Introduction
Spinal cord ischemia/reperfusion injury (SCIRI)
refers to the return of the blood supply in recovery following
trauma of the spinal cord. The neurological function of the spinal
cord cannot be improved, and the injury is further aggravated by
ischemia, which leads to the irreversible, delayed death of spinal
neurons (1). SCIRI is a common
complication following thoracic or thoracoabdominal aortic surgery
(2). Previous studies reported
that the incidence of spinal cord injury caused by
ischemia/reperfusion is 3–18% (3),
and patients with SCIRI have a poor prognosis, usually resulting in
severe paralysis or mortality (4,5),
which places burdens on patients and family members. Therefore, the
identification of effective preventative measures is important for
improving the prognosis of SCIRI.
Previous studies have demonstrated that bone marrow
mesenchymal stem cell (BMSC) transplantation effectively attenuates
SCIRI (6–8). However, high levels of apoptosis in
ischemic tissues following cell transplantation severely affect
treatment outcomes. Studies have reported that the survival rate of
autologous transplanted cells in myocardial ischemia patients was
only 1% (9), and although the
survival rate of BMSCs transplanted into ischemic myocardial
tissues on the first day was >99%, the survival rate on the
fourth day decreased sharply to <0.44% (10). Although the cause of transplanted
cell death remains to be elucidated, insufficient blood supply,
hypoxia, inflammation, oxidative stress and accumulation of
cytotoxic substances all have adverse effects on the survival of
transplanted cells (11). To date,
several in vitro and in vivo studies have determined
that hypoxic preconditioning may increase the adaptability of
mesenchymal stem cells to hypoxic environments, increase cell
activity and inhibit apoptosis (12–16).
However, the function of hypoxic preconditioning on the protective
effect of BMSCs in SCIRI have not been reported.
In the present study, the effect of hypoxic
preconditioning on the protective role of BMSCs in SCIRI was
explored. Hypoxic preconditioning is involved in improving the
survival of BMSCs and promoting the recovery of SCIRI. Hypoxic
preconditioning may become a promising adjuvant therapy in the
treatment of SCIRI with BMSCs.
Materials and methods
Experimental animals
A total of 30 eight week-old male healthy adult
Sprague-Dawley (SD) rats weighing 200–250 g were purchased from the
Experimental Animal Center of the China Medical University
(Shenyang, China). Prior to experimentation, the rats were housed
separately at 22°C with 40–50% humidity and a 12 h light/dark cycle
in animal rooms for adaptive feeding for 1 week. The rats had
access to food and water ad libitum. The care and treatment
of the animals and present study was approved by the Experimental
Animal Ethics Committee of the China Medical University.
Isolation, culture, identification and
hypoxic preconditioning of rat BMSCs
According to previously described methods (17,18),
the rats were sacrificed by cervical dislocation, and the femur and
tibia were removed under sterile conditions. The bone marrow cavity
was exposed, and Dulbecco's modified Eagle's medium (DMEM; Gibco
Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; GE Healthcare Life Sciences, Logan, UT, USA) in
a 2 ml syringe was used to wash out the bone marrow. Following
centrifugation at 252 x g for 10 min at room temperature, the
supernatant was discarded. The cells were resuspended by red cell
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) and centrifuged at 112 x g for 10 min at room
temperature. The cells were then filtered with a 200 mesh sieve and
cultured in an incubator at 37°C in an atmosphere containing 5%
CO2 for 24 h, prior to being inoculated at a density of
5×105/flask. After 24 h, the culture medium was replaced
to remove the non-attached cells, and was replaced again after 5
days. Cells with optimal growth at the third generation were used
to identify the expression levels of CD29, CD44, CD45 and CD90
using FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA).
Hypoxic preconditioning of the cells was performed by culturing the
cells in serum-free DMEM in a cell incubator (37°C; 3%
O2, 5% CO2 and 92% N2) for 24 h,
as previously described by Liu et al (15). The cells were placed in DMEM in the
absence of FBS and cultured at 37°C in an atmosphere containing 3%
O2, 5% CO2 and 92% N2 for 24
h.
Experimental grouping
The SD rats were randomly divided into five groups,
with six rats per group. The sham group received simple surgical
manipulation without ischemia/reperfusion treatment; the SPIRI
group (IR group) received SCIRI surgery; the non-preconditioned
BMSC transplantation group (NP-MSC group) was injected with
untreated BMSCs into the spinal cord sheath prior to
ischemia/reperfusion; the hypoxic preconditioned BMSC
transplantation group (HP-MSC group) was injected with hypoxic
preconditioned BMSCs prior to ischemia/reperfusion; and the
phosphate-buffered saline (PBS) group served as a control group.
The PBS group received injection of an equal volume of PBS prior to
ischemia/reperfusion. Based on a method described by Fang et
al (8), cell transplantation
was performed 2 days prior to SCIRI. A 10 µl polyethylene tube was
inserted into the subarachnoid cavity using a 16-gauge needle, and
slowly moved to the cerebrospinal fluid (CSF). A total of
5×105/5 µl cells or an equal volume of PBS was injected
into the CSF, and the tube was removed. The heads of the
experimental animals faced upwards for 60 min, and the rats with
normal fore and hind limb function were included in the present
study.
