Introduction
Multiple myeloma (MM) is a malignant tumor, which
originates from terminally differentiated B lymphocytes and is
characterized by clonal proliferation of a large number of plasma
cells in the bone marrow of patients. MM has a complex multi-step
and multi-stage pathogenesis, and a variety of factors and pathways
are involved in its development and progression (1,2). MM
is the most common primary tumor of the bone marrow in the USA
(3).
Previous studies have focused on the interactions
among cytogenetic abnormalities, the bone marrow microenvironment,
myeloma cells, nuclear factor-κB (NF-κB) signaling pathways and
resistance mechanisms (4,5). The active transcription factor NF-κB
is a heterodimer consisting of two subunits p50 and p65.
Non-activated NF-κB in the heterodimer also inhibits IκB. Following
activation, NF-κB changes into an active heterodimer, which then
enters the nucleus and combines with the promoter region of several
target genes. This triggers transcription of the target genes, thus
increasing the expression of various cytokines, chemical factors,
adhesion molecules and cyclin D, which in turn promotes the growth
and survival of cells (6,7). Suppression of NF-κB enhances the
anti-MM effects of conventional chemotherapeutic agents (5). For example, celastrol, an inhibitor
of NF-κB, has been demonstrated to induce cell cycle arrest and
apoptosis of human MM cells via downregulation of NF-κB (8).
MicroRNAs (miRs) are a type of non-coding small RNA
with a length of ~22 nt, which regulate gene expression following
transcription of genes, involved in the regulation of cell
differentiation, apoptosis and proliferation by inhibiting the mRNA
of specific target genes (9).
Previous studies have demonstrated that miRNAs are extensively
involved in the occurrence, development and prognosis of a tumor.
For example, the expression of miR-29b was increased 10-fold in MM
cells compared with in the plasma cells of healthy individuals
(10). Upregulation of miR-29b has
been demonstrated to induce significant antitumor activity in human
MM (11). For example, Zhang et
al (12) demonstrated that
miRNA-29b-induced apoptosis was found to act antagonistically with
IL-6 in human myeloma cell lines.
Genistein is predominantly found in Leguminosae,
with the largest quantities identified in Fructus Sophorae and
Subprostrate Sophora (13).
Genistein is able to induce programmed cell death, increase the
anti-cancer efficacy and inhibit angiogenesis, and thus is a
promising cancer chemopreventive agent. The anticancer effect of
genistein has broad application prospects (14,15)
and thus requires further investigation. The aim of the present
study was to examine the effects of genistein on the proliferation
and apoptosis of human MM cells.
Materials and methods
Reagents and chemicals
The chemical structure of genistein is shown in
Fig. 1. Genistein (Sigma-Aldrich,
St. Louis, MO, USA; with a purity >98%) was dissolved in
physiological saline according to the manufacturer's instructions
(16,17). RPMI-1640 and fetal bovine serum
(FBS) were purchased from Invitrogen Life Technologies (Carlsbad,
CA, USA). The Caspase-3 Activity Assay kit and BCA protein assay
kit were purchased from Shanghai Sangon Biotechnology Co., Ltd.
(Shanghai, China). The Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) Apoptosis Detection kit was purchased
from BestBio (Shanghai, China). TRIzol reagent and quantitative
polymerase chain reaction (qPCR) assays were purchased from Tiangen
Biotech (Beijing) Co., Ltd. (Beijing, China).
Cell lines and cell culture
The human MM cell line U266 was purchased from the
cell bank of the Chinese Academy of Sciences (Shanghai, China). The
cells were maintained in RPMI-1640 culture medium supplemented with
10% FBS, 100 units/ml penicillin and 100 mg/ml streptomycin in a
humidified atmosphere in a 37°C incubator with 5%
CO2.
Cell viability assay
Cells were seeded (1×104 cells) in
96-well plates and treated with the indicated dose of genistein (0,
10, 20 and 40 µM) for 24, 48 and 72 h. Cell viability was
measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Sigma-Aldrich). Absorbance was measured at λ=570 nm
using a microplate reader (iMark™; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Annexin V-FITC/PI apoptosis assay
Cells were seeded (1×106 cells) in 6-well
plates and treated with the indicated dose of genistein (0, 10, 20
and 40 µM) for 48 h. Briefly, apoptotic cells were measured
using an Annexin V-FITC/PI Apoptosis Detection kit (BestBio). For
flow cytometric analysis, a Cytomics FC500 flow cytometer with CXP
software (Beckman Coulter, Fullerton, CA, USA) was used.
