Introduction
Acute myeloid leukemia (AML) is characterized by the
blood cells being contained within an abnormal state at an early
stage of their development, and their failure to differentiate into
functional mature cells. The introduction of
all-trans-retinoic acid (ATRA) into the regime of acute
promyelocytic leukemia (APL) therapy verified the concept of
differentiation therapy (1), and
this has prompted scientists to search for effective
differentiation agents. Similar, and promising,
differentiation-inducing effects have also been identified for the
physiologically active form of vitamin D, 1,25-dihydroxyvitamin
D3 (VD3) (2–4). Encouraging results from clinical
trials have suggested that VD3 may be effective as a
differentiation agent in the treatment of AML and myelo-dysplastic
syndrome; however, hypercalcemia has limited its usefulness
(5–8). Therefore, two approaches have been
adopted to solve this problem: One was to develop novel VD3
derivatives that exhibited similar differentiation effects,
although without the adverse calcemia-increasing activity; the
other was to use VD3 at a lower concentration in combination with
other agents. Indeed, numerous agents have been demonstrated to
enhance VD3-induced cell differentiation (9–15).
Reactive oxygen species (ROS) are broadly defined as
a group of highly reactive molecules, which are generated in redox
reactions in the cells. The representative ROS are super-oxide
anion, hydroxyl radical, hydrogen peroxide
(H2O2) and thiyl radical. Since ROS were
identified, the predominant focus has been directed at studying the
oxidative damage caused to biological macromolecules, including
proteins, DNA and lipids. Previous studies have demonstrated that
ROS are able to modulate the activation of signal transduction
pathways involved in several cellular functions, including cell
differentiation and proliferation (16,17).
In the mammalian hematopoietic system, it has been reported that
modulating the levels of ROS may be important in terms of the
control of myeloid precursor differentiation (17,18).
With regards to VD3-induced cell differentiation, numerous reports
have demonstrated an involvement of ROS. Various antioxidants,
including curcumin, ebselen and silibinin, have been shown to
potentiate the differentiation of HL-60 cells induced by VD3
(14,19). However, other groups have reported
that increasing the levels of ROS by iron chelation or by
inhibiting the antioxidant enzyme catalase, could also enhance
VD3-induced monocytic differentiation (20,21).
Therefore, the functional role of ROS in VD3-induced cell
differentiation remains to be fully elucidated.
Studies from our laboratory demonstrated that
adenanthin, a natural diterpenoid, was able to induce
differentiation in leukemia cells via targeting the peroxiredoxin I
(Prx I)/ROS axis (22,23). Prx I is a small antioxidant
protein, which belongs to the peroxiredoxin family and functions
primarily to reduce H2O2 and other peroxide
substrates via conserved cysteine residues using thiol-containing
proteins, including glutathione or thioredoxin, as electron donors
(24). Currently, no direct
evidence has demonstrated whether Prx I exerts a role in
VD3-induced cell differentiation. Considering the possible role of
ROS in VD3-induced cell differentiation, the present study
hypothesized that an increase in the levels of ROS via the
targeting of Prx I may regulate VD3-induced cell
differentiation.
In the present study, using adenanthin as a chemical
tool, it was demonstrated that inhibition of Prx I does enhance
VD3-induced cell differentiation. The present study revealed a
novel role for Prx I in VD3-induced cell differentiation, and
confirmed the hypothesis that targeting Prx I improves the efficacy
of VD3.
Materials and methods
Cell culture and agents
The APL cell line, NB4, was obtained from Dr Michel
Lanotte (Hospital Saint Louis, Paris, France). Leukemic U937 and
HL-60 cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). The cells were grown in RPMI-1640 medium
(BioWhittaker Europe, Verviers, Belgium) supplemented with 10%
fetal calf serum (EuroClone S.p.A, Milan, Italy) at 37°C in a
humidified atmosphere containing 5% CO2. Adenanthin was
isolated from the dried aerial parts of Isodon adenanthus
(Diels) Hara, as described previously (22).
Patient samples
Patients' samples were obtained following the
collection of informed consent under a procurement protocol that
was approved by the Clinical Investigational Review Board of
Shanghai Jiao Tong University School of Medicine (Shanghai, China).
Mononuclear cells were isolated according to the manufacturer's
instructions (Ficoll-Paque PLUS; 17-1440-03; GE Healthcare,
Buckinghamshire, UK) from the bone marrow of one APL patient
(female, 29 years old, 46,XX, PML-RARa positive).
