Introduction
The number of newly diagnosed cases of head and neck
cancer worldwide is ~600,000 annually (1). In addition, head and neck cancer is
the sixth most common type of solid cancer diagnosed worldwide
(2). Since the majority of
patients present with locally advanced or metastatic disease,
intensive surgical therapy followed by adjuvant radiochemotherapy
is often required. Usually, cisplatin is used as a powerful
radiosensitizer, and therefore remains an integral part of head and
neck cancer therapy (3). However,
the cure rate of head and neck cancer remains at ~50% (4); therefore, there is an urgent
requirement to improve treatment and survival rates. One possible
approach involves the inhibition of epidermal growth factor
receptor (EGFR)-related signaling. More than 90% of head and neck
cancers exhibit EGFR overexpression (5,6).
Notably, increased levels of EGFR are associated with a poorer
prognosis (7), and less
differentiated head and neck cancers exhibit higher levels of EGFR
(8). Furthermore, EGFR expression
is associated with resistance to radiotherapy, locoregional
treatment failure, and an increased rate of distant metastases
(9). These findings led to the
development of agents directed against EGFR. In 2006, cetuximab, a
chimeric immunoglobulin G1 monoclonal antibody, was
approved by the Food and Drug Administration for the treatment of
recurrent/metastatic head and neck cancer (10). In addition to targeting EGFR by
inhibiting the extracellular ligand binding domain, tyrosine kinase
inhibitors (TKIs) suppress intracellular tyrosine kinase activity.
Particularly in metastatic non-small cell lung cancer (NSCLC),
agents such as gefitinib and erlotinib are widely used (11). However, in contrast to NSCLC, where
tumors frequently harbor EGFR kinase domain mutations, head and
neck cancer lesions predominantly possess wild-type EGFR (12,13).
This finding may partly explain why erlotinib failed to improve the
complete response rate or progression-free survival of locally
advanced head and neck cancers treated with a
cisplatin/radiotherapy-based regimen (14). At present, there are no markers
predictive of response to anti-EGFR therapies, including monoclonal
antibodies and TKIs. However, ongoing research may identify such
markers, and more accurate patient selection will improve the
benefits of TKI-based therapy in a subset of patients with head and
neck cancer. Another aspect regarding the treatment failure of
anti-EGFR approaches could be lateral signaling, as well as the
activation of alternative receptors and downstream molecules,
including other members of the ErbB family. Wheeler et al
(15) demonstrated that human
epidermal growth factor receptor (HER)2 and HER3 are strongly
activated in cetuximab-resistant cells. Furthermore, EGFR
upregulation led to increased dimerization with HER2 and HER3.
Consequently, the inhibition of EGFR and HER2 resulted in decreased
HER3 and phosphoinositide 3-kinase activity (15). At present, few in vitro
studies concerning dual blockade of EGFR and HER2 have been
conducted in head and neck cancer. Schütze et al (16) demonstrated a clear
antiproliferative effect of afatinib, an EGFR/HER2/HER4 TKI.
Notably, the radiosensitizing effect was only marginal; however,
the study was conducted with only one cell line (FaDu) (16). To date, only one clinical trial
regarding afatinib for the treatment of head and neck cancer has
been published. Seiwert et al (17) compared afatinib treatment with
cetuximab treatment in patients with recurrent or metastatic head
and neck cancer who progressed after platinum-based therapy. The
antitumor activity of afatinib was comparable to that of cetuximab;
however, more patients in the afatinib group discontinued treatment
due to adverse events.
In the present study, the rationale for combining
afatinib with cisplatin was based on three considerations. Firstly,
cisplatin serves as a radiosensitizer, and chemoradiotherapy
remains the gold standard for the adjuvant treatment of head and
neck cancer. Secondly, EGFR overexpression is known to contribute
to radiotherapy resistance. Finally, EGFR overexpression leads to
the activation of other ErbB family members, including HER2 and
HER4.
To the best of our knowledge, the present study is
the first to investigate the efficacy of afatinib in combination
with cisplatin in wild-type EGFR head and neck cancer cell
lines.