Establishment of an SCIRI rat model
An aortic cross-clamping method was used to
establish an SCIRI model in the rats, as previously described
(19). Prior to experimentation,
the rats were fasted (no food or water) for 6 h and anesthetized
using 3% halothane (China Sinopharm international Co., Ltd.,
Shanghai, China). Body temperature was monitored using DT-K101A
rectal thermometers (Hangzhou Hua'an Medical & Health
Instruments Co., Ltd., Hangzhou, China) and during the experimental
process, the body temperature was maintained at 37.5±0.5°C using
electric blankets and heating lamps. The rats were placed in a
supine position, and a longitudinal incision was made along the
left vertex of the sternum to the second rib bone. The left
superior vena cava and internal mammary artery were carefully
avoided. The aortic arch was separated from the left common carotid
and subclavian arteries, and the aortic arch and left subclavian
artery were clamped at the same time using micro-artery clamps
(Jinzhong, Shanghai, China). The artery clamps were opened after 10
min, and the surgical wound was sutured layer by layer with 3-0
sutures (Shanghai Xincheng Medical Instruments Co., Ltd., Shanghai,
China). An intraperitoneal injection of 5 ml of 0.9% saline was
performed following surgery, and the rats were placed in 28°C
incubators for 3 h prior to being returned to their cages.
Neurological function scoring
Neurological function scoring in each group was
performed at 6, 12, 24, 48 h and 7 days following reperfusion using
the Tarlov criteria (8,20,21).
The scoring criteria were as follows: 0 points for the absence of
evident hind limb motor function; 1 point for perceivable autonomic
joint movement; 2 points for free movement of hind limbs, but an
inability to stand; 3 points for an ability to stand but an
inability to walk; and 4 points for total recovery of hind limb
function and the ability to walk.
Detection of the integrity of the blood
spinal cord barrier (BSCB)
A total of 2% Evans blue (Sigma-Aldrich, St. Louis,
MO, USA) staining solution (2 ml/kg) was slowly injected into the
tail vein of the rats. After 1 h, the rats were anesthetized using
3% halothane, and infusion needles were inserted through the
ascending aorta to infuse 500 ml/kg saline. Spinal cord tissue
samples of the L4-L6 were removed and sectioned transversely into
two parts. Following weighing, a section of the spinal cord tissues
of ~100 mg was mixed with 1 ml formamide (Amresco, LLC, Solon, OH,
USA) and then immersed in 37°C prior to being centrifuged at 260 x
g for 10 min at room temperature in order to collect the
supernatant, and the absorbance was measured at 632 nm using a
ELX-800 microplate reader (Bio-Tek Instruments, Inc., Winooski, VT,
USA). Simultaneously, different concentrations (0.05, 0.1, 0.2,
0.4, 0.8, 1.6, and 3.2 µg/ml) of Evans blue solution were prepared,
and a standard curve was plotted to calculate the concentration of
Evans blue in each spinal cord tissue sample. The other section of
spinal cord tissue was fixed in 10% paraformaldehyde (China
Sinopharm international Co., Ltd.) to prepare 10 µm sections. The
stained tissue samples were observed under an IX53 fluorescence
microscope (Olympus Corporation, Tokyo, Japan) and images were
captured.
Hematoxylin and eosin (HE) staining
The injured rat spinal cord tissue samples from each
group were fixed in 10% formaldehyde (China Sinopharm international
Co., Ltd.) overnight and embedded in paraffin (Shanghai Hushi
Laboratorial Equipment Co., Ltd, Shanghai, China) in order to
obtain 5 µm thick sections. HE staining (Beijing Solarbio Science
& Technology Co., Ltd.) was performed, according to standard
procedures. Following staining, the slides were observed under a
DP73 light microscope (Olympus Corporation) and images were
captured.