Cell differentiation assay
The morphological features of the NB4, HL60,U937 and
primary APL cells were examined under a light microscope (BX-51;
Olympus, Tokyo, Japan) following Wright's staining (BASO
Diagnostic, Inc., Guangdong, China). In addition, cell
differentiation was evaluated by the expression of cell surface
differentiation antigens, CD11b and CD14. In brief, following
treatment with adenanthin and/or VD3, NB4 cells were incubated with
monoclonal mouse anti-human PE-conjugated anti-CD14 (cat. no.
557742; BD Biosciences, San Jose, CA, USA; 1:1,000 dilution),
monoclonal mouse anti-human FITC-conjugated anti-CD11b (cat. no.
562793; BD Biosciences; 1:1,000 dilution) or isotype-matched
control antibody. Following incubation for 15 min at room
temperature in the dark, cells were washed twice with PBS and
10,000 cells were analyzed using FACSCalibur (BD Biosciences) and
CellQuest software (version 3.0; Becton Dickinson, Mountain View,
CA, USA).
Phagocytosis assay
The NB4 cells were treated with adenanthin and/or
VD3 for 3 days. Subsequently, 10 µl Escherichia coli
DH5α (OD620=0.5) cells (Tiangen Biotech, Co., Ltd.,
Beijing, China) were added to the medium (1 ml), and the culture
was incubated for a further 1 h at 37°C. The cells were washed
twice with phosphate-buffered saline (PBS) and spun onto a slide
using a Shandon Cytospin cytocentrifuge (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The cell morphology was examined using
Wright's staining.
Trypan blue exclusion assay
NB4 cells were treated with adenanthin and/or VD3
and cell viability was evaluated by trypan blue exclusion assay. In
brief, cells were plated in 24-well plates and washed with PBS
once, then replaced with PBS, including trypan blue solution at a
final concentration of 0.2% (Sigma-Aldrich, St. Louis, MO, USA),
and mixed thoroughly. Following standing at room temperature for 5
min, cells were washed with PBS again and then maintained in the
well with PBS. The stained and unstained cells in each well were
counted under a microscope (Nikon E400; Nikon, Tokyo, Japan). The
unstained cells represented viable cells.
Detection of intracellular ROS
The oxidation-sensitive fluorescent probe dye,
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA;
Invitrogen Molecular Probes®; Thermo Fisher Scientific,
Inc.), was used to measure the levels of intracellular ROS.
H2DCFDA is deacetylated intracellularly by non-specific
esterases, and is further oxidized by cellular peroxides to the
fluorescent compound, 2′,7′-dichlorofluorescein. Briefly, the NB4
cells were treated or untreated with adenanthin, washed with PBS
and incubated with 20 µM H2DCFDA for 30 min at
37°C, according to the manufacturer's protocol. The fluorescence
signals were detected using a fluorescence-activated cell sorting
flow cytometer (FACSCalibur; BD Biosciences). For each sample,
5,000 or 10,000 events were collected. The levels of
H2O2 were expressed in terms of the mean
fluorescence intensity.
NAC treatment
NB4 cells were pretreated with 2 mM NAC (A9165;
Sigma-Aldrich) for 1 h, then treated with adenanthin in the
presence or absence of VD3 for 3 days. The expression of cell
surface markers and differentiation-associated proteins was
determined.
RNA interference and transfection
Sense sequences against Prx I
(5′-AGATATCAGCCTGTCTGAC-3′), nuclear factor-κB (NF-κB;
5′-GATGAGATCTTCCTACTGT-3′), CCAAT/enhancer binding protein β
(C/EBPβ; 5′-GCCCTGAGTAATCACTTAAAG-3′) and non-target control short
hairpin (sh)RNA (NC; 5′-TCCCGTGAATTGGAATCCT-3′) followed by the
loop sequence (TTCAAGAGA) and the reverse compliment of the
targeting sequence, respectively, were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China), annealed and ligated into the
PSIREN-RetroQ Vector (Clontech Laboratories, Mountainview, CA, USA)
according to the manufacturer's instructions of the Knockout™ RNAi
Systems User Manual (Clontech Laboratories). The shRNA vectors or
negative control pSIREN-RetroQ vector were co-transfected with
packaging plasmids VSV-G and Gag-Pol into 293T cells (American Type
Culture Collection, Manassas, VA, USA) to produce the retrovirus.
Supernatants containing retrovirus were collected 48 h following
transfection and were used to infect NB4 cells. After 48 h, 0.5
µg/ml puromycin (Calbiochem, Darmstadt, Germany) was added
to the medium for selection and the stable transformants were
validated by assessing targeted proteins.
