Introduction
Pancreatic cancer often has a poor prognosis, with
the median survival rates for metastatic disease, which
collectively accounts for >80% of the affected individuals, is
~6 months (1). This process of
metastasis is not random (2). A
cascade of complex interactions between the cancer cell and its
surroundings results in a metastatic cascade (3), in which the tumor cells initially
break signaling contact with neighboring cells, degrade and
penetrate the basement membrane and then invade the interstitial
stroma in order to reach the blood/lymph vessels (4,5).
Therefore, there is an urgent requirement to develop molecular
diagnostic biomarkers and targets to detect pancreatic cancer at an
earlier stage, which may assist in improving the treatment success
and survival rates of patients with pancreatic cancer.
Cyclooxygenase (COX), also known as prostaglandin
endoperoxidase or prostaglandin G/H synthase, is a rate-limiting
enzyme involved in the conversion of arachidonic acid to
prostaglandins (6). Two forms of
COX, COX-1 and COX-2, have been identified. COX-1 is constitutively
expressed in several tissues and is responsible for various
physiological functions, whereas COX-2 is an inducible enzyme,
originally found to be induced by growth factors and other stimuli
(7). Previous studies have
demonstrated the relevance of COX-2 in human cancer, including
colorectal cancer, cholangiocarcinoma, liver cancer, esophageal
carcinoma, gastric cancer and pancreatic cancer (8–13).
Determinants of cell invasion, including cell motility, adhesion to
the extracellular matrix and the gelatinolytic activity of
metalloproteinase, are also modulated in COX-2-positive pancreatic
cancer cells (14). Therefore,
COX-2-specific inhibitors may provide a useful anti-invasive
therapeutic option in the treatment of pancreatic cancer.
NS-398 is a selective inhibitor of COX-2 (15). Previous studies have indicated that
NS-398 resists carcinogenesis through anti-angiogenic and
pro-apoptotic effects (16). Few
studies have focused on the anti-invasive and antimetastatic
effects of NS-398 in cancer, and the effects of NS-398 on
pancreatic cancer cell invasiveness, and its exact underlying
mechanisms remain to be fully elucidated.
In the present study, the proliferative and invasive
effects of NS-398 on pancreatic cancer cells were evaluated. The
corresponding molecular mechanisms underpinning the observed
effects have also been analyzed in depth. The present study has
provided an experimental basis for the joint application of NS-398
and P38 inhibitors in pancreatic cancer cells.
Materials and methods
Reagents
NS-398 was obtained from Sigma-Aldrich (St. Louis,
MO, USA). The NS-398 was dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) at a stock concentration of 100
mM, and stored at −20°C. SB-203580 was obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA) and dissolved in DMSO at a
stock concentration of 10 mM. All stock solutions were wrapped in
foil and maintained at 4°C or −20°C.
Cell culture
The PANC-1 pancreatic cancer cell line was purchased
from the Shanghai Institutes for Biological Sciences (Shanghai,
China). The cells were maintained in humidified room air containing
5% CO2 at 37°C, and were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Cells in the logarithmic phase of growth were used in all
subsequent experiments.
Cell Counting Kit-8 assay
The pancreatic cancer cells were seeded at a
concentration of 5×103 cells/200 µl/well in
96-well culture plates for the cell proliferation assay. Briefly,
the cultured wells were treated with 20 µl/well of Cell
Counting Kit-8 reagent (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) during the final 2 h of a 24 h incubation period
at 37°C, and the optical density (OD) of each well was measured at
450 nm using a Model 680 Bio-Rad microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The results of cell
proliferation measurement were expressed as the absorbance at
OD450.
Enzyme-linked immunosorbent assay
(ELISA)
The pancreatic cancer cells (3×105) were
seeded in each well of a 6-well plate (Corning Incorporated,
Corning, NY, USA) with 1 ml complete medium. The cells were starved
in serum-free DMEM for 24 h at 37°C prior to stimulation. Following
stimulation with NS 398 for the durations indicated subsequently in
the Figure legends and the Results section, the supernatant from
each culture medium was collected by centrifugation (4,000 × g) at
4°C, and the prostaglandin E2 (PGE-2) released into the conditioned
medium (100 µl of the cell supernatant) was quantified using
an ELISA kit for PGE-2 (KGE004b; R&D Systems, Minneapolis, MN,
USA), strictly following the manufacturer's protocol. The OD of
each well was determined within 30 min using a microplate reader
(Thermo Labsystems, Santa Rosa, CA, USA) at 450 nm.
