Introduction
The development of skeletal muscle is controlled by
an evolutionarily conserved transcription factor network involving
in the regulation of genes associated with muscle differentiation,
contractility and growth (1,2).
Myocyte enhancer factor-2 (MEF2) is the longest known myogenic
transcription factor, and most muscle-associated genes are directly
activated by MEF2 through combined interaction with other
transcription factors (3). Studies
have demonstrated that, in addition to activating genes involved in
muscle contraction and differentiation, myogenic transcription
factors regulate the expression of a number of conserved microRNAs
(miRNAs) that function to 'fine-tune' the output of transcriptional
networks, leading to precise cellular responses to pathological,
physiological and developmental signals (4). As individual miRNAs can regulate
hundreds of mRNAs and individual mRNAs are in turn targeted by
numerous miRNAs, miRNAs add complexity and accuracy to the
regulation of the core muscle transcriptional network (5).
miRNAs are endogenous, small, non-coding RNAs of ~22
nt in length that have emerged as powerful negative regulators of
gene expression (6). By
selectively repressing the activity of the 3′-untranslated region
(3′-UTR) of target mRNAs, miRNAs confer appropriate timing and
robustness in differentiation programmes. A set of miRNAs,
including miRNA-1, miRNA-133, miRNA-206, miRNA-208, miRNA-486 and
miRNA-499, have been identified to be highly enriched in skeletal
and/or cardiac muscle (7). Certain
key myogenic regulatory factors (MRFs), including myogenin and
myogenic differentiation 1 (MyoD1), are known to regulate these
miRNAs (8,9). Several miRNAs that are normally
induced during myogenic differentiation can initiate the myogenic
program in C2C12 myoblasts, even in the presence of high serum
(10,11). miRNA-378, a cardiac-enriched miRNA,
has been shown to directly target insulin-like growth factor 1
receptor and regulate post-natal cardiac re-modeling (12). However, the functions of miRNA-378
in the differentiation of skeletal muscle have largely remained
elusive.
To reveal the underlying mechanisms of the roles of
miRNA-378 in myoblast differentiation, the present study employed a
gain-of-function approach by transfecting miRNA-378 mimics into
C2C12 myoblast cells and analyzing the resulting gene expression
profiles by means of genome-wide mRNA deep sequencing. The present
study demonstrated that miRNA-378 promoted myoblast differentiation
by targeting the bone morphogenetic protein 4 (BMP4) gene.
Materials and methods
Cell culture
C2C12 myoblasts (Shanghai Cell Bank of the Chinese
Academy of Sciences, Shanghai, China) were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 20% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
atmosphere with 5% CO2. Transfection with miRNA mimics
or plasmids was performed using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols.
cDNA library construction
C2C12 cells transfected with miRNA-378 mimics or
control RNA (synthesized by Suzhou GenePharma Co., Ltd., Suzhou,
China) were collected, and total RNA was then extracted using
TRIzol reagent (Invitrogen). The sequences of the miRNA-378 mimics
are as follows: Sense, 5′-CUCCUGACUCCAGGUCCUGUGU-3′ and antisense,
5′-ACACAGGACCUGGAGUCAGGAG-3′. The quality of the RNA was determined
with a NanoDrop ND-1000 (Nanodrop Technologies, Wilmington, DE,
USA), and detected with 1% agarose gels (Beyotime Institute of
Biotechnology, Haimen, China). Total RNA (10 µg) was used
for sequencing with an Illumina Hiseq2000 (Illumina, San Diego, CA,
USA). To construct a cDNA library, oligo (dT) beads (Qiagen GmbH,
Hilden, Germany) were used to isolate mRNA. The mRNA was digested
by 10 U/µl EcoRI (Takara Biotechnology Co., Ltd.,
Dalian, China) into short fragments, and first-strand cDNA was
synthesized using random hexamer primers (Takara Biotechnology Co.,
Ltd.). Following synthesis of the second-strand cDNA, the
double-stranded cDNA was purified using a QIAquick PCR Extraction
kit (Qiagen GmbH) following the manufacturer's instructions. The
purified fragments were enriched by polymerase chain reaction (PCR)
to generate a cDNA library (13)
and were then sequenced with the IlluminaHiseq2000.