Western blot analysis
Cells were washed with PBS and lysed with lysis
buffer (50 mM Tris-HCl, pH 6.8, 100 mM DTT, 2% SDS and 10%
glycerol). Cell lysates were centrifuged at 20,000 × g for 10 min
and the supernatants were collected. Protein concentration in the
supernatant was determined by the bicinchoninic acid method
according to the manufacturer's instructions (Pierce Biotechnology,
Inc., Rockford, IL, USA) following trichloroacetic acid
precipitation. Equal amounts of cell lysates (10 µg protein
of each sample) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto an
Amersham ECL®-nitrocellulose membrane (GE Healthcare).
The membranes were stained with 0.2% Ponceau S red to ensure equal
protein loading. Following blocking of the membrane with 5% non-fat
milk in Tris-buffered saline, the membranes were incubated with
monoclonal mouse anti-human Prx I (cat. no. sc-293386; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; dilution 1:1,000), polyclonal
rabbit anti-human p21 (cat. no. sc-397; Santa Cruz Biotechnology,
Inc.; dilution 1:1,000), polyclonal rabbit anti-human p65 (cat. no.
sc-372; Santa Cruz Biotechnology, Inc.; dilution 1:1,000) and
polyclonal rabbit anti-human C/EBPβ (cat. no. sc-150; Santa Cruz
Biotechnology, Inc.; dilution 1:1,000) overnight at 4°C.
Subsequently, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat. no. 7074; dilution
1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA) and
horse anti-mouse IgG (cat. no. 7076; dilution 1:2,000; Cell
Signaling Technology, Inc.) secondary antibodies for 1 h at room
temperature. Detection of the signal was performed using a
SuperSignal West Pico Chemiluminescent Substrate kit (Pierce
Biotechnology, Inc.), according to the manufacturer's protocol.
β-actin (Merck Millipore, Darmstadt, Germany) was used as an
internal loading control. The bands were semiquantified using
Quantity One image analysis software (version 4.6.3; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). Student's
t-test was used to evaluate the difference between two different
treatments. P<0.05 was considered to indicate a statistically
significant value.
Results
Adenanthin plus a low concentration of
VD3 induces monocyte differentiation of NB4 cells
To determine whether adenanthin was able to enhance
VD3-induced cell differentiation, NB4 cells were treated with
either VD3 alone (1 nM) or a combination of VD3 and adenanthin (1
µM) for 5 days. As shown in Fig. 1A, the growth inhibitory effect of
adenanthin or VD3 on the NB4 cells was greatly strengthened by
their co-administration. Compared with treatment with either
adenanthin or VD3 alone, the expression of the cell differentiation
surface markers, CD11b and CD14, was markedly increased by
co-treatment with adenanthin and VD3 (Fig. 1B), which was accompanied by an
enlargement of the cytoplasm, the loss of cytoplasmic basophilia
and azurophilic granules, and the appearance of cytological
modifications of the monocytes (Fig.
1C). In addition, the differentiated cells acquired functional
properties of monocytes, including the engulfment of bacteria
(Fig. 1D). These results suggest
that adenanthin markedly enhances the VD3-induced monocyte
differentiation of NB4 cells.
 | Figure 1Ade enhances VD3-induced monocyte
differentiation of NB4 cells. (A) NB4 cells were treated with 1
µM Ade and/or 1 nM VD3 for the time period indicated, and
cell viability was assessed using the trypan blue exclusion assay.
(B) Expression of the cell surface markers, CD11b and CD14, was
detected by fluorescence-activated cell sorting. All values are
presented as the mean ± standard deviation of three independent
experiments. *P<0.05; **P<0.01,
compared with the single treatment groups (Con, Ade or VD3). (C)
Cell morphology was examined using Wright's staining, and the cells
were observed under a light microscope on day 3 (magnification,
×100). (D) Bacterial phagocytosis assays were performed
(magnification, ×100). Con, control; Ade, adenanthin; VD3,
1,25-dihydroxyvitamin D3. |
Knockdown of Prx I enhances VD3-induced
monocyte differentiation
Since adenanthin induces leukemia cell
differentiation by targeting Prx I, the present study postulated
that suppressing the expression of Prx I may also enhance
VD3-induced cell differentiation. To this end, the NB4 cells were
transfected with non-specific shRNA (NB4shNC) and Prx
I-specific shRNA (NB4shPrx I; Fig. 2A). Subsequently, the cells were
treated with VD3. Following treatment for 72 h, the percentage of
CD11b+CD14+ cells was significantly increased
in the NB4shPrx I cells, as compared with the
NB4shNC cells (Fig. 2B;
P<0.05). Since NF-κB has also been demonstrated to be a target
of adenanthin, whether inhibition of the NF-κB (p65) pathway also
contributed to the cell differentiation-enhancing effects of
adenanthin was examined. However, the specific knockdown of NF-κB
(p65) expression in NB4 cells (Fig.