Materials and methods
Cell lines
The cell lines used in the present study (Table I) were provided by the Cancer
Institute, University of Pittsburgh (Pittsburgh, PA, USA) (18). As described previously, the cells
were cultured in a humidified atmosphere containing 5%
CO2/95% air at 37°C and the medium was changed 2–3 times
per week (19,20). The cells were cultured in
Dulbecco's modified Eagle medium (Gibco; Fisher Scientific
Deutschland, Schwerte, Germany) supplemented with 10% fetal calf
serum (Life Technologies GmbH, Darmstadt, Germany), 1%
penicillin/streptomycin (Life Technologies GmbH) and 1% glutamine
(Biochrom KG, Berlin, Germany).
 | Table IName, origin and TNM status of the
five cell lines used in the present study. |
Table I
Name, origin and TNM status of the
five cell lines used in the present study.
| Cell line | Origin | TNM |
|---|
| PCI-1 | Laryngeal carcinoma
of the glottis of a male patient | pT2N00M0G2 |
| PCI-9 | Primary carcinoma
at the base of the tongue of a male patient | pT4N3M0G2 |
| PCI-13 | Oral squamous cell
carcinoma of the retromolar triangle of a male patient | pT4pN1M0G3 |
| PCI-52 | Primary carcinoma
of the aryepiglottic fold of a male patient | pT2N0M0G2 |
| PCI-68 | Primary tongue
carcinoma of a male patient | pT4N0M0G1 |
Mutational analysis of EGFR tyrosine
kinase domain
For mutational analysis of the EGFR tyrosine kinase
domain, DNA was isolated from the HNSCC cell lines using a Roche
DNA Isolation kit (Roche Diagnostics Deutschland GmbH, Mannheim,
Germany), according to manufacturer's protocol. Subsequently,
isolated DNA samples were amplified by polymerase chain reaction
(PCR) using the following allele-specific primers from Eurofins
Genomics (Ebersberg, Germany): EGFR exon 18, forward (F)
5′-CCATGTCTGGCACTGCTTTCC-3′, EGFR exon 18, reverse (R)
5′-AAGGACTCTGGGCTCCCCACC-3′; EGFR exon 19, F
5′-ACCCAGATCACTGGGCAGCATG-3′, EGFR exon 19, R
5′-AGCAGCTGCCAGACATGAGAAAAG-3′; EGFR exon 20, F
5′-CAGCCCTGCGTAAACGTCCCTG-3′, EGFR exon 20, R 5′-GGA GCG CAG ACC
GCA TGT GAGG-3′; EGFR exon 21, F 5′-ACCCTGAATTCGGATGCAGAGC-3′, and
EGFR exon 21, R 5′-ATACAGCTAGTGGGAAGGCAG-3′. PCR was performed on a
Primus 96 Plus cycler (Peqlab Biotechnologie GmbH, Erlangen,
Germany) with an annealing temperature of 65°C for 35 cycles. All
further PCR reagents, including the Taq DNA polymerase PCR buffer
and the dNTPs were purchased from Thermo Fisher Scientific, Inc.
(Darmstadt, Germany). Subsequently, the amplified DNA was
visualized in 2% agarose gels containing ethidium bromide, and
purified using column affinity chromatography. The purified PCR
products were sequenced using a 16-capillary electrophoresis
instrument (3130XL GeneScan; Thermo Fisher Scientific, Inc.,
Darmstadt, Germany).
Treatment with afatinib and
cisplatin
A total of 1×104 cells from each cell
line were seeded per well. Cisplatin was purchased from TEVA GmbH
(Radebeul, Germany) and stored according to the manufacturer's
protocol. Afatinib was purchased from Selleckchem (distributed by
Absource Diagnostics GmbH, München, Germany) and stored according
to the manufacturer's protocol. The afatinib concentrations used in
the study (0.3125, 0.625, 1.25, 2.5, 5.0, 10.0 µM) were
derived from a log2 dilution. These concentrations are
based on the findings of Mukohara et al (21); the clinically relevant maximum
serum concentration of EGFR TKIs, such as gefitinib, was reported
to be ~1 µM. Cisplatin concentrations were fixed in all
experiments. In our previous analysis, the cisplatin half maximal
inhibitory concentration (IC50) values for all five cell
lines used in the present study (Table II) was investigated (unpublished
data). The IC50 values for cisplatin ranged between 1
and 14 µM. In huge panels of human cancer cells,
concentrations similar to these have been reported (22). Following an overnight incubation in
standard medium, medium containing a fixed concentration of
cisplatin and the variable concentrations of afatinib was added to
the cells, and the cultures were incubated for a further 72 h.
 | Table IICisplatin IC50 values in
various cell lines. |
Table II
Cisplatin IC50 values in
various cell lines.
| Cell line | Cisplatin
IC50 (µM) |
|---|
| PCI-1 | 14 |
| PCI-9 | 14 |
| PCI-13 | 1 |
| PCI-52 | 5 |
| PCI-68 | 14 |
Crystal violet assay
Crystal violet (1 g) was diluted in 1 L
double-distilled water containing 20% methanol. Subsequently, the
drug-containing medium was removed from the cells, and 50 µl
crystal violet was added to the wells. After 15 min, the 96-well
plates were washed with distilled water. Using a microplate reader
(Tecan Spectra Rainbow microplate reader; Tecan Deutschland GmbH,
Crailsheim, Germany), the optical density (OD) was measured at a
wavelength of 595 nm. All experiments were performed at least three
times.
