemotherapy in patients
with advanced bladder cancer. Clin Cancer Res. 13:4407–4414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tolcher AW, Quinn DI, Ferrari A, Ahmann F,
Giaccone G, Drake T, Keating A and de Bono JS: A phase II study of
YM155, a novel small-molecule suppressor of survivin, in
castration-resistant taxane-pretreated prostate cancer. Ann Oncol.
23:968–973. 2012. View Article : Google Scholar
|
|
27
|
Wiechno P, Somer BG, Mellado B, Chłosta
PL, Cervera Grau JM, Castellano D, Reuter C, Stöckle M, Kamradt J,
Pikiel J, et al: A randomised phase 2 study combining LY2181308
sodium (survivin antisense oligonucleotide) with first-line
docetaxel/prednisone in patients with castration-resistant prostate
cancer. Eur Urol. 65:516–520. 2014. View Article : Google Scholar
|
|
28
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ning S, Fuessel S, Kotzsch M, Kraemer K,
Kappler M, Schmidt U, Taubert H, Wirth MP and Meye A:
siRNA-mediated down-regulation of survivin inhibits bladder cancer
cell growth. Int J Oncol. 25:1065–1071. 2004.PubMed/NCBI
|
|
30
|
Sapra P, Wang M, Bandaru R, Zhao H,
Greenberger LM and Horak ID: Down-modulation of survivin expression
and inhibition of tumor growth in vivo by EZN-3042, a locked
nucleic acid antisense oligonucleotide. Nucleosides Nucleotides
Nucleic Acids. 29:97–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wiechno P, Chlosta P, Smok-Kalwat J,
Pikilel J, Henry D, Christianson D, Somer B, Mellado B, Duran I and
Castellano D: Interim results of a randomized phase II study with
window-design to evaluate antitumor activity of the survivin
antisense oligonucleotide (ASO) LY2181308 in combination with
docetaxel for first-line treatment of castrate-resistant prostate
cancer (CRPC). J Clin Oncol. 29(suppl): abstr 4592. 2011.
|
|
32
|
Song J, Cao L and Li Y: RNA
interference-mediated inhibition of survivin and VEGF in pancreatic
cancer cells in vitro. Mol Med Rep. 7:1651–1655. 2013.PubMed/NCBI
|
|
33
|
Liu Q, Fu H, Xing R, Tie Y, Zhu J, Sun Z
and Zheng X: Survivin knockdown combined with apoptin
overexpression inhibits cell growth significantly. Cancer Biol
Ther. 7:1053–1060. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao Y, Liu Y, Shao C, Hu J, Li X, Li F,
Zhang L, Zhao D, Sun L, Zhao X, et al: Enhanced tumor suppression
in vitro and in vivo by co-expression of survivin-specific siRNA
and wild-type p53 protein. Cancer Gene Ther. 17:844–854. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baron M, Aslam H, Flasza M, Fostier M,
Higgs JE, Mazaleyrat SL and Wilkin MB: Multiple levels of Notch
signal regulation. Mol Membr Biol. 19:27–38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang YS, Dai Y, Yu XF, Bao SY, Yin YB,
Tang M and Hu CX: Microarray analysis of microRNA expression in
hepatocellular carcinoma and non-tumorous tissues without viral
hepatitis. J Gastroenterol Hepatol. 23:87–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen PF, Chen XQ, Liao YC, Chen N, Zhou Q,
Wei Q, Li X, Wang J and Zeng H: MicroRNA-494-3p targets CXCR4 to
suppress the proliferation, invasion and migration of prostate
cancer. Prostate. 74:756–767. 2014. View Article : Google Scholar : PubMed/NCBI
|