Data assembly and annotation
Raw data were generated by sequencing using the
Illumina Hiseq2000. The data were assembled into transcripts,
unigenes and contigs with Trinity de novo software (version
3.1; http://www.blast2go.com), excluding the
low-quality sequences, adaptors, sequences with uncertain bases and
sequences of >50 bp. To identify differentially expressed genes
between miRNA-378-transfected samples and control RNA-transfected
samples, expression ratios were calculated using the Limma
algorithm in R, applying moderated t-tests. Benjamini-Hochberg
correction was then used for correcting multiple hypothesis
testing, in which the q-value was calculated for each P-value.
Genes with an absolute log2 expression ratio of >0.6 between
miRNA-378-transfected and control RNA-transfected group and a
q-value of <0.005 were considered to be significant under the
corresponding treatment conditions. In addition, to identify
enriched Gene Ontology (GO) terms (http://www.geneontology.org) in the sets of
differentially expressed genes, the Database for Annotation,
Visualization and Integrated Discovery Bioinformatics Resource
(14) was used, where P<0.01
was considered to indicate a statistically significant difference.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) database
(http://www.genome.jp/kegg/) was used to
analyze the metabolic pathways of the genes.
Target gene prediction
Miranda databases (www.microrna.org/) and TargetScan version 4.0
(www.targetscan.org/) were employed to
identify potential target genes of miRNA-378 that were
downregulated in miRNA-378-transfected C2C12 cells compared with
control RNA-transfected cells. Following identification of
potential target genes, SigTerm software (version 7.0) was used to
confirm the prediction results.
Plasmid construction
The BMP4 or transforming growth factor beta 2
(TGFB2) 3′-UTR sequences were PCR-amplified from C2C12 genomic DNA
and cloned into the pGL3-control vector (Promega Corporation,
Madison, WI, USA). Amplification was conducted using primers from
Sangon Biotech Co., Ltd., Shanghai, China) in a PTC240 PCR system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction
mixture was incubated at 95°C for 30 sec, followed by 30 cycles of
95°C for 30 sec, 56°C for 30 sec and 72°C for 60 sec. The products
were sequenced to verify the results. Mutagenesis was performed by
PCR (15), followed by DpnI
(10 U/µl; Takara Biotechnology Co., Ltd.) digestion to
remove parental template DNA and obtain the mutated sequences
BMP4-mut or TGFB2-mut.
Reverse-transcription quantitative
(RT-q)PCR
Cells were lysed and total RNA was extracted using
TRIzol reagent (Invitrogen) following the manufacturer's
instructions. cDNA synthesis was performed using the Superscript
III first-strand synthesis system (Invitrogen). qPCR was then
performed using SYBR Green PCR master mix in an ABI cycler (Thermo
Fisher Scientific, Inc.). ABI 7300 software (Thermo Fisher
Scientific, Inc.) was used for quantitative analysis.
Dual luciferase assay
To assess whether BMP4 or TGFB2 are direct targets
of miRNA-378, firefly luciferase reporter vectors (2 ng; Promega
Corporation) driven by fragments from the respective gene's 3′-UTR
or their mutants together with miRNA-378 mimics and a Renilla
luciferase control vector (1 ng) were co-transfected into C2C12
cells according to the manufacturer's protocols. Experiments were
analyzed using a dual-luciferase reporter assay system (Promega
Corporation) following the manufacturer's protocols. The luciferase
signal was quantified using a luminometer (Monolight 3020; BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
At two days following transfection with miRNA-378
mimics or control RNA (100 nM), C2C12 cells were lysed in
radioimmunoprecipitation buffer [Beyotime Institute of
Biotechnology; 150 mM sodium chloride, 1% Nonidet P-40, 0.1% sodium
dodecyl sulfate, 50 mM Tris-HCl (pH 8.0) and 0.5% sodium
deoxycholate] supplemented with 1% protease inhibitor cocktail
stock solution (Roche Diagnostics GmbH, Mannheim, Germany). Protein
concentration was determined by BCA Protein assay kit (Beyotime
Institute of Biotechnology). Proteins (20 µg) were subjected
to sodium dodecyl sulfate polyacrylamide gel electrophoresis
(Beyotime Institute of Biotechnology). After electroblotting onto a
polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica,
MA, USA), Tris-buffered saline/0.05% Tween-20 (TBST; Beyotime
Institute of Biotechnology) containing 1% skimmed milk was used to
block non-specific binding sites. The PVDF membrane was then
incubated with rabbit polyclonal anti-BMP4 (1:500 dilution; Abcam,
Cambridge, MA, USA; cat. no. ab39973), rabbit polyclonal anti-TGFB2
(1:200 dilution; Abcam; cat. no. ab66043), rabbit polyclonal
anti-MyoR (1:200 dilution; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA; cat. no. sc-366698), rabbit polyclonal anti-MyoD1 (1:200
dilution; Abcam; cat. no. ab64159), anti-MyHC (1:200 dilution;
Abcam; cat. no. ab185967) or with rabbit monoclonal
anti-glyceraldehyde 3-phosphate dehydrogenase (1:2,000 dilution;
Sigma-Aldrich, St. Louis, MO, USA; cat. no. G9545) for 1 h at room
temperature prior to washing three times for 10 min with TBST. The
membrane was then incubated with horseradish peroxidase-conjugated
goat anti-rabbit immunoglobulin G secondary antibodies (1:2,000
dilution; Abcam, cat. no. ab6721) for 1 h at room temperature.