Statistical analysis
Statistical analysis of the data was performed using
Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA) and
Prism 6.04 (GraphPad Software, Inc., La Jolla, CA, USA). Two
statistical aspects were investigated. Initially, the treatment
efficacy was compared between cisplatin monotherapy and combination
therapy with afatinib in each cell line. Secondly, significant
differences in treatment efficacy between the five cell lines were
determined. Due to the lack of a normal distribution and the number
of measurements, a nonparametric Mann-Whitney test was performed.
All of the experiments were repeated at least three times. P≤0.05
was considered to indicate a statistically significant
difference.
Results
Mutational analysis of the EGFR tyrosine
kinase domain
Wild-type exons 18, 19 and 21 were detected in all
cell lines. The silent mutation Q787Q was identified in exon 20 in
all cell lines. In addition, PCI-9 harbored the T785T mutation,
which, similar to Q787Q, is a silent mutation with no functional
relevance (Table III).
 | Table IIIMutation status of the epidermal
growth factor receptor tyrosine kinase domain. |
Table III
Mutation status of the epidermal
growth factor receptor tyrosine kinase domain.
| Cell line | Exon 18 | Exon 19 | Exon 20 | Exon 21 |
|---|
| PCI-1 | wt | wt | wt (Q787Q) | wt |
| PCI-9 | wt | wt | wt (T785T,
Q787Q) | wt |
| PCI-13 | wt | wt | wt (Q787Q) | wt |
| PCI-52 | wt | wt | wt (Q787Q) | wt |
| PCI-68 | wt | wt | wt (Q787Q) | wt |
Treatment efficacy in PCI-1 cells
The fraction of viable cells following treatment
with a fixed concentration of cisplatin (14 µM) and 0.3125
µM afatinib was 100.4% [standard deviation (SD)±2.6%]. When
the cells were treated with cisplatin and 0.625 µM afatinib,
the viable fraction was 103.7% (SD±4.9%). The fraction of viable
cells following treatment with 1.25 µM afatinib and the
fixed concentration of cisplatin was 81.1% (SD±6.1%), which was
significantly reduced, as compared with the control cells
(P<0.0001). Following treatment with cisplatin and 2.5 µM
afatinib, the viable fraction was 69.3% (SD±8.8%), as compared with
the control cells. The fraction of viable cells when treated with
fixed cisplatin and 5 µM afatinib was 65.9% (SD±10.9%).
Following treatment with cisplatin and the highest concentration of
afatinib (10 µM), the viable fraction was only 57.0%
(SD±12.3%) (Fig. 1A).
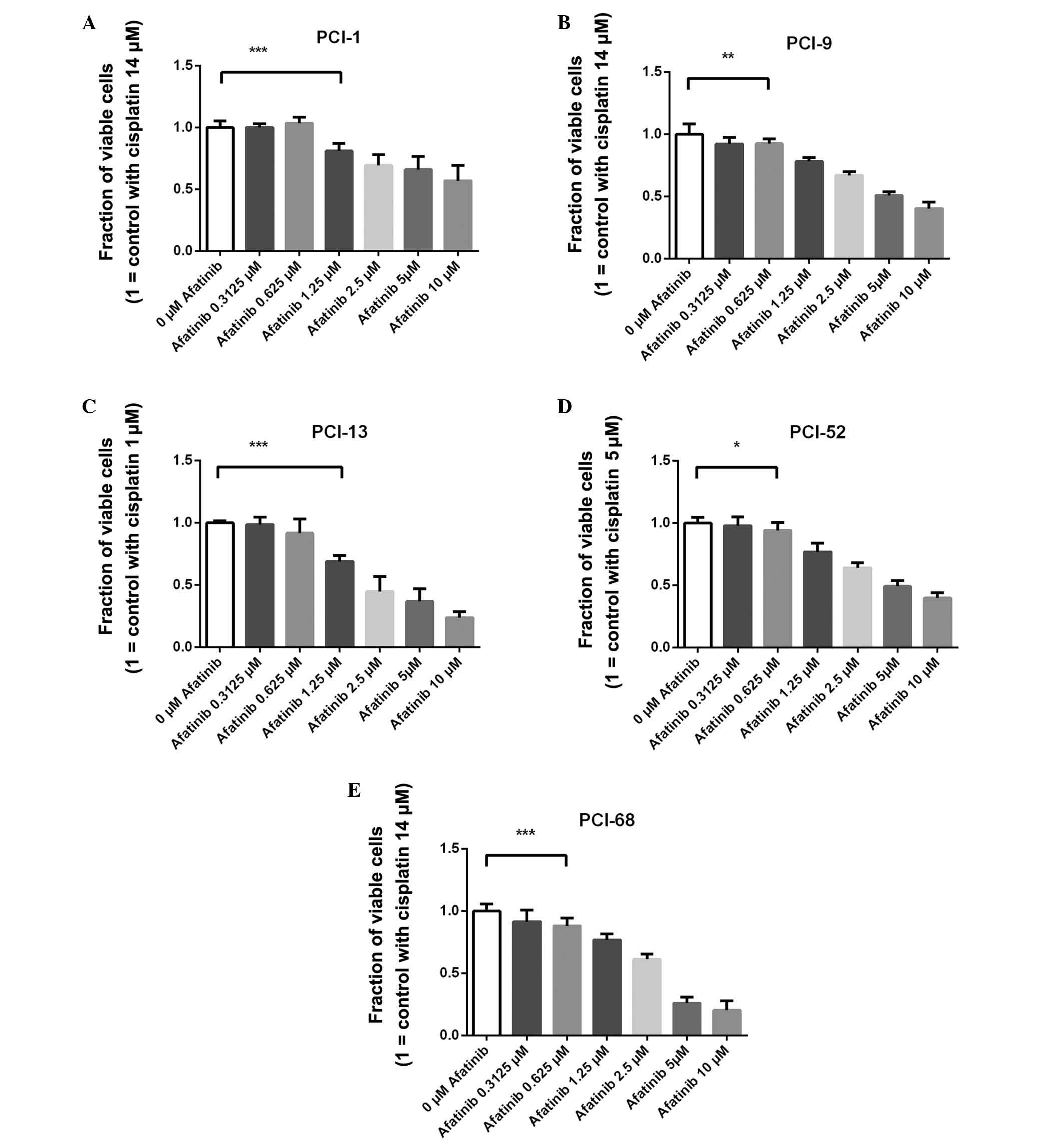 | Figure 1Treatment efficacy of afatinib in
combination with the cell line-specific fixed concentrations of
cisplatin, as measured by crystal violet assay. The white bar