Detection of apoptosis using terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
The levels of apoptosis were detected using an In
Situ Cell Death Detection kit (Roche Diagnostics GmbH, Mannheim,
Germany), according to the manufacturer's instructions. Paraffin
sections were inactivated using H2O2
solution, prior to incubation with 50 µl TUNEL reaction solution
(Roche Diagnostics GmbH) at 37°C for 60 min. Following washing with
PBS, the sections were incubated with 50 µl converter-POD working
solution (Roche Diagnostics GmbH) at 37°C for 30 min. Following
development with 3′-diaminobenzidine (Beijing Solarbio Science
& Technology Co., Ltd.), the nuclei were stained with
hematoxylin. The sections were observed under a microscope and the
images were captured.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), and RNA
was reverse transcribed into cDNA using a cDNA First Strand
Synthesis Reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer's instructions. The sense and
antisense hypoxia-inducible factor 1α (HIF-1α) primers (synthesized
by Sangon Biotech Co., Ltd., Shanghai, China) were
5′-CTCCCATACAAGGCAGCAGAAAC-3′, and 5′-AGAAACGAAACCCCACAGACAAC-3′,
respectively, and the sense and antisense primers of β-actin were
5′-GGAGATTACTGCCCTGGCTCCTAGC-3′, and
5′-GGCCGGACTCATCGTACTCCTGCTT-3′, respectively. Quantitative
analysis was performed using an Exicycler™ 96 Quantitative
Fluorescence analyzer (Bioneer Corporation, Daejeon, Korea). The
total PCR reaction volume was 20 µl and consisted of 1 µl cDNA, 0.5
µl of each primer, 10 µl SYBR® GREEN master mix (BioTeke
Corporation, Beijing, China) and 8 µl ddH2O. The PCR
reaction thermocycling conditions were as follows: 95°C for 10 min;
40 cycles of 95°C for 10 sec, 58°C for 20 sec, 72°C for 30 sec and
4°C for 5 min. The relative mRNA expression levels were calculated
using the 2−ΔΔCt method (22).
Western blotting
Following tissue homogenization (S10; Scientz,
Ningbo, China), total protein from the spinal cord tissue samples
of each group was extracted using radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
and centrifuged 10,010 x g for 10 min at 4°C, and the protein
concentrations were determined using a bicinchoninic acid assay
(Beyotime Institute of Biotechnology). Total protein (40 µg) from
each group was subjected to 8% SDS-PAGE. Following electrophoresis,
the proteins were transferred onto a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). The membrane was
blocked with 5% non-fat milk at room temperature for 1 h, and then
incubated with polyclonal rabbit anti-rat HIF-1α primary antibody
(1:400; cat. no. BA0912-2; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) at 4°C overnight, followed by incubation with
polyclonal goat anti-rabbit horseradish peroxidase-conjugated
secondary antibody (1:5,000; cat. no. A0208; Beyotime Institute of
Biotechnology) at room temperature for 45 min. The development of
the luminescent substrate was measured using an enhanced
chemiluminescence kit (Shanghai 7Sea Pharmatech Co., Ltd.,
Shanghai, China). Following exposure, images were captured and
scanned into a computer, and Image J 2.1 software (National
Institutes of Health, Bethesda, MA, USA) was used to analyze gray
density. The protein expression levels were quantified using
β-actin as an internal control.
Statistical analysis
The data are presented as the mean ± standard
deviation. The comparison between groups was performed using
one-way analysis of variance and multiple comparisons were
performed using the Bonferroni post-hoc test. The processing of the
data and figures was performed using Graphpad Prism 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Isolation, culture and identification of
rat BMSCs
Rat BMSCs were isolated using density gradient
centrifugation. Third generation BMSCs were harvested to perform an
identification of the surface markers using flow cytometry
(Fig. 1). The expression of CD29,
CD44 and CD90 were positive, and the expression of CD45 was
negative. These results were concordant with those of a previous
study (23), indicating that rat
BMSCs had been obtained successfully.
Hypoxic preconditioning increases the
protective effects of BMSCs on neurological function
The neurological function score of the rats was
determined in each group 6, 12, 24, 48 h and 7 days following
SCIRI. The average score of each group is shown in Fig. 2. The hypoxic preconditioned and the
untreated BMSCs promoted the recovery of neurological function
following SCIRI; however, the neurological function in the HP-MSC
group was significantly higher, compared with that of the NP-MSC
group. From 12 h post-ischemia/reperfusion, the neurological
function score in the HP-MSC group was already significantly
higher, compared with that of the IR group (P<0.05); however,
the neurological function of the NP-MSC and IR groups were not
significantly different, indicating that hypoxic preconditioning
increased the protective effect of BMSCs on neurological
function.