Signals were analyzed using enhanced chemiluminescence reagent, ECL
Start (GE Healthcare, Little Chalfont, UK) and visualized with a
Bio-Rad ChemiDoc MP system (Bio-Rad Laboratories, Inc.).
Small interfering RNA (siRNA)
transfection
An siRNA against BMP4, termed si-BMP4 (Suzhou
GenePharma Co., Ltd.) was used to endogenously inhibit BMP4. The
sequences were as follows: Sense, 5′-AGGGCCAGGAAGAAGAAUAAUUUU-3′
and antisense, 5′-AUUAUUCUUCUUCCUGGCCCUUUU-3′. Cells were
transfected with 100 nM of si-BMP4, miRNA-378 or negative control
(NC) RNAs using Lipofectamine 2000 according to the manufacture's
protocols. After 24 h transfection, the cells were harvested and
subjected to the creatine kinase assay.
Creatine kinase (Ck) assay
Ck enzymatic activity was measured in cell lysates
using the EnzyChrom Creatine Kinase assay kit (ECPK-100; BioAssay
Systems LLC, Hayward, CA, USA) according to the manufacturer's
protocol and as described previously (16). In brief, cells were washed twice
with phosphate-buffered saline, lysed on ice for 10 min and scraped
to remove cellular debris. The supernatant was then enriched by
centrifuging at 8,000 × g for 10 min, and a 2.5-fold volume of
H2O was added, of which 10 µl was used for Ck
analysis. Evaluation was performed using the Bio-Rad BCA Protein
assay kit (Bio-Rad Laboratories, Inc.). The absorbance was measured
at 595 nm using a UV-1780 UV spectrophotometer (Shimadzu
Corporation, Kyoto, Japan) and the results were expressed in
arbitrary units with normalization to total protein content.
Statistical analysis
Values are expressed as the mean ± standard
deviation of at least three independent experiments. The two-tailed
Student's t-test was used for performing statistical
analysis in Excel software (version 2007). P<0.05 was considered
to indicate a statistically significant difference.
Results
Transcriptome analysis for the assessment
of aberrant genome-wide mRNA expression following miRNA-378
overexpression
To enhance the current understanding of the role of
miRNA-378 in C2C12-cell differentiation, the present study
performed a genome-wide gene expression analysis of
miRNA-378-transfected cells in comparison with control
RNA-transfected cells. A total of 2,802 genes that were
differentially expressed by >1.5-fold following miRNA-378
treatment for 24 h were identified. To evaluate the potential
functional significance of the differentially expressed genes, this
set of genes was subjected to gene ontology (GO) pathway enrichment
analysis. The results revealed a significant enrichment of a number
of GO terms associated with the cell cycle (Fig. 1A). To further explore the canonical
pathways involved, the genes were then subjected to signaling
pathway enrichment analysis with the KEGG. As shown in Fig. 1B, the 15 most significantly
upregulated pathways included the p53 signaling pathway and Wnt
signaling pathway.
The 706 downregulated genes were then compared with
the 182 predicted target genes of miRNA-378 obtained using the
TargetScan and Miranda databases (Fig.