indicates the control cells treated with cell-specific cisplatin
half maximal inhibitory concentration values (=1.0). The fraction
of viable (A) PCI-1, (B) PCI-9, (C) PCI-13, (D) PCI-52 and (E)
PCI-68 cells. In all cell lines, a concentration-dependent effect
of combined afatinib and cisplatin treatment was detected. In the
PCI-9, PCI-52 and PCI-68 cells, ≥0.625 µM afatinib resulted
in a significantly lower viable fraction, as compared with the
control. In the PCI-1 and PCI-13 cells, ≥1.25 µM afatinib
resulted in a singificantly lower viable fraction. Data are
presented as the mean ± standard deviation. The horizontal bracket
indicates the first concentration with a significantly different
number of viable cells, as compared with the control.
Concentrations above the first significantly different
concentration were also significant. *P≤0.05,
**P≤0.01, ***P≤0.001. |
Treatment efficacy in PCI-9 cells
The fraction of viable cells following treatment
with a fixed concentration of cisplatin (14 µM) and 0.3125
µM afatinib was 92.3% (SD±5.4%). Following treatment with
cisplatin and 0.625 µM afatinib, the viable fraction was
92.4% (SD±3.8%), which was significantly reduced, as compared with
the control (P=0.003). The fraction of viable cells treated with
1.25 µM afatinib and the fixed concentration of cisplatin
was 78.2% (SD±3.2%). Following treatment with cisplatin and 2.5
µM afatinib, the viable fraction was 67.1% (SD±2.8%), as
compared with the control cells. The fraction of viable cells
following treatment with 5 µM afatinib and the fixed
concentration of cisplatin was 51.0% (SD±2.9%). When the cells were
treated with cisplatin and the highest concentration of afatinib
(10 µM), the viable fraction was only 40.4% (SD±5.1%)
(Fig. 1B).
Treatment efficacy in PCI-13 cells
The fraction of viable cells following treatment
with a fixed concentration of cisplatin (1 µM) and 0.3125
µM afatinib was 98.7% (SD±6.1%). When the cells were treated
with cisplatin and 0.625 µM afatinib, the viable fraction
was 91.7% (SD±11.3%). Following treatment with 1.25 µM
afatinib and the fixed concentration of cisplatin, the fraction of
viable cells was 69.1% (SD±4.8%), which was significantly reduced,
as compared with the control (P<0.0001). Following treatment
with cisplatin and 2.5 µM afatinib, the viable fraction was
44.8% (SD±11.8%), as compared with the control cells. The fraction
of viable cells following treatment with 5 µM afatinib and
the fixed concentration of cisplatin was 36.8% (SD±10.4%). When the
cells were treated with cisplatin and the highest concentration of
afatinib (10 µM), the viable fraction was only 23.8%
(SD±4.8%) (Fig. 1C).