Caspase-3 activation assay
Cells were seeded (1×104 cells) in
96-well plates and treated with the indicated dose of genistein (0,
10, 20 and 40 µM) for 48 h. Briefly, caspase-3 activation
was measured using the Caspase-3 Activity Assay kit (Shanghai
Sangon Biotechnology Co., Ltd.). Protein cell lysate (10 µl
per sample) was added to 80 µl reaction buffer with 10
µl substrate (Asp-Glu-Val-Asp-p-nitroanilide) and incubated
at 37°C for 4–6 h. Caspase-3 activation was measured using a
microplate reader (Bio-Rad Laboratories, Inc.) at an absorbance of
405 nm.
Western blotting
Cells were seeded (1×106 cells) in 6-well
plates and treated with the indicated dose of genistein (0, 10, 20
and 40 µM) for 48 h. Protein concentration was determined
using the BCA protein assay kit (Shanghai Sangon Biotechnology Co.,
Ltd.). Protein samples were analyzed using 12% SDS-polyacrylamide
gel electrophoresis followed by semi-dry transfer onto a
polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc.). The
membrane was blocked with 5% non-fat milk in Tris-buffered saline
and Tween-20 (TBST) buffer at 4°C for 4 h. The membrane was
incubated with rabbit anti-human anti-NF-κB p65 (cat. no. BA0610-2;
1:500 dilution; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) and rabbit anti-human anti-GAPDH (cat. no. PB0141; 1:2,000
dilution; Wuhan Boster Biological Technology, Ltd.) overnight at
4°C. The membrane was washed with TBST and proteins were detected
using a BCIP-NBT kit (Promega Corporation, Madison, WI, USA).
Reverse transcription (RT)-qPCR analysis
of miR-29b expression
Cells were seeded (1×106 cells) in 6-well
plates and treated with the indicated dose of genistein (0, 10, 20
and 40 µM) for 48 h. Total RNA was extracted from cells
using TRIzol reagent [Tiangen Biotech (Beijing) Co., Ltd.] and
miRNAs were specifically amplified for individual miRNA TaqMan qPCR
analysis [Tiangen Biotech (Beijing) Co., Ltd.]. Quantification of
the miRNAs was performed using TaqMan miRNA qPCR assays [Tiangen
Biotech (Beijing) Co., Ltd.]. The primers used were as follows:
Forward, 5′-GGG GGTACCCTTCAGGAAGCTGGTTTC-3′ and reverse,
5′-GGGGATATCTACATGTGAGGCAGGTTCTCAC-3′ for miR-29b; forward,
5′-CGCTTCGGCAGCACATATACTA-3′ and reverse
5′-CGCTTCACGAATTTGCGTGTCA-3′ for U6.
Transfection of miR-29b and
anti-miR-29b
miR-29b and anti-miR-29b plasmids were designed and
purchased from Shanghai Sangon Biotechnology Co., Ltd. When the
U266 cells reached 70–80% confluence, Lipofectamine 2000
(Invitrogen Life Technologies) was used to transfect miR-29b (100
nmol/l) or anti-miR-29b (100 nmol/l) into U266 cells according to
the manufacturer's instructions. Cells were seeded
(1×106 cells) in 6-well plates and treated with the
indicated dose of genistein (20 µM) for 48 h.