Small interfering (si)RNA
The human CD147 siRNA and negative control siRNA
were purchased from GenePharma (Shanghai, China). The siRNA
sequence of CD147 was as follows: Sense 5′-GGUUCUUCGUGAGUUCCUCTT-3′
and antisense 5′-GAGGAACUCACGAAGAACCTG-3′. The negative control (NC
siRNA sequence was as follows: Sense 5′-UUCUCCGAACGUGUCACGUTT-3′
and antisense 5′-ACGUGACACGUUCGGAGAATT-3′. DharmaFECT-4
transfection reagent were purchased from Dharmacon, Inc.
(Lafayette, CO, USA). The PANC-1 cells were seeded in 6-well plates
at a density of 2.5×105 cells/well in MEM, and were
grown for 16 h at 37°C. Transfection was performed according to the
manufacturer's instructions, using 4 µl of DharmaFECT-4
reagent and siRNA, at a final concentration of 100 nM/well. The
cells were cultured for a further 24 h at 37°C, following which the
total protein extracts (see the 'western blot analysis' section
below) were isolated and analyzed using rabbit anti-human CD147
(cat. no. 13287; 1:1,000) and rabbit anti-human β-actin (cat. no.
4970; 1:1,000) antibodies (both obtained from Cell Signaling
Technology, Danvers, MA, USA) for immunoblotting. The cells were
collected, washed twice with pre-cooled phosphate-buffered saline
(PBS), and invasion was measured.
Western blot analysis
The cells were lysed in SDS lysis buffer on ice for
30 min. Cell debris was removed by centrifugation at 14,000 g at
4°C for 5 min, and the protein contents of the cell lysates were
determined using a Quick Start™ Bio-Rad Protein Assay kit (cat. no.
CA5000201; Bio-Rad Laboratories, Inc.). The cell lysates with equal
protein content (20 or 40 µg) were then loaded and separated
by 12% SDS-PAGE (cat. no. PG113; Promoton, Nantong, Jiangsu,
China). The protein bands were then electro-transferred onto a
polyvinylidene fluoride membrane (Merck Millipore, Billerica, MA,
USA) and blocked with 5% non-fat milk in Tris-buffered saline
containing 0.1% Tween 20 (pH 7.4) for 1 h at 37°C. Subsequently,
the membrane was incubated with the following antibodies, as
appropriate: anti-c-Jun N-terminal kinase (JNK; cat. no. 9252;
1:1,000; rabbit anti-human), anti-phosphorylated (p-)JNK (cat. no.
9251; 1:1,000; rabbit anti-human), anti-extracellular
signal-regulated kinase (ERK; cat. no. 9102; 1:1,000; rabbit
anti-human), anti-p-ERK (cat. no. 9101; 1:1,000; rabbit
anti-human), anti-p38 (cat. no. 9212; 1:1,000; rabbit anti-human),
anti-p-p38 mitogen-activated protein kinases (MAPKs; cat. no. 9211;
1:1,000; rabbit anti-human; all antibodies from Cell Signaling
Technology, Inc.) and anti-MMP-2 (cat. no. sc-13594; 1:500; mouse
anti-human; Santa Cruz Biotechnology, Dallas, TX, USA) for at least
4 h. After washing three times, the membranes were incubated for 1
h at room temperature with species-specific horseradish peroxidase
(HRP)-conjugated secondary antibodies (goat anti-mouse antibody,
cat. no. 115-035-003; or goat anti-mouse antibody, cat. no.
111-035-003; 1:2,000; Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA). Immunoreactive bands were visualized using
Immobilon Western chemiluminescent HRP substrate (Merck Millipore).
As an internal control, the contents of β-actin in the samples were
also immunoblotted using polyclonal anti-actin antibody as a
primary antibody. The quantitative analysis of the proteins was
performed by normalizing against β-actin by using Image Pro Plus
6.0 software (Media Cybernetics, Inc., Silver Spring, MD, USA).