2C) did not significantly enhance VD3-induced cell
differentiation (Fig. 2D,
P>0.05). These results indicate that targeting Prx I enhances
VD3-induced cell differentiation.
 | Figure 2Knockdown of Prx I enhances
VD3-induced monocyte differentiation in NB4 cells. (A) NB4 cells
were transfected with pSIREN-RetroQ-derived retroviruses carrying
Prx I-specific shRNA (shPrx I) or scrambled RNA (shNC), which was
used as a control. The expression levels of the indicated proteins
were assessed by western blot analysis. (B) Transfected cells were
treated with VD3 for 3 days, and the percentage of
CD11b+ and CD14+ cells were detected by
fluorescence-activated cell sorting. (C) Similarly, p65 was
specifically knocked down in NB4 cells, and the protein expression
levels were determined by western blotting. (D) Cells were treated
with or without VD3, and the percentage of CD11b+ and
CD14+ cells were evaluated by fluorescence-activated
cell sorting. All data are presented as the mean ± standard
deviation of three independent experiments. *P<0.05,
compared with the NB4shNC cells. N.S., no significant
difference compared with the NB4shp65 cells. shRNA,
short hairpin RNA; Con, control; Prx I, peroxiredoxin I; VD3,
1,25-dihydroxyvitamin D3. |
Adenanthin potentiates VD3-induced
differentiation via activation of the ROS-C/EBPβ pathway
Since Prx I functions as a
H2O2 scavenger (22), the present study determined whether
ROS may have an important role in the differentiation-enhancing
effects of adenanthin on VD3. As shown in Fig. 3A, adenanthin treatment alone led to
an increase in the levels of ROS in the NB4 cells, and the levels
were further increased by co-treatment with VD3. These results
suggest the ROS is involved in the combinatorial effects of
adenanthin and VD3. In support of this hypothesis,
N-acetylcysteine (NAC), a ROS scavenger, significantly
inhibited the adenanthin plus VD3-induced upregulation of CD11b and
CD14 (Fig. 3B). Since C/EBPβ has
been shown to exert an important role in monocyte differentiation,
the expression levels of C/EBPβ were detected following
co-treatment with adenanthin and VD3. Treatment of the cells with
adenanthin or VD3 alone increased the expression of C/EBPβ, whereas
co-treatment of the cells with adenanthin and VD3 led to a further
increase in the expression levels of C/EBPβ (Fig. 3C). The expression levels of p21, a
downstream target of C/EBPβ, were also upregulated. Notably, in the
presence of NAC, the effects of co-treatment with adenanthin and
VD3 on C/EBPβ expression were abrogated (Fig. 3C). In addition, the knockdown of
C/EBPβ abrogated adenanthin plus VD3-induced cell differentiation
(Figs. 3D and E). These results
suggest that adenanthin may enhance VD3-induced cell
differentiation via the ROS-C/EBPβ axis.