Treatment efficacy in PCI-52 cells
The fraction of viable cells following treatment
with a fixed concentration of cisplatin (5 µM) and 0.3125
µM afatinib was 98.1% (SD±6.7%). When the cells were treated
with cisplatin and 0.625 µM afatinib, the viable fraction
was 94.2% (SD±6.5%), which was significantly reduced, as compared
with the control cells (P=0.0213). The fraction of viable cells
following treatment with 1.25 µM afatinib and the fixed
concentration of cisplatin was 77.0% (SD±7.1%). Following treatment
with cisplatin and 2.5 µM afatinib, the fraction of viable
cells was 64.1% (SD±4.0%), as compared with the control cells. The
fraction of viable cells following treatment with 5 µM
afatinib and the fixed concentration of cisplatin was 49.2%
(SD±4.4%). Following treatment with cisplatin and the highest
concentration of afatinib (10 µM), the fraction of viable
cells was only 40.0% (SD±3.9%) (Fig.
1D).
Treatment efficacy in PCI-68 cells
The fraction of viable cells following treatment
with the fixed concentration of cisplatin (14 µM) and 0.3125
µM afatinib was 91.6% (SD±9.5%). When the cells were treated
with cisplatin and 0.625 µM afatinib, the viable fraction
was 88.2% (SD±6.4%), which was significantly reduced, as compared
with the control (P=0.0001). The fraction of viable cells following
treatment with 1.25 µM afatinib and the fixed concentration
of cisplatin was 77.0% (SD±4.7%). Following treatment with
cisplatin and 2.5 µM afatinib, the fraction of viable cells
was 61.4% (SD±4.3%), as compared with the control cells. The
fraction of viable cells following treatment with 5 µM
afatinib and the fixed concentration of cisplatin was 26.0%
(SD±5.0%). Following treatment with cisplatin and the highest
concentration of afatinib (10 µM), the viable fraction was
only 20.2% (SD±7.6%) (Fig.
1E).
Statistical analysis of the highest and
lowest efficacy of combination therapy in all cell lines
For all of the investigated concentrations, the
highest and lowest efficacy of afatinib treatment in combination
with cell line-specific cisplatin concentration differed
significantly. In all experiments, afatinib exhibited the lowest
efficacy in the PCI-1 cell line. The treatment efficacy ranges
between the cell lines increased with the higher concentrations of
afatinib. Comparing the effect of 0.3125 µM afatinib (in
combination with the cell-specific fixed dose of cisplatin) the
Mann-Whitney test revealed a significant difference (P=0.0115)
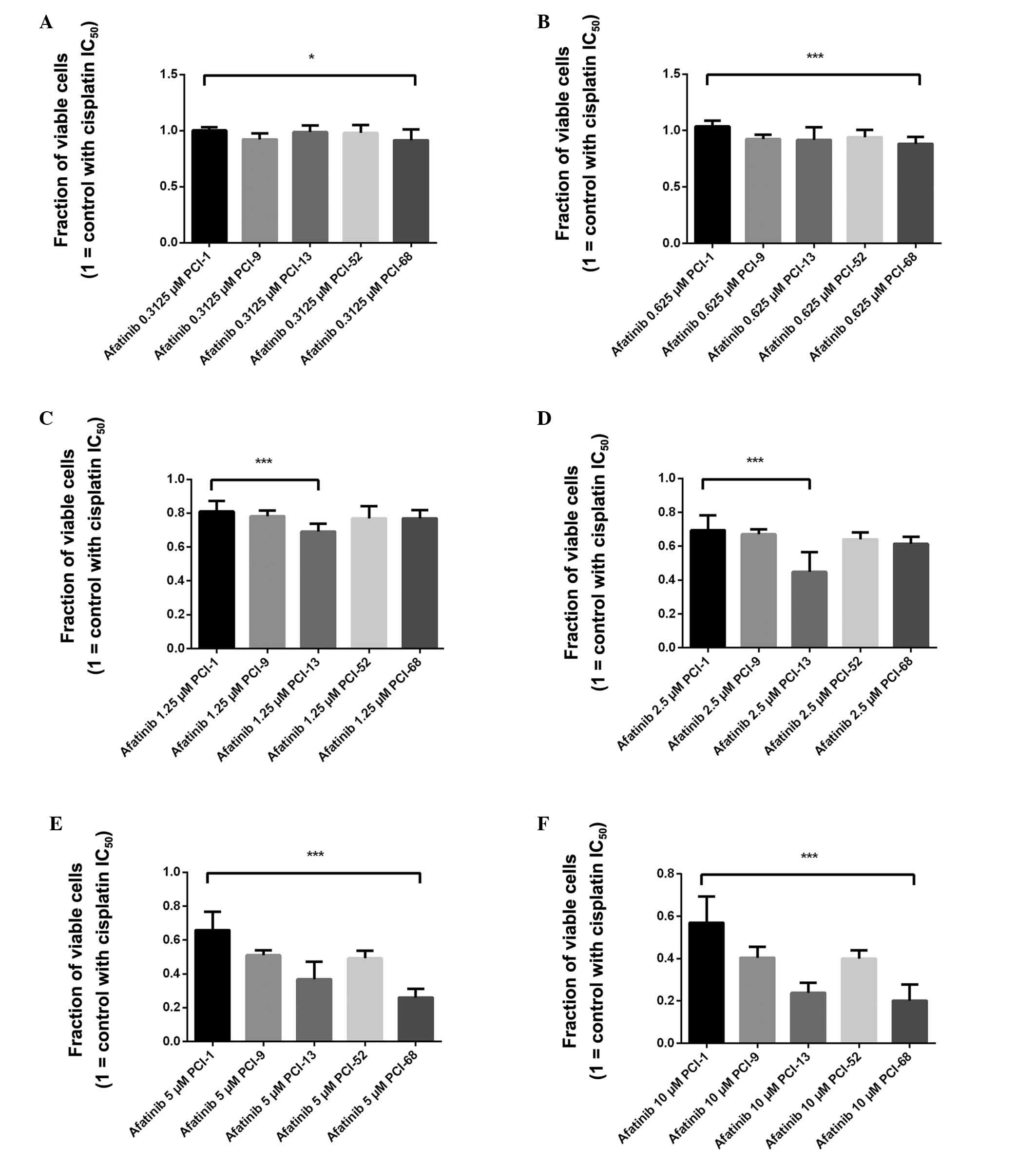
between the PCI-68 (91.6%) and PCI-1 (100.4%) cell lines (Fig. 2A). In addition, a highly