Invasion assay
The invasive abilities of the pancreatic cancer
cells were measured using a BD BioCoat™ BD Matrigel™ Invasion
Chamber (BD Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocol. Briefly, the PANC-1 cells were treated
with inhibitor or siRNA for 24 h, as described above. The cells
were then seeded onto the membrane of the upper chamber of the
Transwell, at a concentration of 3–5×105/ml in 2 ml
DMEM. The medium in the upper chamber was serum-free, and the
medium in the lower chamber contained 5% fetal calf serum as a
source of chemoattractants. The number of cells that passed through
the Matrigel-coated membrane were then counted on the undersides of
the filters using a Nikon Eclipse Ti light microscope (Nikon
Corporation, Tokyo, Japan).
Statistical analysis
Each sample was analyzed in triplicate, and
experiments were repeated three times. All data are presented as
the mean ± standard deviation. All statistical analyses were
performed using Microsoft Office Excel (Microsoft, Albuquerque, New
Mexico, USA) and STASTISCA (StatSoft, Tulsa, OK, USA). Differences
between mean values were evaluated using a paired t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
NS-398 enhances invasion and activates
P38 signaling molecules in pancreatic cancer cells
The expression of PGE-2, a product of COX-2, is
downregulated by NS-398 (17). In
the present study, treatment of the PANC-1 cells with 5 µM
NS-398 significantly reduced the levels of PGE-2, and 50 µM
NS-398 decreased PGE-2 to the lowest levels observed. However, the
CCK-8 kit, which was used to determine the effect of NS-398 on the
proliferation of the PANC-1 pancreatic cancer cells, found that
NS-398 had no significant inhibitory effect at either low or high
concentrations (between 5 and 100 µM). To investigate why
NS-398 was able to inhibit COX-2 activity, but did not lead to the
inhibition of pancreatic cancer cell proliferation, 50 µM
NS-398 was used to treat the pancreatic cancer cells over a
specific period of time, and the expression levels of MAPK
signaling molecules were analyzed. The results showed that the
p-P38 protein was significantly upregulated, however, the p-JNK and