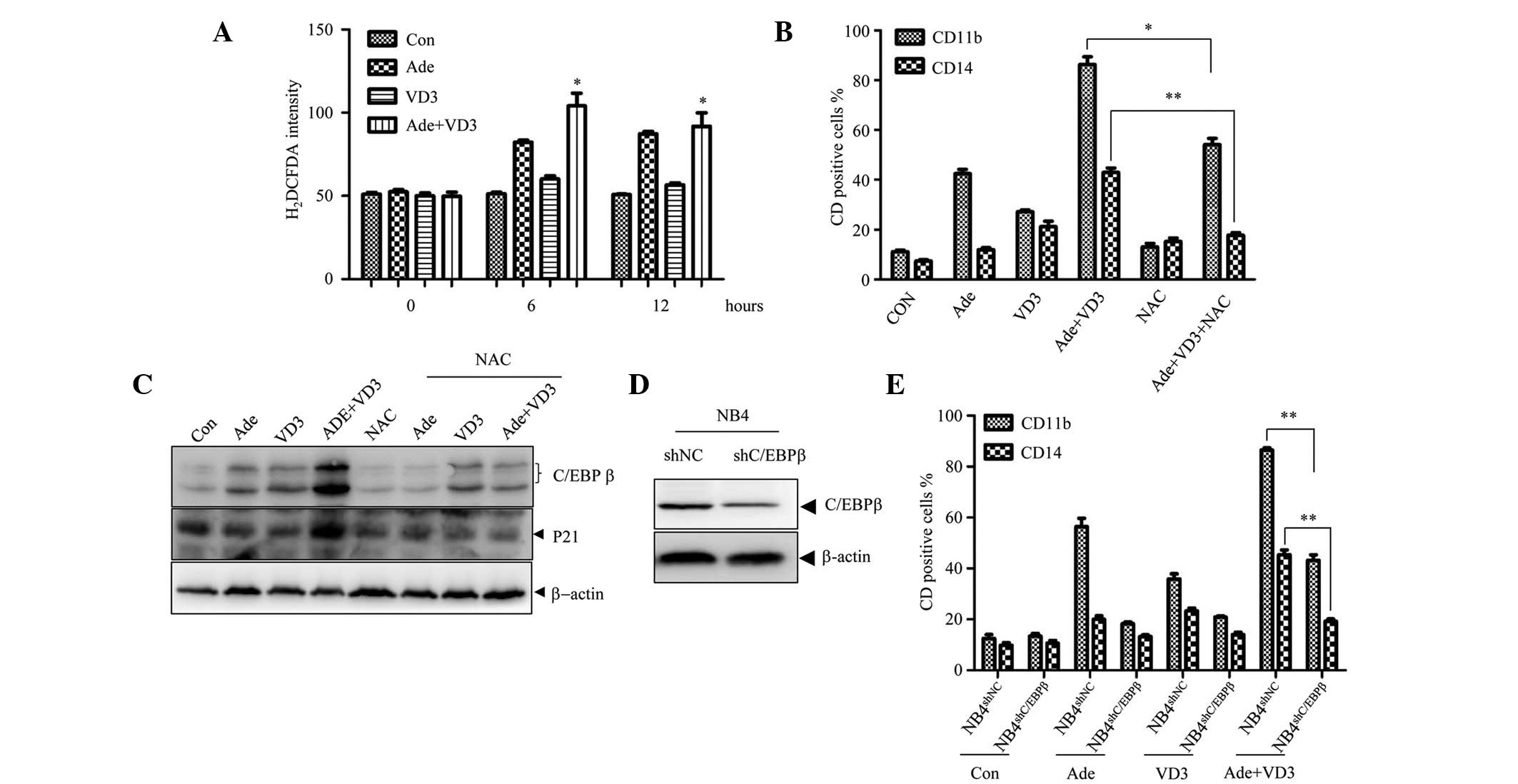 | Figure 3Activation of the ROS-C/EBPβ axis is
important in enhancing the effects of Ade on VD3-induced monocyte
differentiation. (A) NB4 cells were treated with Ade and/or VD3 for
the indicated time periods, and the levels of ROS were determined
using the H2DCFDA staining assay. (B) Following
pretreatment with or without 2 mM NAC for 1 h, the NB4 cells were
treated with Ade in the presence or absence of VD3, and the
percentage of CD11b- and CD14-positive cells was assessed by FACS.
(C) Expression levels of the indicated proteins were assessed using
western blotting. (D and E) NB4 cells were transfected with
pSIREN-RetroQ-derived retroviruses carrying C/EBPβ-specific shRNA
(NB4shC/EBPβ) or scrambled shRNA
(NB4shNC), which was used as a control. (D) Expression
levels of the indicated proteins were assessed using western
blotting. (E) Subsequently, the NB4shC/EBPβ
cells and NB4shNC cells were treated with Ade and/or VD3
for 3 days, and the percentage of CD11b- and CD14-positive cells
was detected by FACS. All data are presented as the mean ± standard
deviation of three independent experiments. *P<0.05;
**P<0.01, compared with the VD3 and Ade-co-treated
NB4shNC cells. Con, control; shRNA, short hairpin RNA;
ROS, reactive oxygen species; H2DCFDA,
2′,7′-dichlorodihydrofluorescein diacetate; NAC,
N-acetylcysteine; FACS, fluorescence-activated cell sorting;
C/EBPβ, CCAAT/enhancer binding protein β; Ade, adenanthin; VD3,
1,25-dihydroxyvitamin D3. |
Adenanthin potentiates VD3-induced
differentiation in U937, HL-60 and primary leukemia cells
To examine whether the combinatorial effects of
adenanthin and VD3 were cell-type-specific, U937, HL-60 and primary
APL cells were treated with adenanthin and/or VD3. Adenanthin plus
VD3 also enhanced monocyte differentiation of the U937, HL-60 and
primary APL cell lines, as determined by the increase in the number
of CD11b- and CD14-positive cells (Fig. 4A–C, the upper panels) and the
appearance of the monocytes' morphology (Fig. 4A–C, the lower panels). These
results suggest that the combinatorial effects of adenanthin and
VD3 are not limited to NB4 cells.