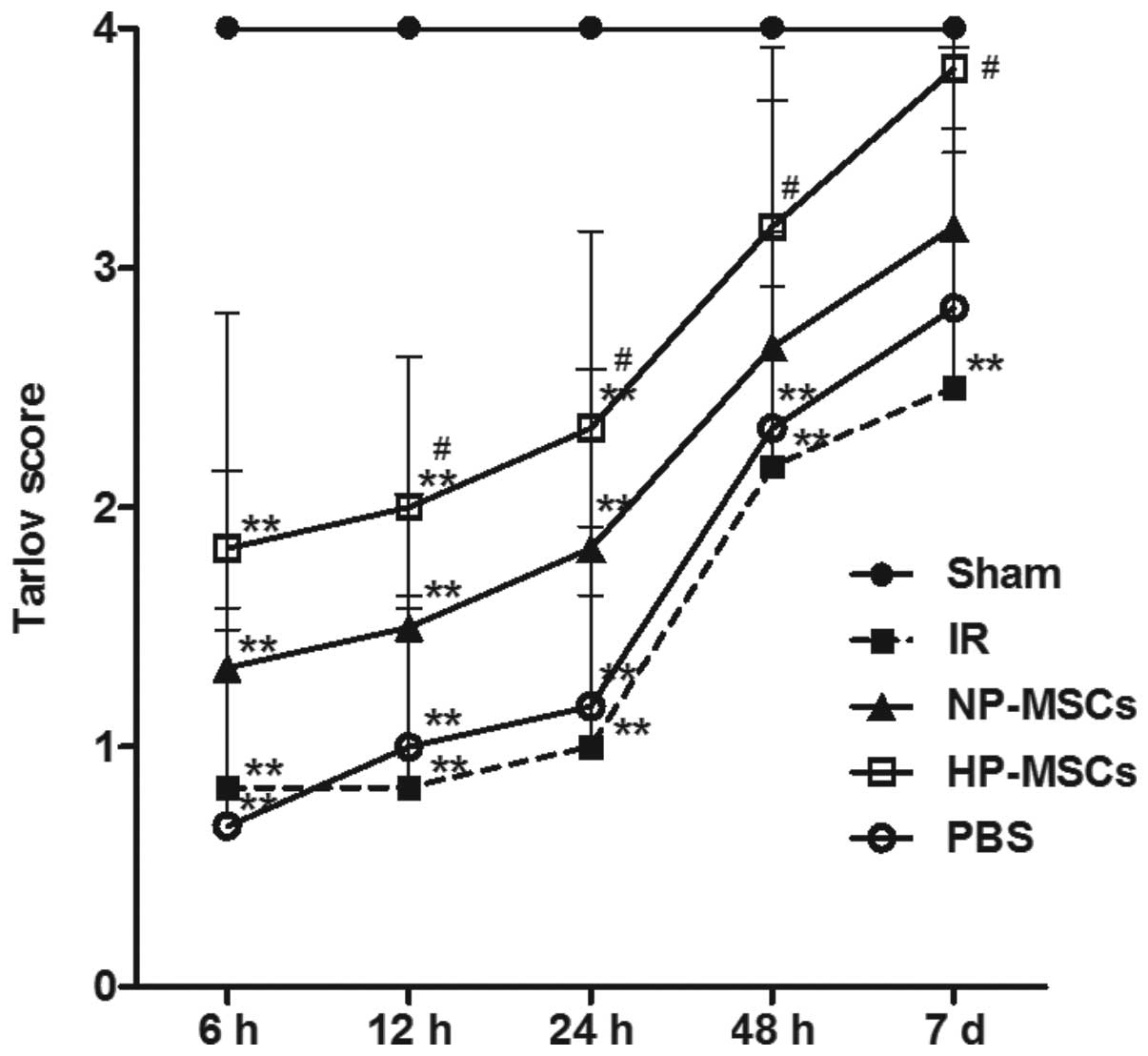 | Figure 2Neurological function scoring using
the Tarlov method. At 6, 12, 24, 48 and 7 days following
ischemia/reperfusion, the neurological function of the rats in each
group was scored. Each group contained six rats and the data are
presented as the mean ± standard deviation. **P<0.01,
vs. sham group; #P<0.05, vs. IR group. BMSC, bone marrow
mesenchymal stem cell; Sham, sham-operated; IR,
ischemia/reperfusion; NP-MSC, non-preconditioned BMSC
transplantation; HP-MSC; hypoxic preconditioned BMSC
transplantation; PBS, phosphate-buffered saline-treated. |
Hypoxic preconditioning increases the
protective effects of BMSCs on the BSCB
Evans blue staining was used to investigate the
protective effects of the treatments of each group on the BSCB
following SCIRI (Fig. 3). The BSCB
was severely damaged in the IR group, and significant Evans blue
leakage (indicated in red) was observed under a fluorescence
microscope. The Evans blue leakage rates in the NP-MSC and HP-MSC
groups significantly weakened. The Evans blue concentration levels
were consistent with the results of the fluorescence microscopy
observations. The Evans blue concentrations in the damaged spinal
cord tissue samples in the IR and PBS groups were significantly
higher, compared with those of the sham group (P<0.01). Compared
with the IR group, the Evans blue concentrations in the NP-MSC and
HP-MSC groups were significantly decreased (P<0.05 and
P<0.01, respectively) and those of the HP-MSC group were
significantly lower, compared with those of the NP-MSC group
(P<0.01). These results indicated that transplantation of BMSCs
provided a certain protective effect on the BSCB, and that hypoxic
preconditioning improved this function.
 | Figure 3Detection of the integrity of the BSCB
using Evans blue staining. The penetration of Evans blue (red) was
observed under a fluorescence microscope (images are representative
results from repeated experiments). The concentration of Evans blue
were measured in the spinal cord tissue samples from each group.
Each group contained six rats and the data are presented as the
mean ± standard deviation. Scale bar, 200 µm.