Statistical analysis
All experiments were repeated at least three times
and were analyzed using SPSS statistical software version 18 (SPSS,
Inc., Chicago, IL. USA). Comparisons between mean values were
assessed using Student's unpaired t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
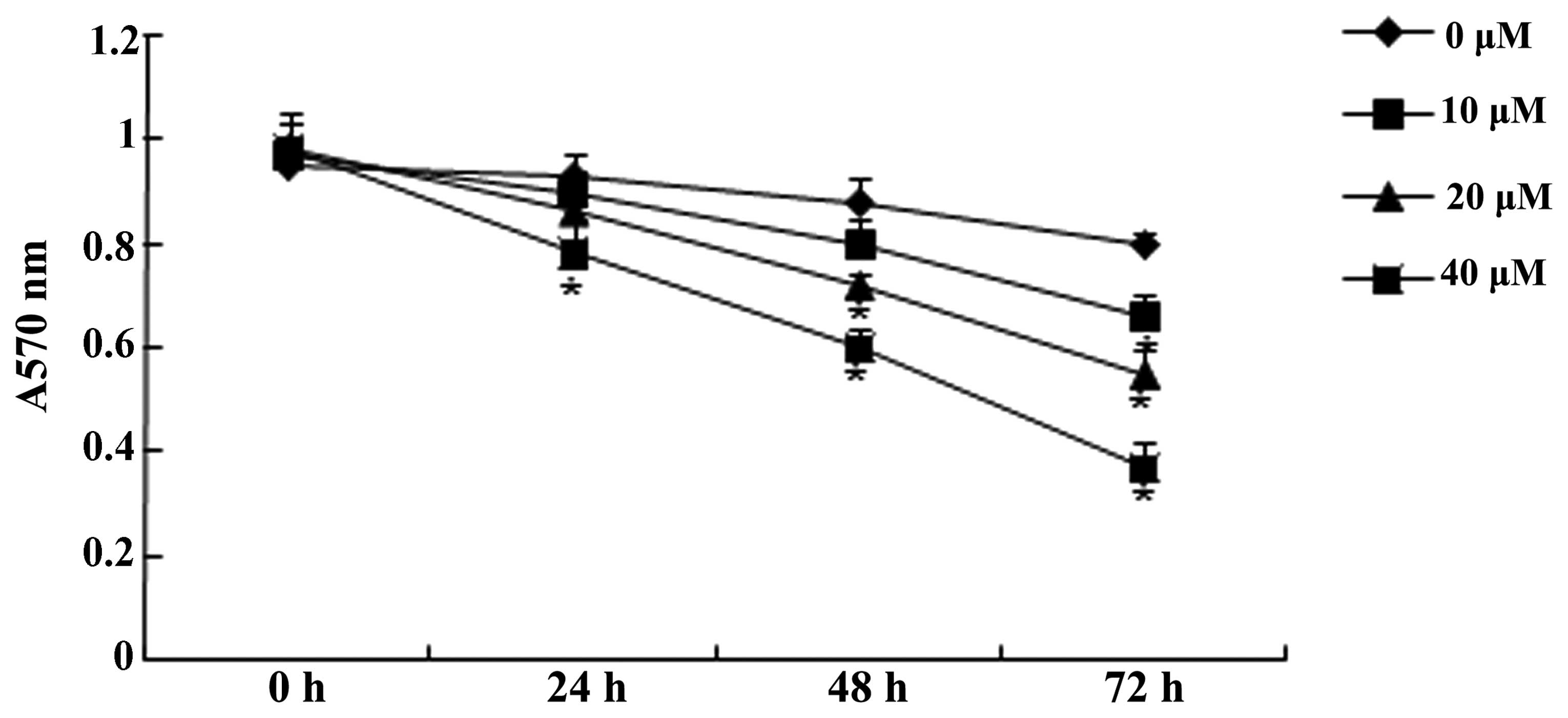
MTT analysis for cell viability
The effect of genistein (0, 10, 20 and 40 µM)
on U266 cell viability was examined by the MTT assay. The results
demonstrated that Genistein could inhibit the proliferation of U266
cells. Following treatment with 10 µM genistein for 72 h, 20
µM genistein for 48 and 72 h and 40 µM genistein for
24, 48 and 72 h, the proliferation of U266 cells was significantly
reduced compared with that of the group treated with 0 µM
genistein (Fig. 2). Thus, 20
µM genistein was selected as the standard treatment for
further experiments.