Discussion
Improving the efficacy and decreasing the side
effects of VD3 has recently garnered a lot of attention in the
scientific community. The present study demonstrated that
adenanthin was able to promote low dose (1 nM) VD3-induced cell
differentiation via the Prx I-ROS-C/EBPβ axis. Therefore, the
present study provides a basis for targeting Prx I to improve the
efficacy of VD3 in leukemia cells.
In mammalian cells, the homeostasis of ROS is
controlled by a diverse set of peroxidases, including catalase,
glutathione peroxidases and peroxiredoxins (25). The levels of ROS change according
to the differentiation status of hematopoietic cells (26); therefore, modulating the expression
or activity of these enzymes may regulate cell differentiation. For
example, in inducing the monocytic differentiation of U937 cells,
treatment with 12-O-tetradecanoylphorbol-13-acetate (TPA) was shown
to decrease the expression of catalase and increase the levels of
ROS, whereas overexpression of catalase inhibited TPA-induced
differentiation (18). Conversely,
Ding et al (27) reported
that catalase co-operates with ATRA to induce the differentiation
of human THP-1 monocytes into macrophages via a reduction in the
generation of H2O2. Therefore, these results
suggested that the functional role of ROS in cell differentiation
may be dependent on the nature of the differentiation induction. In
terms of VD3, Callens et al (21) demonstrated that an increase in ROS
enhanced VD3-induced cell differentiation, whereas Bondza-Kibangou
et al (19) reported that
antioxidants, including catalase, enhanced VD3-induced cell
differentiation. Therefore, further studies are required to fully
delineate the role of ROS in VD3-induced cell differentiation.
Peroxiredoxins comprise a family of six ubiquitous
peroxidases that reduce peroxides; their major functions include
protection against oxidative stress, and the induction of cell
signaling and proliferation (28).
Compared with catalase, peroxiredoxins are more abundant, and have
a higher degree of affinity for H2O2. An
aberrantly high expression of peroxiredoxins has been detected in
various types of cancer, which may contribute towards resistance to
chemotherapy or radiotherapy (24,29).
A previous study in our laboratory demonstrated that adenanthin is
a Prx I inhibitor that may induce partial differentiation of APL
cells in a ROS-dependent manner (22). However, whether the modulation of
Prx I may alter VD3-induced cell differentiation has yet to be
elucidated. The results of the present study demonstrated that
targeting Prx I by adenanthin or RNA interference enhanced
VD3-induced cell differentiation. On the basis of these findings,
it is proposed that an increase in the levels of ROS by targeting
antioxidant enzymes, such as Prx I, may represent a novel method
for enhancing the VD3-induced monocyte differentiation of leukemia
cells.
C/EBPβ, which is a basic leucine zipper
transcription factor, is a member of the C/EBP family (30). C/EBPβ is known to have an important
role in the control of monocytic differentiation (31). It has previously been reported that
VD3 enhances the mRNA and protein expression levels of C/EBPβ via
activation of Raf, mitogen-activated kinase kinase/extracellular
signal-regulated kinase and c-Jun N-terminal kinase (32,33).
In the present study, adenanthin plus VD3 was shown to markedly
increase the expression levels of C/EBPβ, and knocking down C/EBPβ
expression inhibited adenanthin plus VD3-induced cell
differentiation, thus suggesting that C/EBPβ fulfills a critical
role in the combinatorial effects of adenanthin and VD3. However,
the precise mechanisms underlying C/EBPβ upregulation remain to be
fully elucidated. The present study demonstrated that increases in
ROS were associated with the upregulation of C/EBPβ, since NAC, a
well-known ROS scavenger, markedly inhibited the combinatorial
effects of adenanthin and VD3 on C/EBPβ expression. Therefore, the
activation of the ROS-C/EBPβ axis has been demonstrated to fulfill
an important role in monocytic differentiation, which may provide
the basis for further studies to combine low doses of VD3 with
other ROS-increasing agents in order to induce leukemia cell
differentiation.