**P<0.01, vs. sham group; #P<0.05 and
##P<0.01, vs. IR group; and
&&P<0.01, vs. NP-MSC group. BSCB, blood
spinal cord barrier; BMSC, bone marrow mesenchymal stem cell; Sham,
sham-operated; IR, ischemia/reperfusion; NP-MSC, non-preconditioned
BMSC transplantation; HP-MSC; hypoxic preconditioned BMSC
transplantation; PBS, phosphate-buffered saline-treated. |
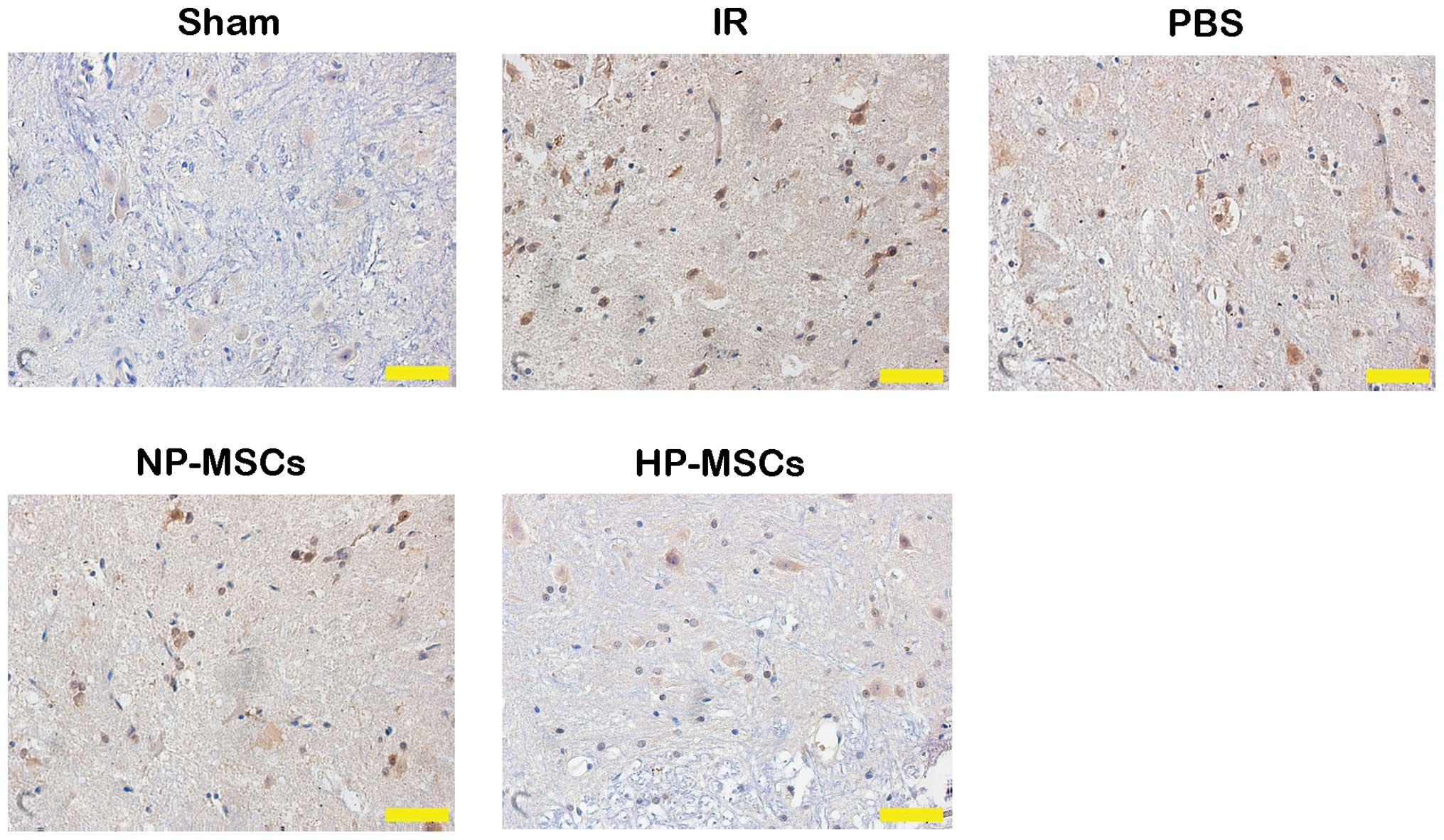
Hypoxic preconditioning increases the
protective effects of BMSCs on spinal cord tissue injury
HE staining was performed on the tissue samples from
each group to observe the effects of the various treatments on
spinal cord injury (Fig. 4).
Tissue injury in the IR and PBS groups was more severe, with nuclei
fragmentation, formation of a large number of vacuoles and tissue
bleeding accompanied by inflammatory cell infiltration. The NP-MSC
and HP-MSC groups had fewer vacuoles and markedly decreased degrees
of injury, and the tissue morphology of the HP-MSC group was
similar to that of normal spinal cord tissues. Detection of the
apoptotic levels in the spinal cord tissue samples using a TUNEL
assay (Fig. 5) determined that an
increased number of apoptotic cells were present in the IR and PBS
groups, compared with the sham group, whereas the number of
apoptotic cells in the NP-MSC group was decreased. The HP-MSC group
exhibited apoptotic cells, however, their number was significantly
lower than that in the NP-MSC group. These results suggested that
hypoxic preconditioning increased the protective effect of the
BMSCs on SCIRI.