Flow cytometric analysis of cell
apoptosis and measurement of caspase-3 activity
In order to further assess the effect of genistein
(0, 10, 20 and 40 µM) on the apoptosis of U266 cells,
apoptosis and the activity of caspase-3 were analyzed. Following
treatment with genistein (0, 10, 20 and 40 µM) for 48 h,
apoptosis of U266 cells increased in a concentration-dependent
manner (Fig. 3A and B). Apoptosis
of U266 cells was significantly increased following treatment with
genistein (20 and 40 µM) for 48 h (Fig. 3A and B). In addition, following
treatment with genistein (20 and 40 µM) for 48 h, the
activity of caspase-3 in U266 cells was significantly increased,
compared with that of the group treated with 0 µM genistein
(Fig. 3C).
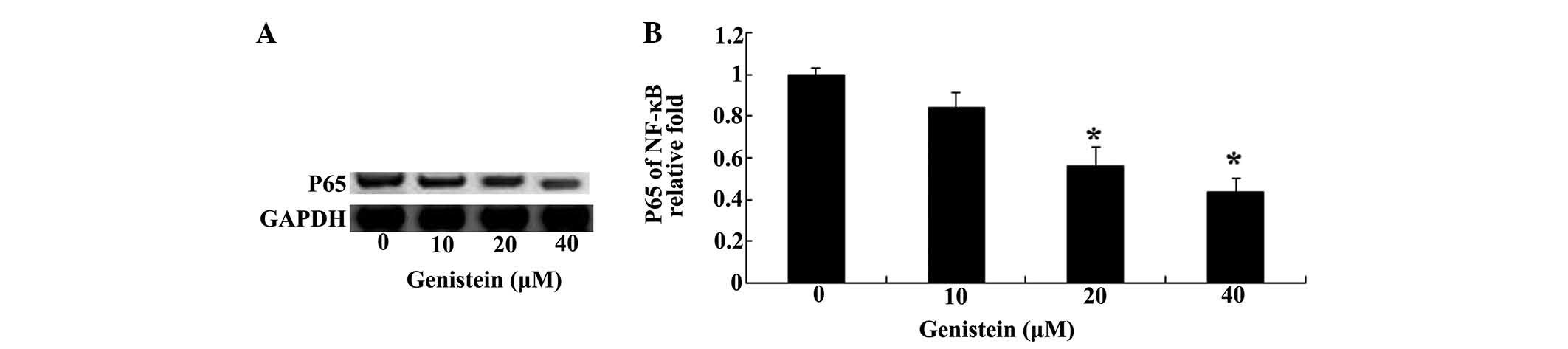
Inhibition of NF-κB by genistein
Based on the abovementioned results, western
blotting was used to analyze the protein level of NF-κB in U266
cells (Fig. 4A). Notably,
treatment with genistein (0, 10, 20 and 40 µM) for 48 h had
a pronounced inhibitory effect on the protein level of NF-κB in
U266 cells (Fig. 4B).
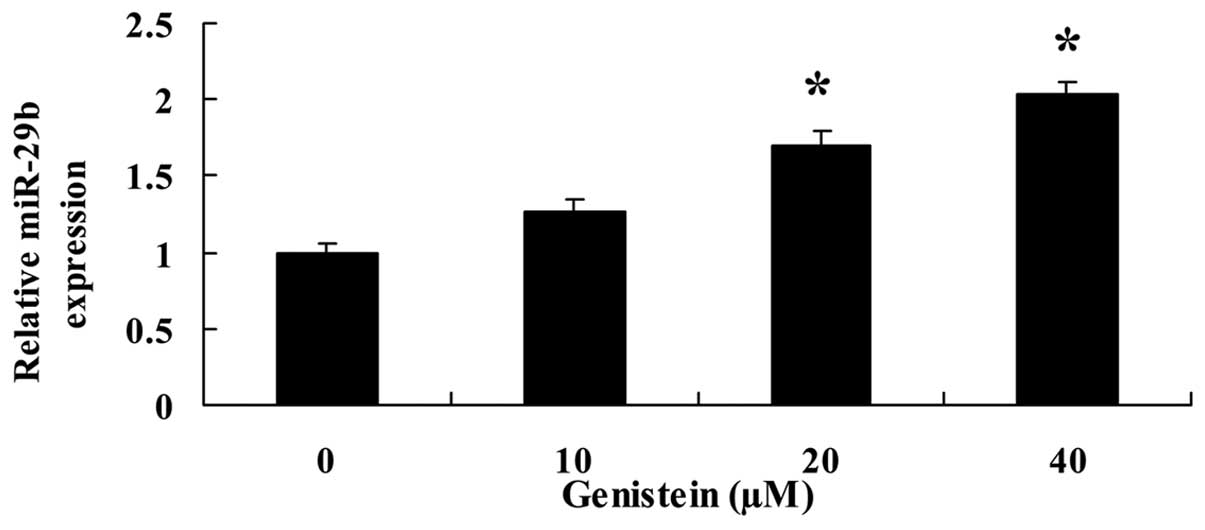
Genistein activates miR-29b
expression
To further investigate the effect of genistein on
miR-29b expression, qPCR was used to examine the expression of
miR-29b in U266 cells. Following treatment with genistein (20 and
40 µM) for 48 h, the expression of miR-29b in U266 cells was
significantly promoted (Fig.