In conclusion, the present study demonstrated that
adenanthin was able to enhance VD3-induced cell differentiation in
leukemia cells via activation of the Prx I-ROS-C/EBPβ axis. These
results suggested that targeting Prx I may be considered a novel
strategy to enhance the efficacy of VD3. The development of novel
Prx I-targeting agents warrants further investigation.
Acknowledgments
The present study was supported in part by grants
from the National Basic Research Program of China (973 Program)
(grant nos. 2015CB910403 and 2013CB910903), the National Natural
Science Foundation of China (grant nos. 91313303, 81272886 and
31100980), the Science and Technology Committee of Shanghai (grant
nos. 11JC1406500, 13431900501 and 13ZR1456900), the Shanghai Talent
Development Project of Shanghai Human Resource and the Social
Security Bureau.
References
|
1
|
Gocek E and Marcinkowska E:
Differentiation therapy of acute myeloid leukemia. Cancers (Basel).
3:2402–2420. 2011. View Article : Google Scholar
|
|
2
|
Hughes PJ, Marcinkowska E, Gocek E,
Studzinski GP and Brown G: Vitamin D3-driven signals for myeloid
cell differentiation – implications for differentiation therapy.
Leuk Res. 34:553–565. 2010. View Article : Google Scholar :
|
|
3
|
Marchwicka A, Cebrat M, Sampath P,
Snieżewski L and Marcinkowska E: Perspectives of differentiation
therapies of acute myeloid leukemia: The search for the molecular
basis of patients' variable responses to 1,25-dihydroxyvitamin D
and vitamin D analogs. Front Oncol. 4:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trump DL, Deeb KK and Johnson CS: Vitamin
D: Considerations in the continued development as an agent for
cancer prevention and therapy. Cancer J. 16:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gocek E, Kiełbiński M, Baurska H, Haus O,
Kutner A and Marcinkowska E: Different susceptibilities to
1,25-dihydroxyvitamin D3-induced differentiation of AML cells
carrying various mutations. Leuk Res. 34:649–657. 2010. View Article : Google Scholar
|
|
6
|
Gocek E, Marchwicka A, Baurska H, Chrobak
A and Marcinkowska E: Opposite regulation of vitamin D receptor by
ATRA in AML cells susceptible and resistant to vitamin D-induced
differentiation. J Steroid Biochem Mol Biol. 132:220–226. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HJ, Muindi JR, Tan W, Hu Q, Wang D,
Liu S, Wilding GE, Ford LA, Sait SN, Block AW, et al: Low 25(OH)
vitamin D3 levels are associated with adverse outcome in newly
diagnosed, intensively treated adult acute myeloid leukemia.
Cancer. 120:521–529. 2014. View Article : Google Scholar :
|
|
8
|
Donovan PJ, Sundac L, Pretorius CJ,
d'Emden MC and McLeod DS: Calcitriol-mediated hypercalcemia: Causes
and course in 101 patients. J Clin Endocrinol Metab. 98:4023–4029.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bae JY, Kim JW and Kim I: Low-dose
1,25-dihydroxyvitamin D(3) combined with arsenic trioxide
synergistically inhibits proliferation of acute myeloid leukemia
cells by promoting apoptosis. Oncol Rep. 30:485–491.
2013.PubMed/NCBI
|
|
10
|
Clark CS, Konyer JE and Meckling KA:
1alpha,25-dihydroxyvitamin D3 and bryostatin-1 synergize to induce
monocytic differentiation of NB4 acute promyelocytic leukemia cells
by modulating cell cycle progression. Exp Cell Res. 294:301–311.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim DS, Kim SH, Song JH, Chang YT, Hwang
SY and Kim TS: Enhancing effects of ceramide derivatives on
1,25-dihydroxyvitamin D(3)-induced differentiation of human HL-60
leukemia cells. Life Sci. 81:1638–1644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SH, Yoo JC and Kim TS: Nargenicin
enhances 1,25-dihydroxyvitamin D(3)- and all-trans retinoic
acid-induced leukemia cell differentiation via PKCbetaI/MAPK
pathways. Biochem Pharmacol. 77:1694–1701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Q, Harrison JS, Uskokovic M, Kutner A
and Studzinski GP: Translational study of vitamin D differentiation
therapy of myeloid leukemia: Effects of the combination with a p38
MAPK inhibitor and an antioxidant. Leukemia. 19:1812–1817. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Salman H, Danilenko M and
Studzinski GP: Cooperation between antioxidants and
1,25-dihydroxyvitamin D3 in induction of leukemia HL60 cell
differentiation through the JNK/AP-1/Egr-1 pathway. J Cell Physiol.