Hypoxic preconditioning upregulates the
expression of HIF-1α and increases the protective effects of BMSCs
on SCIRI
To investigate the possible mechanisms underlying
the increased protective effects of BMSCs on spinal cord injury,
the expression levels of HIF-1α in the spinal cord tissue samples
of each group were determined. Compared with the IR group, the mRNA
expression levels of HIF-1α in the NP-MSC and HP-MSC groups were
markedly increased, and the mRNA expression of HIF-1α in the HP-MSC
group was significantly higher, compared with that in the NP-MSC
group (Fig. 6A). The protein
expression levels of HIF-1α in each group were similar to the mRNA
expression levels of HIF-1α (Fig.
6B). Compared with the IR group, the protein expression of
HIF-1α in the NP-MSC group increased, while that of the HP-MSC
group was significantly higher, compared with the levels detected
in the IR and NP-MSC groups (P<0.01). These results suggested
that hypoxic preconditioning upregulated the expression of HIF-1α
in the BMSCs.
Discussion
The transplantation of BMSCs exerts protective
effects on SCIRI (6–8), however, the survival rate of
transplanted BMSCs is usually low, which severely affects treatment
outcome. Previous studies have hypothesized that hypoxic
preconditioning prior to transplantation increases the survival
rate of BMSCs (15,16); however, its function in SCIRI
remains to be fully elucidated. In the present study, rat BMSCs
were isolated and transplanted into the spinal cord tissues of
SCIRI rats following hypoxic preconditioning. Compared with the
other treatment groups, hypoxic preconditioning increased the
protective effects of BMSCs on neurological function, the BSCB and
tissue damage. These effects may be associated with upregulation of
the expression of HIF-1α. Therefore, hypoxic preconditioning has
the potential to become an effective measure to increase the
function of stem cells in SCIRI.
BMSCs secrete several cytokines, chemokines and
growth factors, in order to promote tissue repair (24). Hypoxic conditions can promote the
ability of BMSCs to secrete individual factors (25–27).
Studies on cerebral ischemia/reperfusion injuries have demonstrated
that hypoxic preconditioning increases the secretion levels of
hepatocyte growth factor (HGF) and vascular endothelial growth
factor (VEGF) by BMSCs, thus increasing cognitive function and
promoting neurogenesis in rats (16,28).
The neurological function score of the HP-MSC group in the present
study was significantly higher, compared with that of the NP-MSC
group, indicating that hypoxic preconditioning increased the
protective effects of BMSCs on neurological function; however,
whether the increased protective effects were associated with the
secretion of HGF and VEGF requires further detailed
investigation.
The BSCB regulates and limits molecules entering the
central nervous system and maintains a normal microenvironment in
the spinal cord (29). Primary
injury can immediately induce BSCB damage, and the damaged BSCB
changes the permeability of proteins so that inflammatory
substances can enter freely, thus inducing and aggravating spinal
cord injury (30). Therefore, the
destruction of the BSCB is one of the central links to SCIRI. The
present study demonstrated that, compared with the NP-MSC group,
the concentration of Evans blue in the spinal cord tissues samples
of the HP-MSC group were significantly decreased, indicating that
hypoxic preconditioning increased the protective effect of BMSCs on
the BSCB. Notably, this mechanism was important in the reduction of
secondary injury following SCIRI.
Hypoxic preconditioning increases the repair
capacity of BMSCs in myocardial tissues and can promote
angiogenesis (31); the ability of
hypoxic preconditioned BMSCs to inhibit apoptosis in ischemic
tissues is was also increased, compared with BMSCs without
preconditioning (32,33). The present study determined that
the effects of hypoxic preconditioned BMSCs on the repair of spinal
cord tissues following ischemia/reperfusion injury, and on the
inhibition of apoptosis, were significantly increased, which
further confirmed that hypoxic preconditioning increased the
protective effect of BMSCs on SCIRI.
HIF-1α is a nuclear transcription factor, the
expression of which is upregulated under hypoxic conditions, which
can increase the tolerance of tissues and cells to hypoxic
environments (34). Several in
vitro studies have revealed that hypoxic preconditioning
promotes the secretion of HIF-1α by BMSCs (35,36).
The present study determined that the expression levels of HIF-1α
in the spinal cord tissue samples of the HP-MSC group were
significantly higher, compared with those of the NP-MSC group,
indicating that hypoxic preconditioning increased the protective
effect of BMSCs on SCIRI by promoting the expression of HIF-1α.
These results were concordant with those of a previous study
investigating renal ischemia/reperfusion injury (37).