significant difference (P<0.0001) was noted between the PCI-68
(88.2%) and PCI-1 (103.7%) cell lines following treatment with
0.625 µM afatinib and cisplatin (Fig. 2B). Following treatment with
cisplatin and 1.25 µM afatinib, a significant difference
(P<0.0001) was detected between the cell line with the highest
efficacy (PCI-13: 69.1%) and the cell line with the lowest efficacy
(PCI-1: 81.1%) (Fig. 2C).
Comparing the effects of 2.5 µM afatinib (in combination
with the cell-specific fixed dose of cisplatin), the Mann-Whitney
test revealed a significant difference (P<0.0001) between the
PCI-13 (44.8%) and PCI-1 (69.3%) cells (Fig. 2D). Following treatment with 5
µM afatinib and cisplatin, a significant difference
(P<0.0001) was detected between the cell line with the highest
efficacy (PCI-68: 26.0%) and the cell line with the lowest efficacy
(PCI-1: 65.9%) (Fig. 2E).
Furthermore, a significant difference (P<0.0001) was detected
between PCI-1 (57.1%) and PCI-68 (20.2%) cells (Fig. 2F) in response to cisplatin and the
highest concentration of afatinib (10 µM).
Discussion
Squamous cell carcinoma of the oral cavity is the
most common type of head and neck cancer (23). Similar to other malignancies,
overall survival is associated with the extent of local tumor
spread, regional lymph node metastases and distant metastases.
Based on the literature, the cumulative five-year survival rate for
head and neck cancer has been ~50% for more than 30 years (24). Unfortunately, ~60% of patients
present with locally advanced or metastatic disease (25). In these patients, multimodal
treatment, which typically comprises surgery, radiotherapy and
chemotherapy, is commonly applied. In terms of targeted therapies,
the EGFR has an important role in head and neck cancer (5); however, single agent therapy against
EGFR with cetuximab in patients with recurrent or metastatic head
and neck cancer exhibits limited response rates of ~13% (26). At present, this treatment failure
is attributed to alternative receptor activation, mainly by other
members of the ErbB family, including HER2 (15,27).
In addition to monoclonal antibodies, including cetuximab and
trastuzumab, which inhibit ErbB family receptors, the TKI are
powerful agents that inhibit signaling. Unfortunately, erlotinib, a
reversible first-generation EGFR TKI, failed to improve treatment
results in patients with locally advanced head and neck cancer
(14). In this context, afatinib,
an irreversible EGFR/HER2/HER4 second-generation TKI, may improve
outcome in head and neck cancer therapy.