5).
Overexpression of miR-29b and NF-κB
expression
To improve our understanding of the expression of
miR-29b and NF-κB in U266 cells, miR-29b was transfected into U266
cells and the expression of NF-κB in U266 cells was detected. These
data indicated that transfection of miR-29b plasmids significantly
increased the expression of miR-29b in U266 cells (Fig. 6A). Furthermore, overexpression of
miR-29b significantly inhibited NF-κB expression in U266 cells
(Fig. 6B).
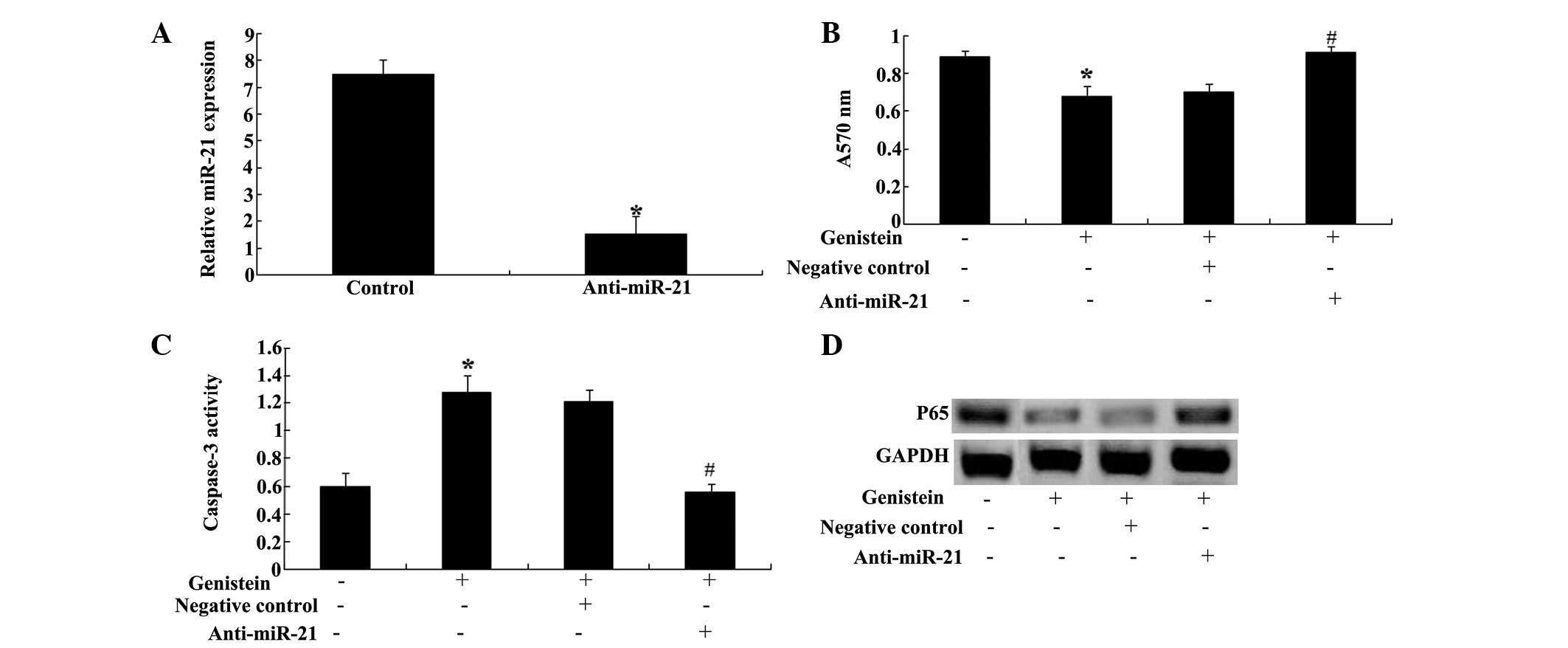
Anti-miR-29b reverses the effect of
genistein
To further investigate the correlation between the
expression of miR-29b and the effect of genistein, the effect of
genistein on MM was examined. The results demonstrated that
transfection of anti-miR-29b plasmids into U266 cells significantly
reduced the expression of miR-29b in U266 cells (Fig. 7A). Furthermore, anti-miR-29b
plasmids could significantly reduce the effect of genistein on cell
proliferation (Fig. 7B) and
apoptosis of U266 cells (Fig. 7C).