p-ERK signaling molecules were unaffected. The increase in p-P38
was observed in the pancreatic cancer cells treated with NS-398 for
1 h, following which the levels of p-ERK gradually increased,
peaked at 12 h and were maintained to 24 h (Fig. 1A–D).
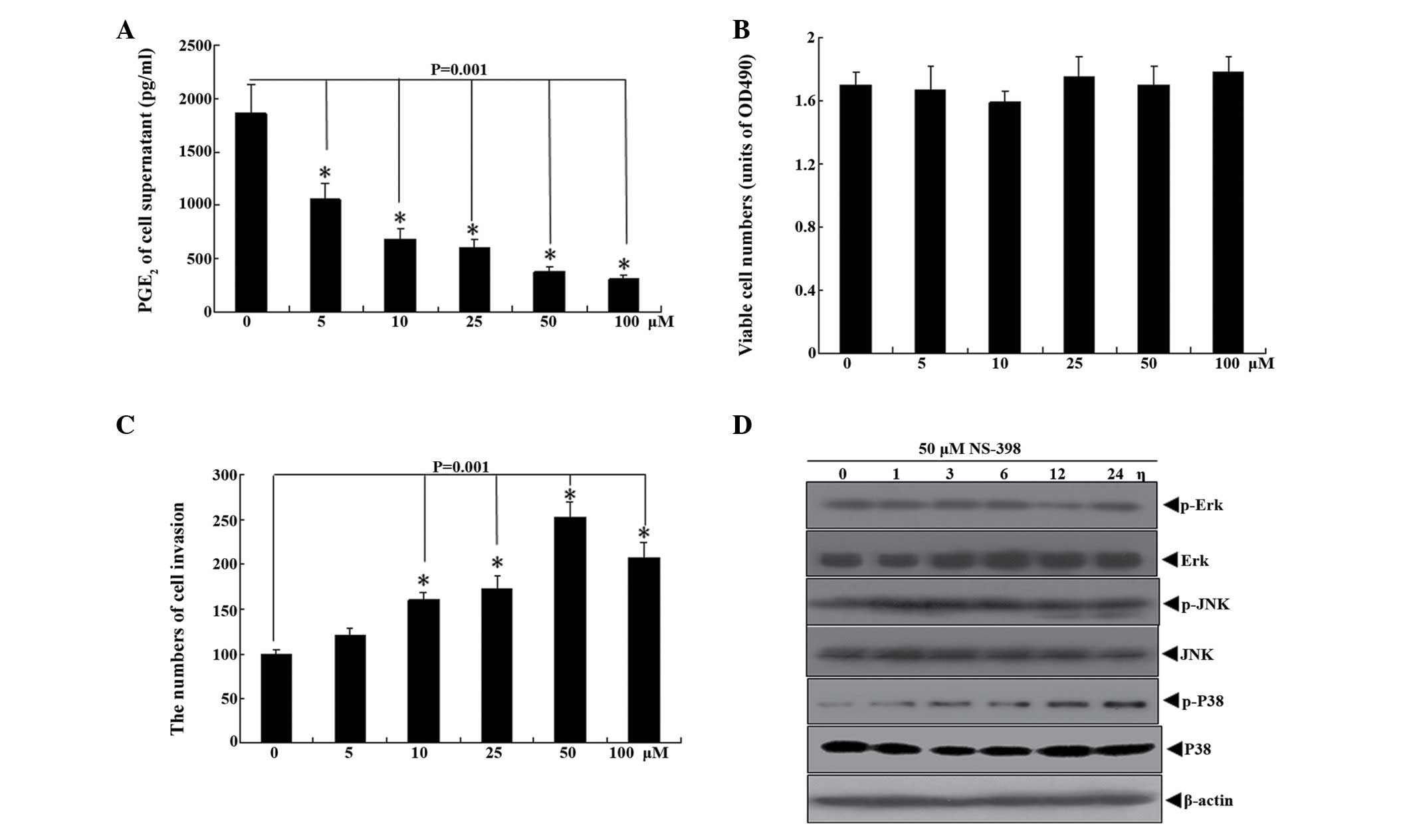 | Figure 1NS-398 enhances the invasiveness of
pancreatic cancer cells and activates P38. The PANC-1 pancreatic
cancer cells were treated with NS-398 (0, 5, 10, 25, 50 and 100
µM) for 24 h. (A) Supernatant containing PANC-1 cells
treated with NS-398 for 24 h was collected, following which the
expression of PGE-2 was detected using an enzyme-linked
immunosorbent assay. (B) Cell viability was detected using a
Cell-Counting-Kit 8 assay. (C) PANC-1 cells were treated with
NS-398 (0, 5, 10, 25, 50 and 100 µM) for 24 h, following
which the invasiveness of the PANC-1 cells was determined. The
number of invasive cells was determined under an inverted
microscope. (D) PANC-1 cells were treated with 50 µM NS-398
for 0, 1, 3, 6, 12 and 24 h. The expression levels of Erk, JNK,
P38, P-Erk, P-JNK and P-P38 were analyzed using western blotting.
Data are presented as the mean ± standard deviation. PGE-2,
prostaglandin E2; Erk, extracellular signal-regulated kinase; JNK,
c-Jun-N-terminal kinase; P-, phosphorylated. |
SB-203580 restores the inhibitory effect
of NS-398 on pancreatic cancer cell proliferation and inhibits the
enhancement of invasion induced by NS-398
Pretreatment of the cells with SB-203580, a P38
signaling molecule inhibitor (18), was observed to inhibit
NS-398-induced P38 activation. Accordingly, treatment with
SB-203580 led to NS-398 inhibiting the proliferation activity of
the pancreatic cancer cells. Invasive in vitro experiments
were also performed, which demonstrated that treatment with 50
µM NS-398 increased the invasiveness of the pancreatic
cancer cells, whereas the P38 inhibitor, SB-203580, significantly
reduced pancreatic cancer cell invasion, compared with the
NS-398-treated cells. SB-203580 was observed to completely inhibit
the NS-398-induced increase in pancreatic cancer cell invasiveness
(Fig. 2A and B).