Given the extensive research being conducted on
afatinib monotherapy in human cancer cell lines (28), the present study aimed to explore
the effects of combination therapy with a widely used agent. By
investigating five wild-type EGFR head and neck cancer cell lines,
the present study demonstrated that afatinib enhances
platinum-based chemotherapy. In all cell lines used in the present
study, the growth inhibiting effects of afatinib were
concentration-dependent.
Notably, differences were noted between the cell
lines. In all cell lines, significant treatment effects could be
observed using concentrations achieved for other EGFR TKIs in a
clinical setting (21). By
comparing the results of the present study to those of previous
studies by our group, we observed the lowest efficacy of afatinib
in the PCI-1 cell line, which exhibits the best response to EGFR
antibodies, including cetuximab and panitumumab (19). In our previous study, the impact of
EGFR knockdown on cetuximab and panitumumab efficacy was
investigated. Notably, knockdown of EGFR expression enhanced
anti-EGFR treatment efficacy (20), and this effect was strongest in
PCI-1 cells. In addition, PCI-1 cells also exhibited the best
response to erlotinib and gefitinib in the same panel of cell lines
used in the present study (data not shown).
According to the growth assay, afatinib efficacy in
the PCI-52 cell line did not differ, as compared with the other
cell lines. In previous studies, this cell line exhibited the
lowest response to cetuximab, panitumumab, erlotinib and gefitinib
(19,29). This finding indicated that
predictions regarding the efficacy of anti-EGFR treatment in head
and neck cancer remain challenging. However, in cancer exhibiting
cetuximab, erlotinib and gefitinib failure, afatinib may serve as
an additional treatment option. This hypothesis was addressed by
Seiwert et al (17), which
indicated that disease control may be achieved by switching from
cetuximab to afatinib treatment, and vice versa, in cases of
progressive disease.
In conclusion, the present study demonstrated that
afatinib in combination with platinum agents may exhibit
considerable potential to enhance response rates in head and neck
cancer, especially in patients that have previously experienced
cetuximab failure. Further preclinical and clinical investigations
are required to identify predictive markers for anti-EGFR/HER2 and
HER4 treatment, and to identify a subset of patients who will
benefit from targeted therapy.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seiwert TY, Salama JK and Vokes EE: The
chemoradiation paradigm in head and neck cancer. Nat Clin Pract
Oncol. 4:156–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta S, Kong W, Peng Y, Miao Q and
Mackillop WJ: Temporal trends in the incidence and survival of
cancers of the upper aerodigestive tract in Ontario and the United
States. Int J Cancer. 125:2159–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grandis JR and Tweardy DJ: Elevated levels
of transforming growth factor alpha and epidermal growth factor
receptor messenger RNA are early markers of carcinogenesis in head
and neck cancer. Cancer Res. 53:3579–3584. 1993.PubMed/NCBI
|
|
6
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar
|
|
7
|
Chung CH, Ely K, McGavran L,
Varella-Garcia M, Parker J, Parker N, Jarrett C, Carter J, Murphy
BA, Netterville J, et al: Increased epidermal growth factor
receptor gene copy number is associated with poor prognosis in head
and neck squamous cell carcinomas. J Clin Oncol. 24:4170–4176.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maurizi M, Scambia G, Benedetti Panici P,
Ferrandina G, Almadori G, Paludetti G, De Vincenzo R, Distefano M,
Brinchi D, Cadoni G, et al: EGF receptor expression in primary
laryngeal cancer: Correlation with clinico-pathological features
and prognostic significance. Int J Cancer. 52:862–866. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agulnik M: New approaches to EGFR
inhibition for locally advanced or metastatic squamous cell
carcinoma of the head and neck (SCCHN). Med Oncol. 29:2481–2491.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
No authors listed. Cetuximab approved by
FDA for treatment of head and neck squamous cell cancer. Cancer
Biol Ther. 5:340–342. 2006.PubMed/NCBI
|
|
11
|
Melosky B: Review of EGFR TKIs in
metastatic NSCLC, including ongoing trials. Front Oncol. 4:2442014.