In addition, anti-miR-29b plasmids could promote the levels of
NF-κB in U266 cells (Fig. 7D).
Discussion
MM is a malignant disease of plasma cells, of which
the main clinical manifestations include hyperglobulinemia, renal
dysfunction, bone damage and pancytopenia (18). At present there is no cure,
however, there has been significant progress in treatment in
previous years, including thalidomide, proteasome inhibitors and
the application of bone marrow transplantation. However, the
survival time of MM patients has not yet significantly improved.
Therefore, possible treatments for MM are being continuously
investigated. In the present study, genistein (5, 10 and 20
µM) inhibited the proliferation of U266 cells. Genistein
possesses estrogen and anti-estrogen properties, as well as
exerting antioxidant effects to inhibit the activity of protein
tyrosine kinase, which inhibits the activity of topoisomerase II
(19). In the present study,
genistein significantly increased apoptosis and activity of
caspase-3 in U266 cells. A previous study revealed that genistein
combined with doxorubicin had a synergistic cytotoxic effect on
breast cancer cells (20).
Genistein has also been demonstrated to induce apoptosis of MCF-7
and 3T3-L1 cells via regulation of ERα expression (15).
Sustained activation of NF-κB can promote MM cell
proliferation, mediate the secretion of IL-6 and the expression of
adhesion molecules, upregulate anti-apoptotic proteins, inhibit
death receptor pathways and promote angiogenesis, contributing to
the proliferation of malignant myeloma cells and their resistance
to apoptosis (21). Drugs
targeting NF-κB can prevent NF-κB activation, promote apoptosis and
improve prognosis, providing a broad prospect for the treatment of
MM (22). In the present study,
genistein was found to have a pronounced inhibitory effect on the
protein level of NF-κB in U266 cells. In addition, Luo et al
reported that genistein could induce apoptosis in human colon
cancer through inhibiting the NF-κB pathway (23). Chung et al demonstrated that
genistein inhibits phorbol ester-induced NF-κB transcriptional
activity in human mammary epithelial cells (24).
miRNAs comprise ~1–2% of the known eukaryotic genome
and are important in tumor biology, acting as tumor suppressor
genes and proto-oncogenes. Overexpression of miRNA-29b reduces the
level of Mcl-1 protein, thereby inhibiting the growth of MM cells,
suggesting that miRNA-29b may be a tumor suppressor gene.
Furthermore, miR-29b suppresses MM and endothelial cells by
inducing the expression of SOCS-1 (25). Based on this, the present study
found that the expression of miR-29b in U266 cells was also
significantly promoted following treatment with genistein. It has
been reported that genistein exerts its anti-tumor effect through
downregulation of miR-1260b in prostate cancer cells (26). Xia et al reported that
genistein can suppress pancreatic cancer cells through inhibition
of miR-27a (27). The results of
the present study demonstrated that overexpression of miR-29b
significantly inhibited NF-κB expression in U266 cells. miR-29b was
able to alter and control the expression of NF-κB in U266 cells.