2A). A total of 113 candidate target genes of miRNA-378 were
thereby identified, which were also significantly overrepresented
in the downregulated gene sets obtained by SigTerm software
(http://sigterms.sourceforge.net)
analysis (17). The candidate
genes included TGFB2, MKX, PAX7, RXRA and BMP4, which function in
muscle development and muscle cell differentiation (10). Furthermore, of these candidate
genes, the genes associated with development or differentiation
were clustered as a regulatory network with miRNA-378, as
illustrated in Fig. 2B generated
by String software (version 9.0) (18). The interaction network showed that
certain genes, including BMP4, RXRA, ACVR1 and SDC1, were
potentially down-regulated by miRNA-378, whereas other genes,
including QK, ATG7, CAV2 and GJC1, were upregulated by
miRNA-378.
BMP4 is a direct target of miRNA-378
To validate the results of the transcriptome
sequencing, eight genes, GJC1, ATG7, QK, MYOD1, TGFB2, RARB, TGFBR3
and BMP4, were selected for further qPCR analysis, since the
functions of these genes are involved in muscle differentiation or
development. The PCR results demonstrated that the mRNA levels of
four genes, GJC1, ATG7, QK and MYOD1, were significantly
upregulated, while TGFB2 and BMP4 were markedly down-regulated in
miRNA-378-transfected cells compared with the negative control
group. The mRNA expression of RARB and TGFBR3 was not markedly
affected by miRNA-378 (Fig.
3A).
 | Figure 3miR-378 directly targets BMP4. (A)
mRNA expression of eight genes in C2C12 cells transfected with
miR-378 mimics or control RNAs as determined by quantitative
polymerase chain reaction analysis with normalization to GAPDH.
Expression levels relative to those in the negative control group
are shown. Values are expressed as the mean ± standard deviation
from three different experiments. (B) Western blot analysis of the
BMP4 and TGFB2 proteins in miR-378 mimics or control
RNA-transfected C2C12 cells. GAPDH served as protein loading
control. Representative blots of three independent experiments are
shown. (C and D) A dual luciferase reporter assay was performed to
identify direct target genes of miR-378. Cells were transfected
with luciferase vectors driven by a fragment of the 3′-untranslated
region of BMP4 or TGFB2, respectively, or a mutated sequence, as
well as mmu-miR-378. Firefly luciferase/Renilla luciferase activity
was expressed relative to that of the negative control. Values are
expressed as the mean ± standard deviation (n=3) from three
independent experiments. *P<0.05,
**P<0.01 vs. the NC group. miR/miRNA, microRNA; BMP4,
bone morphogenetic protein 4; TGFB2, transforming growth factor
beta 2; NC, negative control; mut, mutated; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; ctrl, control. |
To further examine the potential targets of
miRNA-378, western blot analysis of BMP4 and TGFB2 protein
expression was performed in miRNA-378-transfected C2C12 cells.
Transfection of miRNA-378 resulted in an obvious decrease in
endogenous BMP4 protein, but had no effect on the protein levels of
TGFB2 (Fig. 3B). These results
indicated that miRNA-378 affected BMP4 expression at the mRNA as
well as the protein level. To further validate whether miRNA-378 is
a direct regulator of BMP4 or TGFB2, luciferase plasmids containing
the BMP4 or TGFB2 3′-UTR downstream of the luciferase gene were
constructed and used in a dual luciferase assay with miRNA-378. The
results showed that in miRNA-378-transfected cells, the luciferase
activity of the BMP4 3′-UTR-driven vector was reduced by ~60%
compared with that in the negative control-transfected group;
however, luciferase activity of the mutant luciferase reporter
vector for BMP4 was not affected by miRNA-378 (Fig. 3C). This result confirmed that BMP4
is a direct target of miRNA-378. However, miRNA-378 did not affect
the luciferase activity of the TGFB2 or TGFB2-mut reporter vectors,
suggesting that miRNA-378 did not directly target the TGFB2 gene
(Fig. 3D).