NS-398 induces the expression levels of
CD147 and MMP-2 through the activation of P38 in pancreatic cancer
cells
The activation of the P38 signaling pathway is
closely associated with the activation of the CD147-MMP-2 signaling
pathway (19). In order to
determine the role of P38 signaling molecules in the induction of
CD147 and downstream MMP-2 by NS-398, the PANC-1 cells were treated
with 50 µM NS-398 for the indicated durations. The results
revealed that the levels of CD147 and MMP-2 were upregulated
following activation of the P38 signaling molecules. SB-203580
inhibited NS-398-induced P38 activation, and also inhibited the
expression levels of CD147 and downstream MMP-2 (Fig. 3A and B).
 | Figure 3NS-398 induces the expression of CD147
and MMP-2 via the activation of P38 in pancreatic cancer cells. (A)
PANC-1 cells were treated with 0, 5, 10, 25, 50 and 100 µM
NS-398 for 24 h. The expression levels of P-P38, CD147 and MMP-2
were analyzed using western blotting. (B) PANC-1 cells were
pretreated with 20 µM SB-203580 for 2 h, following which the
cells were incubated with or without 50 µM NS-398 for 24 h.
Western blot analysis was used to detect the expression levels of
P-P38, CD147 and MMP-2. (C and D) The densitometric analysis for
the data shown in (A) and (B) for P-P38, CD147 and MMP-2 is shown.
P-, phosphorylated; CD147, cluster of differentiation 147; MMP-2,
matrix metalloproteinase-2. |
CD147 siRNA inhibits the NS-398-induced
invasion of pancreatic cancer cells and restores anti-proliferation
activity
To further confirm the hypothesis that NS-398
mediates the CD147-MMP-2 signaling pathway, involved in pancreatic
cancer cell invasiveness, through the activation of P38 signaling
molecules, CD147 siRNAs were transfected into PANC-1 cells for 24
h. The cells were then treated with or without 50 µM NS-398,
and western blot analysis of the protein expression levels was
performed. The results indicated that the NS-398-induced expression
of MMP-2 was inhibited by the CD147 siRNAs, however, the activation
induced by NS-398 remained. Invasive in vitro experiments
were also performed, which revealed that the CD147 siRNAs
significantly inhibited the NS-398-induced increase in pancreatic
cancer cell invasiveness. CD147 siRNA treatment also restored the
anti-proliferative effect of NS-398 in the pancreatic cancer cells
(Fig. 4A–C).
 | Figure 4CD147 siRNA inhibits the expression of
MMP-2 induced by NS-398. siRNAs targeted specifically against CD147
or negative control siRNA (NC) were transiently transfected into
PANC-1 cells. At 24 h post-transfection, the cells were incubated
with or without 50 µM NS-398 for 24 h. (A) Expression levels
of CD147, MMP-2, P-P38 and P38 were detected using western
blotting, with β-actin as a loading control. (B) The densitometric
analysis of the western blot in (A) for P-P38, CD147 and MMP-2 is
shown. (C) Cell viability was evaluated using a Cell Counting Kit-8
assay. (D) Invasiveness of PANC-1 was determined using an inverted
microscope, under which number of invasive cells were counted. Data
are presented as the mean ± standard deviation. CD147, cluster of
differentiation 147; siRNA, small interfering RNA; Con, control;
NC, negative control; OD, optical density. |
Discussion
Previous studies have shown that COX-2 is
overexpressed or activated in pancreatic cancer cells (20–23).
In the present study, the effects of the COX-2 inhibitor, NS-398,
on cell viability, invasion and invasion-associated cellular
properties were determined. The resulting data indicated that
NS-398 did not inhibit proliferation, but it promoted pancreatic
cancer cell invasiveness. Furthermore, the results revealed that
treatment with NS-398 led to the activation of P38-mediated
proliferation and invasiveness of the pancreatic cancer cells.
In the present study, following incubation of the
pancreatic cancer cells with NS-398, the levels of PGE-2 in the
culture supernatant were reduced in a NS-398 dose-dependent manner.
These data are consistent with previous relevant reports on the
effects of NS-398 on COX-2 (15–24).
Notably, treatment with NS-398 had no significant effect on
pancreatic cancer cell proliferation or viability. NS-398 can
induce growth inhibition, and even apoptosis of tumor cells,
including gastric cancer and colorectal cancer (25,26).
The results of the present study suggested that NS-398 activated a
signaling molecule, which antagonized the inhibitory effects of
NS-398 on the COX-2 signaling pathway, which led to growth
inhibition. Previous studies have found that the ERK signaling
pathway can be activated by NS-398 (27). A similar result was observed in the
present study, in which NS-398 activated P38, however, the other
MAPK signaling pathways, ERK and JNK, were not significantly
affected. SB-203580, an inhibitor of P38 activation, restored the
inhibitory effect of NS-398 on pancreatic cancer cell growth. This
indicated that the activation of P38 was involved in the antagonism
of NS-398-induced proliferation inhibition, and increased the
invasion of pancreatic cancer cells.