The decreased expression of miR-29b reduced the effect of genistein
on U266 cells and increased the expression of NF-κB in U266 cells.
In conclusion, genistein inhibits the proliferation and induces the
apoptosis of human MM cells through suppressing NF-κB via
upregulation of miR-29b.
References
|
1
|
Kelly T, Børset M, Abe E, Gaddy-Kurten D
and Sanderson RD: Matrix metalloproteinases in multiple myeloma.
Leuk Lymphoma. 37:273–281. 2000.PubMed/NCBI
|
|
2
|
Barillé S, Akhoundi C, Collette M,
Mellerin MP, Rapp MJ, Harousseau JL, Bataille R and Amiot M:
Metalloproteinases in multiple myeloma: Production of matrix
metalloproteinase-9 (MMP-9), activation of proMMP-2 and induction
of MMP-1 by myeloma cells. Blood. 90:1649–1655. 1997.
|
|
3
|
He X, Yang K, Chen P, Liu B, Zhang Y, Wang
F, Guo Z, Liu X, Lou J and Chen H: Arsenic trioxide-based therapy
in relapsed/refractory multiple myeloma patients: A meta-analysis
and systematic review. Onco Targets Ther. 7:1593–1599. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hameed A, Brady JJ, Dowling P, Clynes M
and O'Gorman P: Bone disease in multiple myeloma: Pathophysiology
and management. Cancer Growth Metastasis. 7:33–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fuchs O: Targeting of NF-kappaB signaling
pathway, other signaling pathways and epigenetics in therapy of
multiple myeloma. Cardiovasc Hematol Disord Drug Targets. 13:16–34.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manni S, Brancalion A, Mandato E, Tubi LQ,
Colpo A, Pizzi M, Cappellesso R, Zaffino F, Di Maggio SA, Cabrelle
A, et al: Protein kinase CK2 inhibition down modulates the NF-κB
and STAT3 survival pathways, enhances the cellular proteotoxic
stress and synergistically boosts the cytotoxic effect of
bortezomib on multiple myeloma and mantle cell lymphoma cells. PLoS
One. 8:e752802013. View Article : Google Scholar
|
|
7
|
Gatt ME, Takada K, Mani M, Lerner M, Pick
M, Hideshima T, Carrasco DE, Protopopov A, Ivanova E, Sangfelt O,
et al: TRIM13 (RFP2) downregulation decreases tumour cell growth in
multiple myeloma through inhibition of NF Kappa B pathway and
proteasome activity. Br J Haematol. 162:210–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni H, Zhao W, Kong X, Li H and Ouyang J:
NF-kappa B modulation is involved in celastrol induced human
multiple myeloma cell apoptosis. PLoS One. 9:e958462014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou JJ, Zheng S, Sun LF and Zheng L:
MicroRNA regulation network in colorectal cancer metastasis. World
J Biol Chem. 5:301–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo X, Gu J, Zhu R, Feng M, Zhu X, Li Y
and Fei J: Integrative analysis of differential miRNA and
functional study of miR-21 by seed-targeting inhibition in multiple
myeloma cells in response to berberine. BMC Syst Biol. 8:822014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jagannathan S, Vad N, Vallabhapurapu S,
Vallabhapurapu S, Anderson KC and Driscoll JJ: MiR-29b replacement
inhibits proteasomes and disrupts aggresome+autophagosome formation
to enhance the anti-myeloma benefit of bortezomib. Leukemia.
29:727–738. 2015. View Article : Google Scholar :
|
|
12
|
Zhang YK, Wang H, Leng Y, Li ZL, Yang YF,
Xiao FJ, Li QF, Chen XQ and Wang LS: Overexpression of microRNA-29b
induces apoptosis of multiple myeloma cells through down regulating
Mcl-1. Biochem Biophys Res Commun. 414:233–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qian K, Gao AJ, Zhu MY, Shao HX, Jin WJ,
Ye JQ and Qin AJ: Genistein inhibits the replication of avian
leucosis virus subgroup J in DF-1 cells. Virus Res. 192:114–120.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki R, Kang Y, Li X, Roife D, Zhang R
and Fleming JB: Genistein potentiates the antitumor effect of
5-Fluorouracil by inducing apoptosis and autophagy in human
pancreatic cancer cells. Anticancer Res. 34:4685–4692.