Effect of miRNA-378 on myogenic
differentiation
Finally, the present study investigated the overall
effect of miRNA-378 overexpression on the myogenic differentiation
of C2C12 by assessing the expression of a panel of differentiation
biomarkers. As shown in Fig. 4A,
C2C12 cells transfected with miRNA-378 mimics showed a
significantly increased expression of myosin heavy chain (MyHC) and
MyoD1 proteins, which are commonly detected in post-mitotic muscle
cells and in committed proliferating myoblast cells, respectively,
along with a concomitant decrease in the expression of myogenic
repressor (MyoR), an inhibitor of myogenesis (10). Furthermore, the effect on myogenic
differentiation was evaluated by comparing Ck activity in
miRNA-378-transfected cells and control RNA-transfected cells. Ck
activity in miRNA-378-transfected cells was significantly higher
than that in control RNA-treated cells (Fig. 4B). The present study further
examined whether knockdown of endogenous BMP4 was able to promote
myogenic differentiation in C2C12 cells. As shown in Fig. 4B, transfection of BMP4 siRNA and
miRNA-378 resulted in an increase of Ck activity compared with the
control group. This indicates that the downregulation of BMP4
mimics the function of miRNA-378 on myogenic differentiation of
C2C12 cells, further indicating that miRNA-378 regulates myogenic
differentiation by down-regulating BMP4.
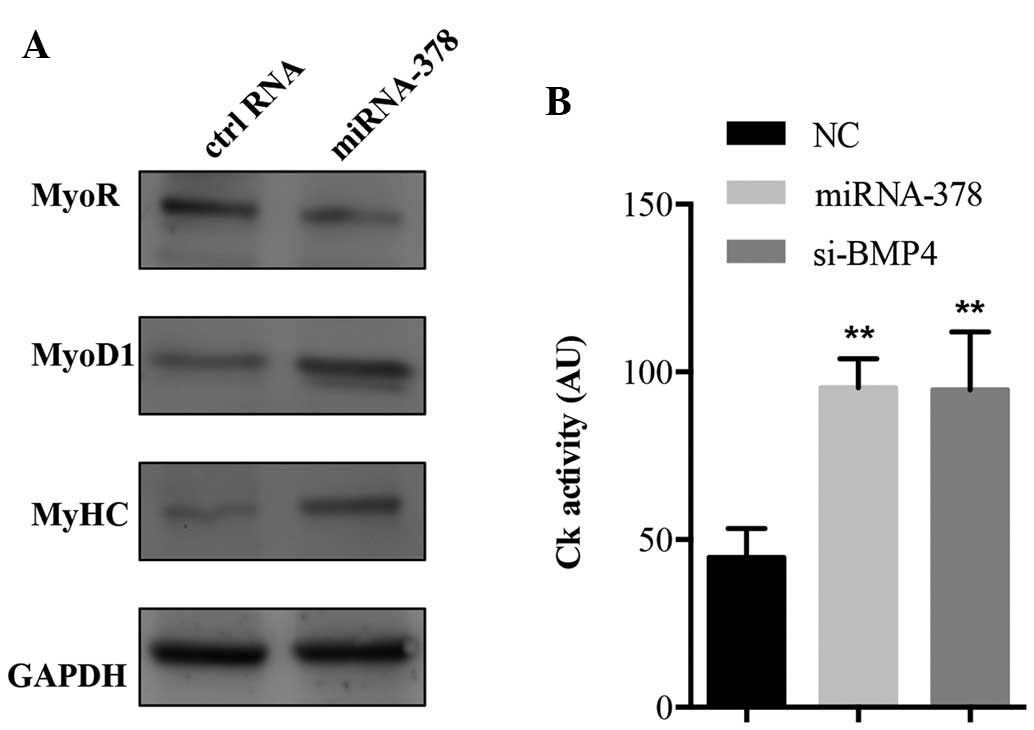 | Figure 4miRNA-378 promotes myogenic
differentiation. (A) Western blot analysis of MyoR, MyoD1 and MyHC
protein in the miRNA-378 mimics or control RNA-transfected C2C12
cells. GAPDH served as protein loading control. Representative
blots of three experiments are shown. (B) C2C12 cells transfected
with miRNA-378 mimics, si-BMP4 or control RNAs were analyzed for Ck
activity. Values are expressed as the mean ± standard deviation of
three independent experiments. **P<0.001 vs. the NC
group. miRNA, microRNA; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; NC, negative control; siRNA, small interfering RNA;
BMP4, bone morphogenetic protein 4; Ck, creatine kinase; MyoR,
myogenic repressor; MyoD1, myogenic differentiation 1; MyHC, myosin
heavy chain. |
Discussion
The present study performed a genome-wide mRNA deep
sequencing analysis to investigate the changes in mRNA expression
in miRNA-378-transfected C2C12 cells and demonstrated that
miRNA-378 promoted myogenic differentiation by directly targeting
the BMP4 gene.