Abnormal activation of the P38 signaling pathway may
trigger the tumor cell CD147 signaling pathway, and CD147, a member
of the immunoglobulin superfamily, has been identified as a
receptor for CyPA (28,29). However, whether NS-398 can induce
CD147 signaling molecules in pancreatic cancer cells remains to be
elucidated. In the present study, treatment of the pancreatic
cancer cells with NS-398 at different concentrations for 24 h led
to upregulation in the levels of CD147 in dose-dependent manner.
NS-398 can activate the P38 signaling pathway, and it has been
reported that the P38 signaling pathway can increase the expression
levels of MMP-2 and CD147 (30–34).
The present study hypothesized that NS-398 upregulates the
expression of CD147-MMP-2 through the activation of P38 by NS-398.
This hypothesis was confirmed following treatment of pancreatic
cells with SB-203580, an ERK inhibitor, which inhibited P38
activation by NS-398, following which the upregulation of MMP-2 and
CD147 were inhibited in the NS-398-treated pancreatic cancer
cells.
CD147-MMP-2 signaling is associated with tumor cell
invasiveness, and MMP-2 is a downstream molecule of CD147 that is
involved in cell migration and invasiveness (34,35).
Notably, NS-398 was observed to increase the invasiveness of
pancreatic cancer cells, whereas the P38 inhibitor, SB-203580,
inhibited the invasive abilities of pancreatic cancer cells induced
by NS-398, indicating that the NS-398-induced increase in
pancreatic cancer cell invasiveness was associated with the
activation of P38.
The CD147 downstream molecule, MMP-2, is an
important invasive factor (36).
The present study found that NS-398 activated the expression of
MMP-2. SB-203580 and CD147 siRNA inhibited the NS-398-induced
increase in pancreatic cancer cell invasion, whereas the
NS-398-induced expression of MMP-2 was restored by CD147-siRNA and
SB-203580. However, P38 remained activated in the CD147 siRNA and
NS-398 co-treated pancreatic cancer cells. According to these data,
the present study hypothesized that NS-398 enhanced pancreatic
cancer cell invasiveness by the P38-CD147-MMP-2 signaling pathway,
and the mechanisms by which NS-398 activates P38 and CD147-MMP-2
warrant further investigation.
In conclusion, the results of the present study
suggested that NS-398 inhibited COX-2 activity in the pancreatic
cancer cells, however, it did not inhibit pancreatic cancer cell
proliferation or vitality. By contrast, NS-398 activated the P38
signaling pathway in the pancreatic cancer cells, which was
involved in pancreatic cancer cell proliferation resistance and
activated CD147-MMP-2, increasing pancreatic cancer cells
invasiveness. These results demonstrated that treatment with NS-398
alone is insufficient in treating patients with pancreatic cancer,
and the application of NS-398 in the treatment of pancreatic cancer
patients may pose a potential risk.
Acknowledgments
This study was supported in part by the National
Natural Science Foundation of China (no. 81172322), the Shanghai
Municipal Education Committee (no. 13ZZ089), the Science and
Technology Committee of Shanghai (no. 14401901500) and the Science
and Technology Committee of Baoshan District (no. 12-E-2).
References
|
1
|
Nentwich MF, Bockhorn M, König A, Izbicki
JR and Cataldegirmen G: Surgery for advanced and metastatic
pancreatic cancer-current state and trends. Anticancer Res.