2014.PubMed/NCBI
|
|
15
|
Choi EJ, Jung JY and Kim GH: Genistein
inhibits the proliferation and differentiation of MCF-7 and 3T3-L1
cells via the regulation of ERα expression and induction of
apoptosis. Exp Ther Med. 8:454–458. 2014.PubMed/NCBI
|
|
16
|
Li J, Li J, Yue Y, Hu Y, Cheng W, Liu R,
Pan X and Zhang P: Genistein suppresses tumor necrosis factor
α-induced inflammation via modulating reactive oxygen
species/Akt/nuclear factor κB and adenosine monophosphate-activated
protein kinase signal pathways in human synoviocyte MH7A cells.
Drug Des Devel Ther. 8:315–323. 2014. View Article : Google Scholar
|
|
17
|
Jamadar-Shroff V, Papich MG and Suter SE:
Soy-derived isoflavones inhibit the growth of canine lymphoid cell
lines. Clin Cancer Res. 15:1269–1276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen HF, Li ZY, Tang JQ, Shen HS, Cui QY,
Ren YY, Qin LM, Jin LJ, Zhu JJ, Wang J, et al: Clinical study of
thalidomide combined with dexamethasone for the treatment of
elderly patients with newly diagnosed multiple myeloma. Asian Pac J
Cancer Prev. 13:4777–4781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura H and Wang Y, Xue H, Romanish MT,
Mager DL, Helgason CD and Wang Y: Genistein versus ICI 182, 780: An
ally or enemy in metastatic progression of prostate cancer.
Prostate. 73:1747–1760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue JP, Wang G, Zhao ZB, Wang Q and Shi Y:
Synergistic cytotoxic effect of genistein and doxorubicin on
drug-resistant human breast cancer MCF-7/Adr cells. Oncol Rep.
32:1647–1653. 2014.PubMed/NCBI
|
|
21
|
Zhang GJ and Zhang Z: Effect of Bcl-2 on
apoptosis and transcription factor NF-κB activation induced by
adriamycin in bladder carcinoma BIU87 cells. Asian Pac J Cancer
Prev. 14:2387–2391. 2013. View Article : Google Scholar
|
|
22
|
Li Z, Yang Z, Peng X, Li Y, Liu Q and Chen
J: Nuclear factor-κB is involved in the protocadherin-10-mediated
pro-apoptotic effect in multiple myeloma. Mol Med Rep. 10:832–838.
2014.PubMed/NCBI
|
|
23
|
Luo Y, Wang SX, Zhou ZQ, Wang Z, Zhang YG,
Zhang Y and Zhao P: Apoptotic effect of genistein on human colon
cancer cells via inhibiting the nuclear factor-kappa B (NF-κB)
pathway. Tumour Biol. 35:11483–11488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung MH, Kim DH, Na HK, Kim JH, Kim HN,
Haegeman G and Surh YJ: Genistein inhibits phorbol ester-induced
NF-κB transcriptional activity and COX-2 expression by blocking the
phosphorylation of p65/Rel in human mammary epithelial cells. Mutat
Res. 768:74–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amodio N, Bellizzi D, Leotta M, Raimondi
L, Biamonte L, D'Aquila P, Di Martino MT, Calimeri T, Rossi M,
Lionetti M, et al: miR-29b induces SOCS-1 expression by promoter
demethylation and negatively regulates migration of multiple
myeloma and endothelial cells. Cell Cycle. 12:3650–3662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Tanaka Y, Tabatabai ZL and Dahiya R: Genistein downregulates
onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation
and histone modification in prostate cancer cells. Br J Cancer.
110:1645–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia J, Cheng L, Mei C, Ma J, Shi Y, Zeng F
and Wang Z and Wang Z: Genistein inhibits cell growth and invasion
through regulation of miR-27a in pancreatic cancer cells. Curr
Pharm Des. 20:5348–5353. 2014. View Article : Google Scholar : PubMed/NCBI
|