The process of muscle differentiation occurring in
embryonic as well as adult muscle precursors is accompanied by the
induction of a specific class of miRNAs, whose expression is driven
by MRFs and other myogenic-dependent transcription factors
(8,19). Functionally, two different groups
of miRNAs are found in muscle tissue: i) Non-muscle-specific miRNAs
which are present in muscles, but are also expressed in other
tissues (20) and ii)
muscle-specific miRNAs, which are present in cardiac and skeletal
muscle tissues in greater amounts compared with other tissues
(20,21). Muscle-specific miRNAs were indeed
shown to act on various levels in the regulation of muscle
homoeostasis and differentiation, and their expression was found to
be aberrant in certain muscular disorders, including Duchenne
muscular dystrophy, myocardial infarction and other types of
myopathy (22,23). Among muscle-specific miRNAs, the
miR-1/206 and miR-133 families, which originate from three
different chromosomes (10), have
been most extensively studied and have been shown to take multiple
roles in the modulation of muscle differentiation (24). In addition, several
non-muscle-specific miRNAs, including miRNA-24, miRNA-214 and
miRNA-26a have been shown to participate in the differentiation
process into muscle cells (25–27).
Studies have indicated that miRNA-378 is involved in
skeletal muscle development (28,29).
In the C2C12 myoblast cell line, upregulation of miRNA-378 has been
shown to promote efficient myotube formation by repressing
antagonists of differentiation, such as MyoR (29). Likewise, miRNA-378 expression in
porcine longissimus muscles was shown to be implicated in the
modulation of myogenesis, mainly with regard to fibre formation
(28). Recently, miRNA-378 has
been shown to induce skeletal muscle differentiation in the RH30
human alveolar rhabdomyosarcoma cell line (13). In accordance with these findings,
the present study demonstrated that miRNA-378 promoted myogenic
differentiation of C2C12 cells via the BMP4 gene. Aberrant
expression of specific myogenic biomarkers indicated the induction
of myogenic differentiation. Transfection of miRNA-378 resulted in
a significant increase of MyoD1 and MyHC protein and a decrease of
MyoR protein. Furthermore, transfection of C2C12 cells with siRNA
against BMP4 mimicked the effect generated by miRNA-378, suggesting
that miRNA-378 activated myogenic differentiation by targeting
BMP4.
In conclusion, the present study demonstrated that
miRNA-378 promoted myogenic differentiation by down-regulating
BMP4. These findings enhanced the current understanding of the
biological mechanisms underlying muscle differentiation.
Acknowledgments
The present study was supported by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions, Jiangsu Co-innovation Center for Prevention and
Control of Important Animal Infectious Diseases and Zoonoses, and
the National Natural Science Foundation of China (grant nos.
31101683 and 31272405).
References
|
1
|
Buckingham M: Skeletal muscle formation in
vertebrates. Curr Opin Genet Dev. 11:440–448. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckingham M: Myogenic progenitor cells
and skeletal myogenesis in vertebrates. Curr Opin Genet Dev.
16:525–532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satou Y and Satoh N: Gene regulatory
networks for the development and evolution of the chordate heart.
Genes Dev. 20:2634–2638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams AH, Liu N, van Rooij E and Olson
EN: MicroRNA control of muscle development and disease. Curr Opin
Cell Biol. 21:461–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang F, Niu G, Chen X and Cao F: Molecular
imaging of microRNAs. Eur J Nucl Med Mol Imaging. 38:1572–1579.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goljanek-Whysall K, Sweetman D and
Munsterberg AE: MicroRNAs in skeletal muscle differentiation and
disease. Clin Sci (Lond). 123:611–625. 2012. View Article : Google Scholar
|
|
8
|
Rao PK, Kumar RM, Farkhondeh M,
Baskerville S and Lodish HF: Myogenic factors that regulate
expression of muscle-specific microRNAs. Proc Natl Acad Sci USA.
103:8721–8726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenberg MI, Georges SA, Asawachaicharn
A, Analau E and Tapscott SJ: MyoD inhibits Fstl1 and Utrn
expression by inducing transcription of miR-206. J Cell Biol.