32:1999–2002. 2012.PubMed/NCBI
|
|
2
|
Fidler IJ: Origin and biology of cancer
metastasis. Cytometry. 10:673–680. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walsh N, O'Donovan N, Kennedy S, Henry M,
Meleady P, Clynes M and Dowling P: Identification of pancreatic
cancer invasion-related proteins by proteomic analysis. Proteome
Sci. 7:32009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramer R, Weinzierl U, Schwind B, Brune K
and Hinz B: Ceramide is involved in r(+)-methanandamide-induced
cyclooxygenase-2 expression in human neuroglioma cells. Mol
Pharmacol. 64:1189–1198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mollace V, Muscoli C, Masini E, Cuzzocrea
S and Salvemini D: Modulation of prostaglandin biosynthesis by
nitric oxide and nitric oxide donors. Pharmacol Rev. 57:217–252.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pereira C, Sousa H, Silva J, Brandão C,
Elgueta-Karstegl C, Farrell PJ, Medeiros R and Dinis-Ribeiro M: The
−1195G allele increases the transcriptional activity of
cyclooxygenase-2 gene (COX-2) in colon cancer cell lines. Mol
Carcinog. 53(Suppl 1): E92–E95. 2014. View
Article : Google Scholar
|
|
9
|
Wójcik M, Ramadori P, Blaschke M, Sultan
S, Khan S, Malik IA, Naz N, Martius G, Ramadori G and Schultze FC:
Immunodetection of cyclooxygenase-2 (COX-2) is restricted to tissue
macrophages in normal rat liver and to recruited mono-nuclear
phagocytes in liver injury and cholangiocarcinoma. Histochem Cell
Biol. 137:217–233. 2012. View Article : Google Scholar
|
|
10
|
Young AL, Chalmers CR, Hawcroft G, Perry
SL, Treanor D, Toogood GJ, Toogood GJ, Jones PF and Hull MA:
Regional differences in prostaglandin E2 metabolism in
human colorectal cancer liver metastases. BMC Cancer. 13:922013.
View Article : Google Scholar
|
|
11
|
Shibata-Kobayashi S, Yamashita H, Okuma K,
Shiraishi K, Igaki H, Ohtomo K and Nakagawa K: Correlation among 16
biological factors [p53, p21(waf1), MIB-1 (Ki-67), p16 (INK4A),
cyclin D1, E-cadherin, Bcl-2, TNF-α, NF-κB, TGF-β, MMP-7, COX-2,
EGFR, HER2/neu, ER and HIF-1α] and clinical outcomes following
curative chemoradiation therapy in 10 patients with esophageal
squamous cell carcinoma. Oncol Lett. 5:903–910. 2013.PubMed/NCBI
|
|
12
|
Yan WF, Sun PC, Nie CF and Wu G:
Cyclooxygenase-2 polymorphisms were associated with the risk of
gastric cancer: Evidence from a meta-analysis based on case-control
studies. Tumour Biol. 34:3323–3330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hillion J, Smail SS, Di Cello F, Belton A,
Shah SN, Huso T, Schuldenfrei A, Nelson DM, Cope L, Campbell N, et
al: The HMGA1-COX-2 axis: A key molecular pathway and potential
target in pancreatic adenocarcinoma. Pancreatology. 12:372–379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okami J, Nakamori S, Hiraoka N, Tsujie M,
Hayashi N, Yamamoto H, Fujiwara Y, Nagano H, Dono K, Umeshita K, et
al: Suppression of pancreatic cancer cell invasion by a
cycloox-ygenase-2-specific inhibitor. Clin Exp Metastasis.
20:577–584. 2003. View Article : Google Scholar
|
|
15
|
Duan DP, Dang XQ, Wang KZ, Wang YP, Zhang
H and You WL: The cyclooxygenase-2 inhibitor NS-398 inhibits
proliferation and induces apoptosis in human osteosarcoma cells via
downregulation of the survivin pathway. Oncol Rep. 28:1693–1700.
2012.PubMed/NCBI
|
|
16
|
Youns M, Efferth T and Hoheisel JD:
Transcript profiling identifies novel key players mediating the
growth inhibitory effect of NS-398 on human pancreatic cancer
cells. Eur J Pharmacol. 650:170–177. 2011. View Article : Google Scholar
|
|
17
|
Araki E, Forster C, Dubinsky JM, Ross ME
and Iadecola C: Cyclooxygenase-2 inhibitor ns-398 protects neuronal
cultures from lipopolysaccharide-induced neurotoxicity. Stroke.
32:2370–2375. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar S, Boehm J and Lee JC: p38 MAP
kinases: Key signalling molecules as therapeutic targets for
inflammatory diseases. Nat Rev Drug Discov. 2:717–726. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Lin J, Kanekura T, Su J, Lin W,
Xie H, Wu Y, Li J, Chen M and Chang J: A small interfering
CD147-targeting RNA inhibited the proliferation, invasiveness and
metastatic activity of malignant melanoma. Cancer Res.