View Article : Google Scholar
|
|
12
|
Loeffler-Ragg J, Witsch-Baumgartner M,
Tzankov A, Hilbe W, Schwentner I, Sprinzl GM, Utermann G and
Zwierzina H: Low incidence of mutations in EGFR kinase domain in
Caucasian patients with head and neck squamous cell carcinoma. Eur
J Cancer. 42:109–111. 2006. View Article : Google Scholar
|
|
13
|
Shigematsu H and Gazdar AF: Somatic
mutations of epidermal growth factor receptor signaling pathway in
lung cancers. Int J Cancer. 118:257–262. 2006. View Article : Google Scholar
|
|
14
|
Martins RG, Parvathaneni U, Bauman JE,
Sharma AK, Raez LE, Papagikos MA, Yunus F, Kurland BF, Eaton KD,
Liao JJ, et al: Cisplatin and radiotherapy with or without
erlotinib in locally advanced squamous cell carcinoma of the head
and neck: A randomized phase II trial. J Clin Oncol. 31:1415–1421.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wheeler DL, Huang S, Kruser TJ,
Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT and
Harari PM: Mechanisms of acquired resistance to cetuximab: Role of
HER (ErbB) family members. Oncogene. 27:3944–3956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schütze C, Dörfler A, Eicheler W, Zips D,
Hering S, Solca F, Baumann M and Krause M: Combination of EGFR/HER2
tyrosine kinase inhibition by BIBW 2992 and BIBW 2669 with
irradiation in FaDu human squamous cell carcinoma. Strahlenther
Onkol. 183:256–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seiwert TY, Fayette J, Cupissol D, Del
Campo JM, Clement PM, Hitt R, Degardin M, Zhang W, Blackman A,
Ehrnrooth E and Cohen EE: A randomized, phase II study of afatinib
versus cetuximab in metastatic or recurrent squamous cell carcinoma
of the head and neck. Ann Oncol. 25:1813–1820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heo DS, Snyderman C, Gollin SM, Pan S,
Walker E, Deka R, Barnes EL, Johnson JT, Herberman RB and Whiteside
TL: Biology, cytogenetics, and sensitivity to immunological
effector cells of new head and neck squamous cell carcinoma lines.
Cancer Res. 49:5167–5175. 1989.PubMed/NCBI
|
|
19
|
Hartmann S, Kriegebaum U, Küchler N,
Lessner G, Brands RC, Linz C, Schneider T, Kübler AC and
Müller-Richter UD: Efficacy of cetuximab and panitumumab in oral
squamous cell carcinoma cell lines: Prognostic value of MAGE-A
subgroups for treatment success. J Craniomaxillofac Surg.
41:623–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartmann S, Seher A, Brands RC, Linz C,
Lessner G, Böhm H, Kübler AC and Müller-Richter UD: Influence of
epidermal growth factor receptor expression on the cetuximab and
panitumumab response rates of head and neck carcinoma cells. J
Craniomaxillofac Surg. 42:1322–1328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mukohara T, Engelman JA, Hanna NH, Yeap
BY, Kobayashi S, Lindeman N, Halmos B, Pearlberg J, Tsuchihashi Z,
Cantley LC, et al: Differential effects of gefitinib and cetuximab
on non-small-cell lung cancers bearing epidermal growth factor
receptor mutations. J Natl Cancer Inst. 97:1185–1194. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tardito S, Isella C, Medico E, Marchiò L,
Bevilacqua E, Hatzoglou M, Bussolati O and Franchi-Gazzola R: The
thioxotriazole copper(II) complex A0 induces endoplasmic reticulum
stress and paraptotic death in human cancer cells. J Biol Chem.
284:24306–24319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Belcher R, Hayes K, Fedewa S and Chen AY:
Current treatment of head and neck squamous cell cancer. J Surg
Oncol. 110:551–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vermorken JB and Specenier P: Optimal
treatment for recurrent/metastatic head and neck cancer. Ann Oncol.
21(Suppl 7): vii252–vii261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vermorken JB, Trigo J, Hitt R, Koralewski
P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A and
Baselga J: Open-label, uncontrolled, multicenter phase II study to
evaluate the efficacy and toxicity of cetuximab as a single agent
in patients with recurrent and/or metastatic squamous cell
carcinoma of the head and neck who failed to respond to
platinum-based therapy. J Clin Oncol. 25:2171–2177. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yonesaka K, Zejnullahu K, Okamoto I, Satoh
T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda
M, et al: Activation of ERBB2 signaling causes resistance to the
EGFR-directed therapeutic antibody cetuximab. Sci Transl Med.
3:99ra862011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cha MY, Lee KO, Kim M, Song JY, Lee KH,
Park J, Chae YJ, Kim YH, Suh KH, Lee GS, et al: Antitumor activity
of HM781-36B, a highly effective pan-HER inhibitor in
erlotinib-resistant NSCLC and other EGFR-dependent cancer models.
Int J Cancer. 130:2445–2454. 2012. View Article : Google Scholar
|
|
29
|
Hartmann S, Neckel N, Seher A, Mutzbauer
G, Brands RC, Linz C, Kübler A and Müller-Richter UD: Erlotinib and
gefitinib responsiveness in head and neck cancer cell lines - a
comparing analysis with cetuximab. Clin Oral Investig. Aug
23–2015.Epub ahead of print. View Article : Google Scholar
|