175:77–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JF, Mandel EM, Thomson JM, Wu Q,
Callis TE, Hammond SM, Conlon FL and Wang DZ: The role of
microRNA-1 and microRNA-133 in skeletal muscle proliferation and
differentiation. Nat Genet. 38:228–233. 2006. View Article : Google Scholar
|
|
11
|
Kim HK, Lee YS, Sivaprasad U, Malhotra A
and Dutta A: Muscle-specific microRNA miR-206 promotes muscle
differentiation. J Cell Biol. 174:677–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Knezevic I, Patel A, Sundaresan NR, Gupta
MP, Solaro RJ, Nagalingam RS and Gupta M: A novel
cardiomyocyte-enriched microRNA, miR-378, targets insulin-like
growth factor 1 receptor: Implications in postnatal cardiac
remodeling and cell survival. J Biol Chem. 287:12913–12926. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Megiorni F, Cialfi S, McDowell HP, Felsani
A, Camero S, Guffanti A, Pizer B, Clerico A, De Grazia A, Pizzuti
A, et al: Deep Sequencing the microRNA profile in rhabdomyosarcoma
reveals down-regulation of miR-378 family members. BMC Cancer.
14:8802014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada A: PCR-based site-directed
mutagenesis. Methods Mol Biol. 57:157–165. 1996.PubMed/NCBI
|
|
16
|
Hupkes M, Sotoca AM, Hendriks JM, van
Zoelen EJ and Dechering KJ: MicroRNA miR-378 promotes BMP2-induced
osteogenic differentiation of mesenchymal progenitor cells. BMC Mol
Biol. 15:12014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Creighton CJ, Nagaraja AK, Hanash SM,
Matzuk MM and Gunaratne PH: A bioinformatics tool for linking gene
expression profiling results with public databases of microRNA
target predictions. RNA. 14:2290–2296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
Peer, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39:D561–568. 2011. View Article : Google Scholar :
|
|
19
|
Zhao Y, Samal E and Srivastava D: Serum
response factor regulates a muscle-specific microRNA that targets
Hand2 during cardiogenesis. Nature. 436:214–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCarthy JJ: MicroRNA-206: The skeletal
muscle-specific myomiR. Biochim Biophys Acta. 1779:682–691. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenberg I, Eran A, Nishino I, Moggio M,
Lamperti C, Amato AA, Lidov HG, Kang PB, North KN,
Mitrani-Rosenbaum S, et al: Distinctive patterns of microRNA
expression in primary muscular disorders. Proc Natl Acad Sci USA.
104:17016–17021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cacchiarelli D, Martone J, Girardi E,
Cesana M, Incitti T, Morlando M, Nicoletti C, Santini T, Sthandier
O, Barberi L, et al: MicroRNAs involved in molecular circuitries
relevant for the Duchenne muscular dystrophy pathogenesis are
controlled by the dystrophin/nNOS pathway. Cell Metab. 12:341–351.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu N, Williams AH, Kim Y, McAnally J,
Bezprozvannaya S, Sutherland LB, Richardson JA, Bassel-Duby R and
Olson EN: An intragenic MEF2-dependent enhancer directs
muscle-specific expression of microRNAs 1 and 133. Proc Natl Acad
Sci USA. 104:20844–20849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng Y, Cao JH, Li XY and Zhao SH:
Inhibition of miR-214 expression represses proliferation and
differentiation of C2C12 myoblasts. Cell Biochem Funct. 29:378–383.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Q, Zhang Y, Yang G, Chen X, Zhang Y,
Cao G, Wang J, Sun Y, Zhang P, Fan M, et al: Transforming growth
factor-beta-regulated miR-24 promotes skeletal muscle
differentiation. Nucleic Acids Res. 36:2690–2699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong CF and Tellam RL: MicroRNA-26a
targets the histone methyltransferase Enhancer of Zeste homolog 2
during myogenesis. J Biol Chem. 283:9836–9843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou X, Tang Z, Liu H, Wang N, Ju H and Li
K: Discovery of MicroRNAs associated with myogenesis by deep
sequencing of serial developmental skeletal muscles in pigs. PLoS
One. 7:e521232012. View Article : Google Scholar
|
|
29
|
Gagan J, Dey BK, Layer R, Yan Z and Dutta
A: MicroRNA-378 targets the myogenic repressor MyoR during myoblast
differentiation. J Biol Chem. 286:19431–19438. 2011. View Article : Google Scholar : PubMed/NCBI
|


