HIF-1α can induce gene expression and resume
function of the cellular internal environment in the absence of
oxygen (38,39). The results of the present study
suggested that hypoxic preconditioning unregulated the protein and
mRNA expression levels of HIF-1α in BMSCs, improving the function
of motor nerve cells following SCIRI. It also inhibited apoptosis
and protected the integrity of the BSCB, all of which can enhance
the repair capacity of BMSCs on tissue injury. These functions may
be associated with upregulation of the expression of HIF-1α
following hypoxic preconditioning. In conclusion, hypoxic
preconditioning was found to be an effective means for increasing
the survival rate of transplanted BMSCs, and for promoting the
protective effects of BMSCs on SCIRI. The results suggest that
hypoxic preconditioning may serve as a promising adjuvant
therapeutic strategy for the treatment of SCIRI.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81401000),
the Science and Technology Foundation of the Liaoning Province
(grant no. 2012408002), the Doctoral Research Foundation of the
Liaoning Province (grant no. 20141035) and the Foundation for
Scientific Research of The First Affiliated Hospital of the China
Medical University (grant no. 2014-07).
References
|
1
|
Weir CJ, Zivin JA and Lyden PD:
Inter-relationships between spinal cord blood flow, neuronal death
and neurological function in rabbit spinal cord ischemia. Brain
Res. 946:43–51. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuniyoshi Y, Koja K, Miyagi K, Shimoji M,
Uezu T, Arakaki K, Yamashiro S, Mabuni K, Senaha S and Nakasone Y:
Prevention of postoperative paraplegia during thoracoabdominal
aortic surgery. Ann Thorac Surg. 76:1477–1484. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacArthur RG, Carter SA, Coselli JS and
LeMaire SA: Organ protection during thoracoabdominal aortic
surgery: Rationale for a multimodality approach. Semin Cardiothorac
Vasc Anesth. 9:143–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sinha AC and Cheung AT: Spinal cord
protection and thoracic aortic surgery. Curr Opin Anaesthesiol.
23:95–102. 2010. View Article : Google Scholar
|
|
5
|
Mauney MC, Blackbourne LH, Langenburg SE,
Buchanan SA, Kron IL and Tribble CG: Prevention of spinal cord
injury after repair of the thoracic or thoracoabdominal aorta. Ann
Thorac Surg. 59:245–252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi E, Kazui T, Jiang X, Washiyama N,
Yamashita K, Terada H and Bashar AH: Intrathecal injection of bone
marrow stromal cells attenuates neurologic injury after spinal cord
ischemia. Ann Thorac Surg. 81:2227–2233; discussion 2233–2224.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi E, Kazui T, Jiang X, Washiyama N,
Yamashita K, Terada H and Bashar AH: Therapeutic benefit of
intrathecal injection of marrow stromal cells on ischemia-injured
spinal cord. Ann Thorac Surg. 83:1484–1490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang B, Wang H, Sun XJ, Li XQ, Ai CY, Tan
WF, White PF and Ma H: Intrathecal transplantation of bone marrow
stromal cells attenuates blood-spinal cord barrier disruption
induced by spinal cord ischemia-reperfusion injury in rabbits. J
Vasc Surg. 58:1043–1052. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pagani FD, DerSimonian H, Zawadzka A,
Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH,
Eisen HJ and Aaronson KD: Autologous skeletal myoblasts
transplanted to ischemia-damaged myocardium in humans. Histological
analysis of cell survival and differentiation. J Am Coll Cardiol.
41:879–888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toma C, Pittenger MF, Cahill KS, Byrne BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hodgetts SI, Beilharz MW, Scalzo AA and
Grounds MD: Why do cultured transplanted myoblasts die in vivo? DNA
quantifi-cation shows enhanced survival of donor male myoblasts in
host mice depleted of CD4+ and CD8+ cells or Nk1.1+ cells. Cell
transplant. 9:489–502. 2000.PubMed/NCBI
|
|
12
|
Huang X, Su K, Zhou L, Shen G, Dong Q, Lou
Y and Zheng S: Hypoxia preconditioning of mesenchymal stromal cells
enhances PC3 cell lymphatic metastasis accompanied by VEGFR-3/CCR7
activation. J Cell Biochem. 114:2834–2841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HW, Haider HK, Jiang S and Ashraf M:
Ischemic preconditioning augments survival of stem cells via
miR-210 expression by targeting caspase-8-associated protein 2. J
Biol Chem. 284:33161–33168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peterson KM, Aly A, Lerman A, Lerman LO
and Rodriguez-Porcel M: Improved survival of mesenchymal stromal
cell after hypoxia preconditioning: Role of oxidative stress. Life
Sci. 88:65–73. 2011. View Article : Google Scholar :
|
|
15
|
Liu H, Liu S, Li Y, Wang X, Xue W, Ge G
and Luo X: The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic
effects of hypoxia-preconditioned mesenchymal stem cells for renal
ischemia/reperfusion injury. PloS one. 7:e346082012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang CP, Chio CC, Cheong CU, Chao CM,
Cheng BC and Lin MT: Hypoxic preconditioning enhances the
therapeutic potential of the secretome from cultured human
mesenchymal stem cells in experimental traumatic brain injury. Clin
Sci (Lond). 124:165–176. 2013. View Article : Google Scholar
|
|
17
|
Carr CA, Stuckey DJ, Tatton L, Tyler DJ,
Hale SJ, Sweeney D, Schneider JE, Martin-Rendon E, Radda GK,
Harding SE, et al: Bone marrow-derived stromal cells home to and
remain in the infarcted rat heart but fail to improve function: An
in vivo cine-MRI study. Am J Physiol Heart Circ Physiol.