66:11323–11330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida S, Ujiki M, Ding XZ, Pelham C,
Talamonti MS, Bell RH Jr, Denham W and Adrian TE: Pancreatic
stellate cells (PSCs) express cyclooxygenase-2 (COX-2) and
pancreatic cancer stimulates COX-2 in PSCs. Mol Cancer. 4:272005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yip-Schneider MT, Barnard DS, Billings SD,
Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS
and Sweeney CJ: Cyclooxygenase-2 expression in human pancreatic
adenocarcinomas. Carcinogenesis. 21:139–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Molina MA, Sitja-Arnau M, Lemoine MG,
Frazier ML and Sinicrope FA: Increased cyclooxygenase-2 expression
in human pancreatic carcinomas and cell lines: Growth inhibition by
nonsteroidal anti-inflammatory drugs. Cancer Res. 59:4356–4362.
1999.PubMed/NCBI
|
|
23
|
Tucker ON, Dannenberg AJ, Yang EK, Zhang
F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT
and Fahey TJ III: Cyclooxygenase-2 expression is up-regulated in
human pancreatic cancer. Cancer Res. 59:987–990. 1999.PubMed/NCBI
|
|
24
|
Wang X, Liang Y, Wang J and Wang M: Effect
of NS-398, a cyclooxygenase-2 selective inhibitor, on the
cytotoxicity of cytotoxic T lymphocytes to ovarian carcinoma cells.
Tumour Biol. 34:1517–1522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Honjo S, Osaki M, Ardyanto TD, Hiramatsu
T, Maeta N and Ito H: COX-2 inhibitor, NS398, enhances Fas-mediated
apoptosis via modulation of the PTEN-Akt pathway in human gastric
carcinoma cell lines. DNA Cell Biol. 24:141–147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Banu N, Buda A, Chell S, Elder D, Moorghen
M, Paraskeva C, Qualtrough D and Pignatelli M: Inhibition of COX-2
with NS-398 decreases colon cancer cell motility through blocking
epidermal growth factor receptor transactivation: Possibilities for
combination therapy. Cell Prolif. 40:768–779. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elder DJ, Halton DE, Playle LC and
Paraskeva C: The MEK/ERK pathway mediates COX-2-selective
NSAID-induced apoptosis and induced COX-2 protein expression in
colorectal carcinoma cells. Int J Cancer. 99:323–327. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yurchenko V, Constant S and Bukrinsky M:
Dealing with the family: CD147 interactions with cyclophilins.
Immunology. 117:301–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yurchenko V, Constant S, Eisenmesser E and
Bukrinsky M: Cyclophilin-CD147 interactions: A new target for
anti-inflammatory therapeutics. Clin Exp Immunol. 160:305–317.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gabison EE, Hoang-Xuan T, Mauviel A and
Menashi S: EMMPRIN/CD147, an MMP modulator in cancer, development
and tissue repair. Biochimie. 87:361–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Lu N, Zhou J, Chen ZN and Zhu P:
Cyclophilin A up-regulates MMP-9 expression and adhesion of
monocytes/macrophages via CD147 signalling pathway in rheumatoid
arthritis. Rheumatology (Oxford). 47:1299–1310. 2008. View Article : Google Scholar
|
|
32
|
Tang Y, Nakada MT, Rafferty P, Laraio J,
McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P and Yan L:
Regulation of vascular endothelial growth factor expression by
EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res.
4:371–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nabeshima K, Iwasaki H, Koga K, Hojo H,
Suzumiya J and Kikuchi M: Emmprin (basigin/CD147): Matrix
metalloproteinase modulator and multifunctional cell recognition
molecule that plays a critical role in cancer progression. Pathol
Int. 56:359–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
35
|
Kanekura T, Chen X and Kanzaki T: Basigin
(CD147) is expressed on melanoma cells and induces tumor cell
invasion by stimulating production of matrix metalloproteinases by
fibroblasts. Int J Cancer. 99:520–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kolli-Bouhafs K, Boukhari A, Abusnina A,
Velot E, Gies JP, Lugnier C and Rondé P: Thymoquinone reduces
migration and invasion of human glioblastoma cells associated with
FAK, MMP-2 and MMP-9 downregulation. Invest New Drugs.
30:2121–2131. 2012. View Article : Google Scholar
|


