204:964–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Ikezoe T, Nishioka C, Ni L,
Koeffler HP and Yokoyama A: Inhibition of mTORC1 by RAD001
(everolimus) potentiates the effects of 1,25-dihydroxyvitamin D(3)
to induce growth arrest and differentiation of AML cells in vitro
and in vivo. Exp Hematol. 38:666–676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Irwin ME, Rivera-Del Valle N and Chandra
J: Redox control of leukemia: From molecular mechanisms to
therapeutic opportunities. Antioxid Redox Signal. 18:1349–1383.
2013. View Article : Google Scholar :
|
|
17
|
Hole PS, Darley RL and Tonks A: Do
reactive oxygen species play a role in myeloid leukemias? Blood.
117:5816–5826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto T, Sakaguchi N, Hachiya M,
Nakayama F, Yamakawa M and Akashi M: Role of catalase in monocytic
differentiation of U937 cells by TPA: Hydrogen peroxide as a second
messenger. Leukemia. 23:761–769. 2009. View Article : Google Scholar
|
|
19
|
Bondza-Kibangou P, Millot C, El Khoury V
and Millot JM: Antioxidants and doxorubicin supplementation to
modulate CD14 expression and oxidative stress induced by vitamin D3
and seocalcitol in HL60 cells. Oncol Rep. 18:1513–1519.
2007.PubMed/NCBI
|
|
20
|
Stixová L, Procházková J, Soucek K,
Hofmanová J and Kozubík A: 5-Lipoxygenase inhibitors potentiate
1alpha,25-dihydroxyvitamin D3-induced monocytic differentiation by
activating p38 MAPK pathway. Mol Cell Biochem. 330:229–238. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Callens C, Coulon S, Naudin J,
Radford-Weiss I, Boissel N, Raffoux E, Wang PH, Agarwal S, Tamouza
H, Paubelle E, et al: Targeting iron homeostasis induces cellular
differentiation and synergizes with differentiating agents in acute
myeloid leukemia. J Exp Med. 207:731–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu CX, Yin QQ, Zhou HC, Wu YL, Pu JX, Xia
L, Liu W, Huang X, Jiang T, Wu MX, et al: Adenanthin targets
peroxiredoxin I and II to induce differentiation of leukemic cells.
Nat Chem Biol. 8:486–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu CX, Zhou HC, Yin QQ, Wu YL and Chen
GQ: Targeting peroxiredoxins against leukemia. Exp Cell Res.
319:170–176. 2013. View Article : Google Scholar
|
|
24
|
Ishii T, Warabi E and Yanagawa T: Novel
roles of peroxiredoxins in inflammation, cancer and innate
immunity. J Clin Biochem Nutr. 50:91–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Owusu-Ansah E and Banerjee U: Reactive
oxygen species prime Drosophila haematopoietic progenitors for
differentiation. Nature. 461:537–541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding Q, Jin T, Wang Z and Chen Y: Catalase
potentiates retinoic acid-induced THP-1 monocyte differentiation
into macrophage through inhibition of peroxisome
proliferator-activated receptor gamma. J Leukoc Biol. 81:1568–1576.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poole LB, Hall A and Nelson KJ: Overview
of peroxiredoxins in oxidant defense and redox regulation. Curr
Protoc Toxicol. Chapter 7: Unit7.9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang B, Wang Y and Su Y: Peroxiredoxins,
a novel target in cancer radiotherapy. Cancer Lett. 286:154–160.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsukada J, Yoshida Y, Kominato Y and Auron
PE: The CCAAT/enhancer (C/EBP) family of basic-leucine zipper
(bZIP) transcription factors is a multifaceted highly-regulated
system for gene regulation. Cytokine. 54:6–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huber R, Pietsch D, Panterodt T and Brand
K: Regulation of C/EBPβ and resulting functions in cells of the
monocytic lineage. Cell Signal. 24:1287–1296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji Y and Studzinski GP: Retinoblastoma
protein and CCAAT/enhancer-binding protein beta are required for
1,25-dihydroxyvitamin D3-induced monocytic differentiation of HL60
cells. Cancer Res. 64:370–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marcinkowska E, Garay E, Gocek E, Chrobak
A, Wang X and Studzinski GP: Regulation of C/EBPbeta isoforms by
MAPK pathways in HL60 cells induced to differentiate by
1,25-dihydroxyvitamin D3. Exp Cell Res. 312:2054–2065. 2006.
View Article : Google Scholar : PubMed/NCBI
|


