295:H533–H542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen
Z, Zhang M, He J and Zheng S: Hypoxia preconditioned bone marrow
mesenchymal stem cells promote liver regeneration in a rat massive
hepatectomy model. Stem Cell Res Ther. 4:832013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lang-Lazdunski L, Heurteaux C, Mignon A,
Mantz J, Widmann C, Desmonts J and Lazdunski M: Ischemic spinal
cord injury induced by aortic cross-clamping: Prevention by
riluzole. Eur J Cardiothorac Surg. 18:174–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, Xie K, Li J, Xu N, Gong G, Wang
G, Yu Y, Dong H and Xiong L: Beneficial effects of hydrogen gas
against spinal cord ischemia-reperfusion injury in rabbits. Brain
Res. 1378:125–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Ding Q, Zhou Y, Gou X, Hou L, Chen
S, Zhu Z and Xiong L: Ethyl pyruvate attenuates spinal cord
ischemic injury with a wide therapeutic window through inhibiting
high-mobility group box 1 release in rabbits. Anesthesiology.
110:1279–1286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary
A, Zhu M, Ashjian P, Benhaim P, Hedrick MH and Fraser JK:
Differential expression of stem cell mobilization-associated
molecules on multi-lineage cells from adipose tissue and bone
marrow. Immunol Lett. 89:267–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kinnaird T, Stabile E, Burnett MS, Lee CW,
Barr S, Fuchs S and Epstein SE: Marrow-derived stromal cells
express genes encoding a broad spectrum of arteriogenic cytokines
and promote in vitro and in vivo arteriogenesis through paracrine
mechanisms. Circ Res. 94:678–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Das R, Jahr H, van Osch GJ and Farrell E:
The role of hypoxia in bone marrow-derived mesenchymal stem cells:
Considerations for regenerative medicine approaches. Tissue Eng
Part B Rev. 16:159–168. 2010. View Article : Google Scholar
|
|
27
|
Rosová I, Dao M, Capoccia B, Link D and
Nolta JA: Hypoxic preconditioning results in increased motility and
improved therapeutic potential of human mesenchymal stem cells.
Stem Cells. 26:2173–2182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bernaudin M, Tang Y, Reilly M, Petit E and
Sharp FR: Brain genomic response following hypoxia and
re-oxygenation in the neonatal rat. Identification of genes that
might contribute to hypoxia-induced ischemic tolerance. J Biol
Chem. 277:39728–39738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan W and Kastin AJ: Cytokine transport
across the injured blood-spinal cord barrier. Curr Pharm Des.
14:1620–1624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Profyris C, Cheema SS, Zang D, Azari MF,
Boyle K and Petratos S: Degenerative and regenerative mechanisms
governing spinal cord injury. Neurobiol Dis. 15:415–436. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang JA, He A, Hu X, Jiang Y, Sun Y, Jiang
J, Gui C, Wang Y and Chen H: Anoxic preconditioning: A way to
enhance the cardiopro-tection of mesenchymal stem cells. Int J
Cardiol. 133:410–412. 2009. View Article : Google Scholar
|
|
32
|
Li JH, Zhang N and Wang JA: Improved
anti-apoptotic and anti-remodeling potency of bone marrow
mesenchymal stem cells by anoxic pre-conditioning in diabetic
cardiomyopathy. J Endocrinol Invest. 31:103–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He A, Jiang Y, Gui C, Sun Y, Li J and Wang
JA: The anti-apoptotic effect of mesenchymal stem cell
transplantation on ischemic myocardium is enhanced by anoxic
preconditioning. Can J Cardiol. 25:353–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kanichai M, Ferguson D, Prendergast PJ and
Campbell VA: Hypoxia promotes chondrogenesis in rat mesenchymal
stem cells: A role for AKT and hypoxia-inducible factor
(HIF)-1alpha. J Cell Physiol. 216:708–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Xue W, Ge G, Luo X, Li Y, Xiang H,
Ding X, Tian P and Tian X: Hypoxic preconditioning advances CXCR4
and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem
Biophys Res Commun. 401:509–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma D, Lim T, Xu J, Tang H, Wan Y, Zhao H,
Hossain M, Maxwell PH and Maze M: Xenon preconditioning protects
against renal ischemic-reperfusion injury via HIF-1alpha
activation. J Am Soc Nephrol. 20:713–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh N, Sharma G and Mishra V: Hypoxia
inducible factor-1: Its potential role in cerebral ischemia. Cell
Mol Neurobiol. 32:491–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirota K: Hypoxia-inducible factor 1, a
master transcription factor of cellular hypoxic gene expression. J
Anesth. 16:150–159. 2002. View Article : Google Scholar
|